Abstract
The heterotrimeric guanine nucleotide-binding protein Gαq transduces signals from heptahelical transmembrane receptors (e.g., α1-adrenergic, endothelin 1A, and angiotensin II) to stimulate generation of inositol-1,4,5-trisphosphate and diacylglycerol. In addition, Gαq decreases cAMP production, through unknown mechanisms, and thus affects physiological responsiveness of cardiac myocytes and other cells. Here, we provide evidence that Gαq expression increases Gαs ubiquitination, decreases Gαs protein content, and impairs basal and β1-adrenergic receptor-stimulated cAMP production. These biochemical and functional changes are associated with Akt activation. Expression of constitutively active Akt also decreases Gαs protein content and inhibits basal and β1-adrenergic receptor-stimulated cAMP production. Akt knockdown inhibits Gαq-induced reduction of Gαs protein. In addition, MDM2, an E3 ubiquitin ligase, binds Gαs and promotes its degradation. Therefore, increased expression of Gαq decreases cAMP production through Akt-mediated Gαs protein ubiquitination and proteasomal degradation.
Keywords: G protein, ubiquitination, proteasomal degradation, Akt, cAMP
INTRODUCTION
Heptahelical transmembrane receptors comprise the largest and most versatile receptor family in the mammalian genome and are ubiquitously expressed. Heterotrimeric guanine nucleotide-binding proteins (G proteins) are pivotal mediators of signaling pathways initiated by these receptors. Each G protein selectively binds to specific receptors and transduces the signal to a specific set of downstream effectors [1]. For instance, Gαs transduces signals from the β1-adrenergic receptor (β1AR) to stimulate cAMP production by adenylyl cyclase, and Gαq transduces signals from the α1–adrenergic receptor (α1AR) to stimulate inositol-1,4,5-trisphosphate and diacylglycerol generation via phospholipase C. These signals, in turn, activate additional downstream effectors, such as protein kinases, to regulate a diverse range of biological functions [1].
In addition to mediating these classical linear signaling pathways, a specific heptahelical G protein-coupled receptor may regulate signaling pathways activated by another receptor. This interaction has been referred to as “cross-talk” between two signaling pathways. One typical example of cross-talk is inhibition of β1AR-mediated cAMP production by α1AR stimulation in the heart [2]. This inhibition of cAMP production is associated with depressed left ventricular (LV) contractility in vivo [2]. Similarly, cardiac-directed expression of Gαq decreases βAR-mediated cAMP production and impairs LV function [3; 4; 5]. These examples underscore the potential importance of Gαq on β1AR-mediated cAMP production, and mandate studies to determine mechanisms underlying these interactions. Previous reports indicate that inhibition of βAR-mediated cAMP production by Gαq activation does not occur via receptor desensitization or reduced adenylyl cyclase expression [3; 4; 6]. In the current study, we tested the hypothesis that Gαq activation inhibits β1AR-mediated cAMP production at the Gαs level. Our results reveal a novel mechanism by which Gαq influences Gαs signaling by regulating Gαs protein levels.
MATERIALS AND METHODS
Animals
Transgenic mice with cardiac-directed expression of Gαq (initial transgenic mouse line provided by Dr. G.W. Dorn, University of Cincinnati) were used. Left ventricles from 10-week-old Gαq mice (Gαq) and transgene negative littermates (CON) were snap frozen with liquid nitrogen, and stored at –80°C. This study was approved by Animal Use and Care Committee of VA San Diego Healthcare System, in accordance with AAALAC guidelines.
Cell culture
Cardiac myocytes and fibroblasts were isolated from 1-day-old Sprague-Dawley rats by Percoll centrifugation as previously described [7]. HEK293 cells were from ATCC. Cells were maintained in DMEM supplemented with 10% fetal bovine serum in a humidified incubator with 5% CO2 at 37°C. DNA transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Forty-eight hours after transfection, cells were harvested for Western blotting.
Reagents
Protease inhibitors MG132 (carbobenzyloxy-L-leucinyl-L-leucinyl-L-leucinal), lactacystin, ALLM (N-acetyl-L-leucinyl-L-leucinyl-methioninal), MDL (carbobenzoxyl-valyl-phenylalanial), and E64 (trans-epoxysuccinyl-L-leucylamido(4-guanidino)butane) were from Calbiochem. Calcium activators ionomycin and thapsigarin, PKC activator phorbol 12-myristate 13-acetate (PMA), and PKC inhibitors bisindolylmaleimide (BIM) and Et-8-OCH3 were also from Calbiochem. ON-TARGETplus SMARTpool small interfering RNA (siRNA) specific for human Akt1 was obtained from Dharmacon.
Antibodies to Gαs(sc-383), Gαq(sc-393), and ubiquitin(sc-8017) were obtained from Santa Cruz Biotechnology; antibody to MDM2(clone SMP14) was purchased from BD Biosciences; antibodies to phosphor-Akt(S473)(#4051), Akt(#9272), and phosphor-MDM2(S166)(#3521) were obtained from Cell Signaling Technology; and antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; RDI-TRK5G4-6C5) was obtained from Research Diagnostic Inc.
Mammalian expression vectors encoding MDM2 (provided by Dr. J. Chen, University of South Florida) and Gαq (pcDNA-Gαq, University of Missouri-Rolla cDNA Resource Center) were used. Full length Gαq cDNA was subcloned adenovirus shuttle vector pE1Z for generating adenovirus encoding Gαq (Ad.Gαq). Empty adenovirus vector (Ad.NULL) was also constructed. Adenovirus encoding constitutively active Akt (Ad.Akt) (provided by Dr. K. Walsh, Boston University) was expanded and purified.
Western blotting
LV samples or cultured cells were homogenized and Western blotting was performed as described previously [6]. Equal loading of samples and even transfer efficiency were monitored by reprobing stripped blots with GAPDH antibody. Quantification of protein content was performed using Gel-Pro® Analyzer (Media Cybernetics) as previously described [6].
Co-immunoprecipitation
Cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40) in the presence of protein inhibitor cocktail (Roche). Cell lysates (500 μg total protein) were clarified by centrifugation, precleared by incubation with protein A/G beads (Pierce), and incubated for 5h at 4°C with immunoprecipitation antibodies. The resulting immune complexes were precipitated with protein A/G beads (Pierce), washed with RIPA buffer, and subjected to Western blotting.
Northern blotting
Total cellular RNA was extracted from LV samples using RNA STAT-60 (Tel-Test Inc.). Twenty micrograms of denatured total RNA was fractionated in a formaldehyde-agarose gel, and transferred to a Nytran membrane (Schleicher & Schuell), and hybridized with a 32P-labeled murin Gαs cDNA probe amplified by PCR using primer pairs: 5’-CGTGATGAACCTGCCGAAC-3’ and 5’-CCTCCTGCAGGCGGTTAG-3’. Equal loading of samples and even transfer efficiency were monitored by re-probing stripped blots with β-actin probe. Quantification of mRNA expression was performed using Gel-Pro® Analyzer.
Akt knockdown by siRNA transfection
HEK293 cells grown to 50–60% confluency were transfected with 200 pmol ON-TARGETplus SMARTpool siRNA specific for human Akt1 using the X-tremeGENE siRNA transfection (Roche) reagent according to the manufacturer’s instruction. Twenty-four hours after siRNA transfection, Ad.NULL or Ad.Gαq were added to the cell culture. Cells were harvested 24 h after viral infection and cell homogenates were used for Western blotting.
Statistical analysis
Data represent mean±SE; group differences were detected using one-way ANOVA, followed by Bonferroni t-testing. The null hypothesis was rejected when p<0.05.
RESULTS
Gαq expression decreases Gαs protein but not mRNA content
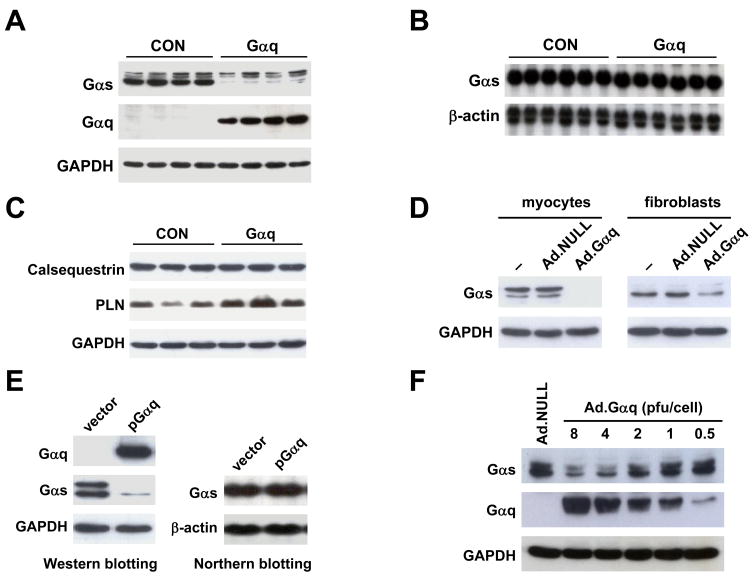
To test the hypothesis that Gαq decreases βAR-mediated cAMP production at the Gαs level, we first compared Gαs protein content in LV samples from transgenic Gαq mice and CON mice by Western blotting. Gαs protein content in LV homogenates from Gαq mice was reduced by 98% (CON: 666±23 densitometric units (du), Gαq: 16±1 du, p<0.0001, n=8; Fig. 1A). Despite such a marked decrease in Gαs protein content, Northern blotting showed no difference in contents of Gαs mRNA in CON and Gαq mouse hearts (CON: 3147±45 du, Gαq: 3208±116 du, p=0.6, n=6; Fig. 1B). These results indicate that Gαq expression affects Gαs protein content post-transcriptionally.
Fig. 1.
Cardiac-directed Gαq expression decreased Gαs protein, but not Gαs mRNA. (A) A representative Western blot showing decreased Gαs protein content in LV samples from 4 CON and 4 Gαq mice. (B) Northern blot showing normal Gαs mRNA content in LV samples from Gαq mice; β-actin was used as an internal control. (C) Western blots showing unchanged calsequestrin and GAPDH expression, and increased phospholamban (PLN) expression, in LV samples from Gαq mice. (D) Western blots showing decreased Gαs protein content in isolated cardiac myocytes and fibroblasts after gene transfer with Ad.Gαq. (E) Gαs protein was decreased in HEK293 cells transfected with pGαq (vs empty plasmid vector pcDNA3.1), despite normal Gαs mRNA content. (F) Increased Gαq expression were associated with progressively reduced amounts of Gαs. GAPDH and β-actin were used as internal controls for Western and Northern blotting respectively.
We then compared contents of other proteins in LV samples from CON and Gαq mouse hearts. Western blotting showed no change in GAPDH and calsequestrin protein levels after Gαq expression (Fig. 1C). Content of phospholamban protein, in contrast to Gαs, was increased by Gαq expression (CON: 611±88 du, Gαq: 1532±169 du, p<0.001, n=6; Figure 1C), indicating that Gαq expression does not result in a general diminution in protein levels in mouse hearts.
To determine whether the effect of Gαq expression was cell type-dependent, we isolated cardiac myocytes and cardiac fibroblasts, and infected these cells with Ad.Gαq (4 pfu/cell). Western blotting showed that Gαs protein content was decreased by Gαq expression in both cardiac myocytes and fibroblasts (Fig. 1D), indicating that reduction in Gαs conferred by increased Gαq expression was not cell type-dependent.
To determine whether Gαs down-regulation by Gαq also occurs in cells not derived from the heart, we measured Gαs protein content in HEK293 cells transfected with pcDNA-Gαq. Western blotting showed that Gαq expression reducedGαs protein content in HEK293 cells (Fig. 1E), as it did in cardiac myocytes and fibroblasts and in vivo. We also found that Gαq expression did not affect Gαs mRNA content by Northern blotting in HEK293 cells (Fig. 1E). In addition, a dose-response relationship between increased Ad.Gαq amount (0.5–8 pfu/cell) and reduction in Gαs protein content (0–74% reduction) was documented (Fig. 1F). These results indicate that Gαq down-regulates Gαs protein content post-transcriptionally in multiple cell types and in proportion to the Gαq expression level.
Gαq decreases Gαs protein by facilitating proteasomal degradation
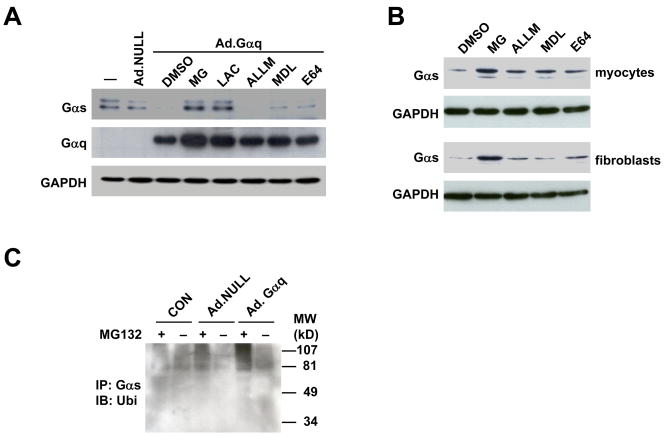
To gain insight into how Gαq expression decreases Gαs protein content, we infected HEK293 cells with Ad.Gαq and treated these cells with protease inhibitors. Western blotting showed that MG132 and lactacystin, two specific proteasomal inhibitors, blocked Gαq-induced reduction of Gαs protein. In contrast, Gαs protein content was not altered by calpain inhibitor ALLM, and was marginally increased by lysosomal protease inhibitors MDL and E64 (Fig. 2A), indicating that Gαq-induced increased Gαs turnover is mediated by proteasomal degradation.
Fig. 2.
Gαq expression increased Gαs ubiquitination and proteasomal degradation. (A) In HEK293 cells, Gαq-induced reduction in Gαs protein content was blocked by specific proteasomal inhibitors, MG132 (MG) and by lactacystin (LAC), but not by calpain inhibitor ALLM or lysosomal protease inhibitors MDL and E64. (B) In cardiac myocytes and fibroblasts, specific proteasomal inhibitor MG132, but not by calpain inhibitor ALLM or by lysosomal protease inhibitors MDL and E64, inhibited Gαs protein reduction in cardiac myocytes and fibroblasts. (C) Increased Gαq expression increased Gαs ubiquitination. Gαs was immunoprecipitated from cell homogenates from parental HEK293 cells or HEK293 cells infected with Ad.NULL or Ad.Gαq. Ubiquitin conjugation was detected by Western blotting using a specific ubiquitin antibody.
We then treated isolated cardiac myocytes and cardiac fibroblasts with MG132, ALLM, MDL, and E64, and measured Gαs protein content. Western blotting showed that only the proteasomal inhibitor MG132 blocked degradation of Gαs protein in both cardiac myocytes and cardiac fibroblasts (Fig. 2B), confirming that Gαs protein turnover in these cardiac-derived cells is also mediated by proteasomal degradation.
Gαq increases Gαs ubiquitination
Proteins are usually first marked by conjugation with ubiquitin before being targeted for proteasomal degradation. To determine whether Gαs underwent ubiquitination, Gαs was immunoprecipitated from HEK293 cell homogenates, and ubiquitin-conjugated Gαs was determined by Western blotting using an antibody against ubiquitin. A smear of ubiquitin-conjugated Gαs was noted in HEK293 cell homogenates, and accumulation of polyubiquitinated Gαs was found in cells incubated with MG132 (Fig. 2C). Infection of HEK293 cells with Ad.Gαq increased the density of the smear of ubiquitin-conjugated Gαs, both with and without MG132 incubation (Fig. 2C). These data indicate that Gαs is subject to polyubiquitination in intact cells, and expression of Gαq increases the amount of polyubiquitinated Gαs.
Akt activation decreases Gαs protein content and function
To determine the mechanism by which Gαq facilitates Gαs proteasomal degradation, we first examined the potential role of Ca2+ and PKC (both activated by Gαq). Treatment with the Ca2+ activators ionomycin and thapsigarin, and the PKC activator PMA did not alter Gαs protein content (Fig. 3A). In addition, treatment with the PKC inhibitors BIM and Et-8-OCH3 did not inhibit Gαq-induced Gαs degradation (Fig. 3A), suggesting that Gαq-facilitated Gαs proteasomal degradation is not mediated by Ca2+ or PKC activation.
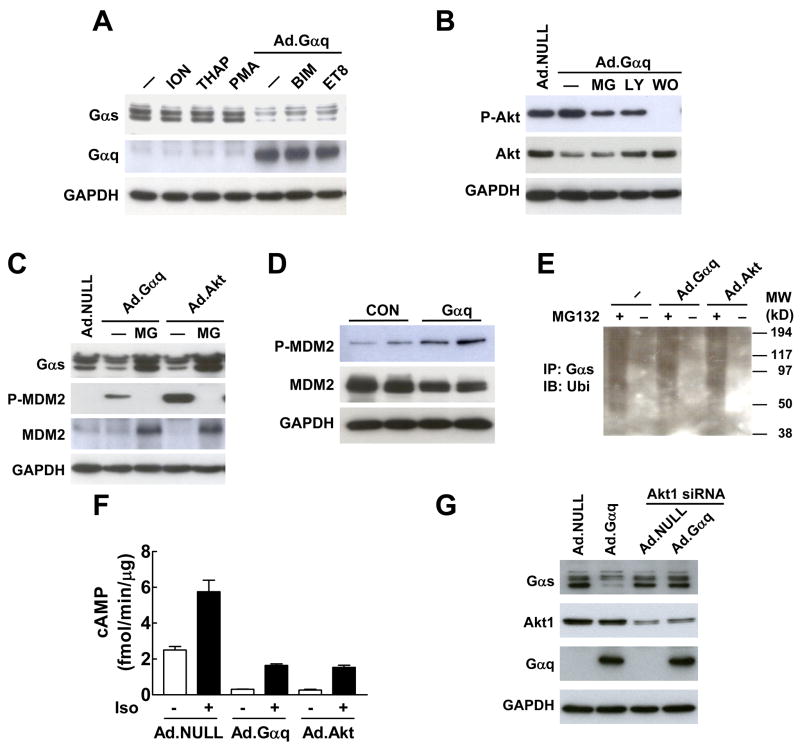
Fig. 3.
Gαq-induced Gαs protein degradation is mediated by Akt. (A) Gαq-induced reduction in Gαs protein was not mediated by Ca2+ activators ionomycin (ION) and thapsigarin (THAP), PKC activator PMA, and PKC inhibitors BMI and Et-8-OCH3 (ET8). (B) PI3K inhibitors LY294002 (LY) and wortmanin (WO) inhibited Gαq-induced Akt activation. MG, MG132. (C) Expression of Gαq and constitutively active Akt in HEK293 cells decreased Gαs protein content and increased MDM2 phosphorylation. Treatment with the proteasomal inhibitor MG132 (MG) blocked the reduction of Gαs protein content. (D) Cardiac-directed Gαq expression increased MDM2 phosphorylation in LV samples. (E) Expression of constitutively active Akt (Ad.Akt) increased Gαs ubiquitination in HEK293 cells. Endogenous Gαs-ubiquitin conjugate was co-immunoprecipitated by Gαs antibody and detected by Western blotting using ubiquitin antibody. (F) Expression of Gαq and constitutively active Akt decreased basal and isoproterenol (Iso)-stimulated cAMP production in isolated neonatal cardiac fibroblasts. Bars represent mean values from six experiments and error bars denote SEM (n=6). (G) Knockdown of Akt1 by siRNA blocked Gαq-induced reduction in Gαs protein.
We then investigated whether Gαq–facilitated Gαs protein degradation was mediated by Akt, another mediator for Gαq signaling [8]. In HEK293 cells, we found that Gαq increased protein content of Ser473-phosphorylated Akt, a surrogate measurement for Akt activation (Fig. 3B). In addition, inclusion of the PI3K inhibitors, LY294002 and wortmannin, inhibited Gαq-induced Akt activation. Furthermore, similar to expression of a constitutively active form of Akt, expression of Gαq increased MDM2 phosphorylation at Ser166, a preferred site for Akt-mediated phosphorylation (Fig. 3C). Increased MDM2 phosphorylation also was observed in LV samples from mice with cardiac-directed Gαq expression (Fig. 3D). These results confirm that Gαq expression activates Akt.
We then explored whether activation of Akt could affect Gαs protein content and function. Expression of the constitutively active form of Akt decreased Gαs protein content, and this decrease was blocked by the proteasomal protein degradation inhibitor MG132 (Fig. 3C). We also found that expression of a constitutively active form of Akt, like expression of Gαq, increased ubiquitinated Gαs protein content (Fig. 3E), a necessary step for facilitated proteasomal degradation. In addition, expression of Gαq and constitutively active Akt not only decreased basal cAMP production (Ad.Gαq vs Ad.NULL: 88% reduction, p<0.001; Ad.Akt vs Ad.NULL: 89% reduction, p<0.001), but also decreased isoproterenol-stimulated cAMP production in isolated cardiac fibroblasts (Ad.Gαq vs Ad.NULL: 72% reduction, p<0.001; Ad.Akt vs Ad.NULL: 73% reduction, p<0.001; Fig. 3F). Finally, knockdown of Akt1 in HEK293 cells using siRNA inhibited Gαq-induced reduction of Gαs protein (Fig. 3G). These data indicate that Akt activation mediates Gαq-facilitated Gαs protein turnover and function.
MDM2 expression decreases Gαs protein content
To explore whether MDM2, an E3 ubiquitin ligase, influences Gαs protein degradation, we first tested whether MDM2 was physically associated with Gαs. We found that Gαs was co-immunoprecipitated by a specific anti-MDM2 antibody from lysate from HEK293 cells expressing Gαq (Fig. 4A). This physical association of Gαs and MDM2 was confirmed by co-immunoprecipitation of the Gαs-MDM2 complex using anti-Gαs antibody and immunoblotting with anti-MDM2 antibody (Fig. 4A). Incubation of HEK293 cells with the proteasomal degradation inhibitor MG132 increased the amount of co-immunoprecipitated Gαs and MDM2 (Fig. 4A). We then examined whether MDM2 affected Gαs protein content. Western blotting showed that increased MDM2 expression decreased Gαs protein content in HEK293 cells (Fig. 4B). Taken together, these data indicate that MDM2 binds to Gαs and has a direct impact on Gαs protein content.
Fig. 4.
MDM2 binds Gαs and decreases its content. (A) Co-immunoprecipitation of MDM2 and Gαs from cell homogenates from MG132-treated HEK293 cells infected with Ad.Gαq. MG, MG132. (B) Expression of MDM2 in HEK293 cells decreased Gαs protein content.
DISCUSSION
In this study, we identified a novel mechanism by which increased Gαq influences the Gαs- mediated signaling pathway. We found that Gαq expression increased Gαs ubiquitination, decreased Gαs protein content, and impaired basal and β1AR-stimulated cAMP production. These biochemical and functional changes were associated with Akt activation. We demonstrated that expression of constitutively active Akt decreased Gαs protein content and inhibited basal and β1AR-stimulated cAMP production, and Akt knockdown inhibited Gαq-induced reduction of Gαs protein. In addition, we provided evidence that MDM2, an E3 ubiquitin ligase, bound Gαs and promoted Gαs protein degradation.
Heterotrimeric G proteins play a major role in transducing signals from hundreds of cell membrane G protein-coupled receptors [1]. Recent studies also describe accessory proteins as new Gα binding partners, which provide additional signal input independent of G protein-coupled receptors and introduce additional functional roles for G proteins [9]. The physiological and pathophysiological responses that are directly regulated by G proteins are diverse, ranging from embryonic development to clinical heart disease and cancer. The functional specificity of G proteins is largely determined by the α subunit, although βγ subunits also play important roles [10]. Although cross-talk between Gαq and Gαs signaling pathways has been proposed [11; 12], those studies only measured transient changes of cAMP production in one cell type in vitro. The effect of long-term Gαq activation on Gαs signaling was established in these studies. Our data provide direct evidence that Gαq reduces Gαs signaling by facilitating Gαs protein proteasomal degradation in multiple cells and also in intact hearts.
The ubiquitin-proteasome system plays an essential role in numerous biological and pathological processes [13]. Gpa1, an α subunit of a G protein in Saccharomyces cerevisiae, appears to undergo ubiquitination and proteasomal degradation [14], but whether this also occurs in mammalian G proteins is unknown. Recently it was shown that MG132 blocks mammalian Gαs degradation [15], but mechanisms have not been established. Our data not only demonstrate that Gαq facilitates Gαs proteasomal degradation and decreases cAMP production, but also provide evidence that Akt mediates this process.
Ubiquitination occurs in a three-step process catalyzed by ubiquitin-activating enzyme (E1), conjugating enzyme (E2), and ubiquitin ligase (E3) [13]. MDM2, an E3 ubiquitin ligase, not only interacts with tumor suppressor protein p53 and other nuclear proteins and regulates their function, but also binds cytoplasmic β-arrestin [16]. Association of MDM2 and β-arrestin brings β2AR, insulin-like growth factor receptor, and G protein-coupled receptor kinase 2 to the vicinity of MDM2. MDM2-catalyzed ubiquitination of these membrane or membrane-bound proteins is essential for their internalization or degradation [16; 17; 18]. In the present study, we showed that MDM2 interacted with Gαs, and promoted Gαs ubiquitination and proteasomal degradation. This binding of MDM2 and Gαs, unlike that with β2AR, insulin-like growth factor receptor, and G protein-coupled receptor kinase 2 [16; 17; 18], does not appear to require the presence of β-arrestin — we could not detect the Gαs/β-arrestin complex by co-immunoprecipitation (data not shown), while the Gαs/MDM2 complex was readily detectable under the same experimental conditions.
Protein ubiquitination and proteasomal degradation can be stimulated or inhibited by protein kinase activation [19]. Akt promotes proteasomal degradation of transcription factors, e.g. p53, FOXO, and NFAT, but inhibits protein turnover of itself and BRF1, a zinc finger protein with mRNA decay properties. Our data suggest a previously unrecognized intracellular signaling event in which Akt activation mediates Gαq-induced Gαs ubiquitination and proteasomal degradation. Alternatively, increased Gαq may sequester Gβγ subunits away from Gαs and subsequently promote selective ubiquitination and degradation of Gαs. However, our findings in this study do not support this notion: (1) Akt expression, in the absence of Gαq overexpression, increased Gαs ubiquitination and proteasomal degradation. (2) Akt knockdown inhibited Gαq-induced reduction of Gαs. Since Gαs contains a potential Akt phosphorylation motif LLRCRVLTSGI (consensus Akt phosphorylation motif: RXRXXS/T) surrounding threonine residue 190, it is possible that Akt promoted Gαs degradation by increasing Gαs phosphorylation, as shown in many other cases [19]. To exclude this possibility, we determined whether the synthesized LLRCRVLTSGI peptide is an Akt substrate. Using an in vitro phosphorylation assay with active recombinant Akt1, we found that this peptide is a poor Akt substrate (Km:123 μM, Vmax: 27 nmol/min/mg) (unpublished data). It is therefore unlikely that direct Gαs phosphorylation by Akt affects Gαs protein stability. We are actively investigating whether Akt-promoted Gαs degradation results from increased MDM2 E3 ligase activity via MDM2 stabilization [20].
Gαq transduces signals from heptahelical transmembrane receptors, e.g. α1AR and angiotensin II receptor. Increased expression of Gαq leads to pathological cardiac hypertrophy and development of heart failure [3; 5]. Reduced Gαq signaling (Gαq deletion or inhibition) appears to prevent maladaptive cardiac hypertrophy [21; 22]. A polymorphism in the Gαq gene promoter region is associated with enhanced promoter activity, increased Gαq expression, higher prevalence of LV hypertrophy, accelerated mortality in heart failure [23; 24]. Since cAMP is a critical regulator for LV contractile function, facilitated Gαs degradation and depressed cAMP production may represent a novel mechanism for Gαq-induced cardiac dysfunction.
In conclusion, our data reveal a novel mechanism by which Gαq decreases basal and βAR-stimulated cAMP production through Akt activation, increased Gαs ubiquitination, and facilitated Gαs proteasomal degradation.
Acknowledgments
This work was supported by Grant-In-Aids from the American Heart Association Western States Affiliate (TT and MHG), NIH grants HL66941, HL088426, and HL081741 (HKH), and a VA Merit Review Award (HKH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunter T. Signaling--2000 and beyond. Cell. 2000;100:113–27. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 2.Akhter SA, Milano CA, Shotwell KF, et al. Transgenic mice with cardiac overexpression of α1B-adrenergic receptors. In vivo α1-adrenergic receptor-mediated regulation of β-adrenergic signaling. J Biol Chem. 1997;272:21253–9. doi: 10.1074/jbc.272.34.21253. [DOI] [PubMed] [Google Scholar]
- 3.Dorn GW, 2nd, Tepe NM, Lorenz JN, et al. Low- and high-level transgenic expression of β2-adrenergic receptors differentially affect cardiac hypertrophy and function in Gαq-overexpressing mice. Proc Natl Acad Sci U S A. 1999;96:6400–5. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth DM, Gao MH, Lai NC, et al. Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation. 1999;99:3099–102. doi: 10.1161/01.cir.99.24.3099. [DOI] [PubMed] [Google Scholar]
- 5.D'Angelo DD, Sakata Y, Lorenz JN, et al. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–6. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang T, Gao MH, Roth DM, et al. Adenylyl cyclase type VI corrects cardiac sarcoplasmic reticulum calcium uptake defects in cardiomyopathy. Am J Physiol Heart Circ Physiol. 2004;287:H1906–12. doi: 10.1152/ajpheart.00356.2004. [DOI] [PubMed] [Google Scholar]
- 7.Gao M, Ping P, Post S, et al. Increased expression of adenylylcyclase type VI proportionately increases β-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1998;95:1038–43. doi: 10.1073/pnas.95.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howes AL, Miyamoto S, Adams JW, et al. Gαq expression activates EGFR and induces Akt mediated cardiomyocyte survival: dissociation from Gαq mediated hypertrophy. J Mol Cell Cardiol. 2006;40:597–604. doi: 10.1016/j.yjmcc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Sato M, Blumer JB, Simon V, et al. Accessory proteins for G proteins: partners in signaling. Annu Rev Pharmacol Toxicol. 2006;46:151–87. doi: 10.1146/annurev.pharmtox.46.120604.141115. [DOI] [PubMed] [Google Scholar]
- 10.Robishaw JD, Berlot CH. Translating G protein subunit diversity into functional specificity. Curr Opin Cell Biol. 2004;16:206–9. doi: 10.1016/j.ceb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, et al. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: cross talk between Gq and Gs. Am J Physiol Cell Physiol. 2000;278:C154–62. doi: 10.1152/ajpcell.2000.278.1.C154. [DOI] [PubMed] [Google Scholar]
- 12.Ostrom RS, Naugle JE, Hase M, et al. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J Biol Chem. 2003;278:24461–8. doi: 10.1074/jbc.M212659200. [DOI] [PubMed] [Google Scholar]
- 13.Haglund K, Dikic I. Ubiquitylation and cell signaling. Embo J. 2005;24:3353–9. doi: 10.1038/sj.emboj.7600808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madura K, Varshavsky A. Degradation of Gα by the N-end rule pathway. Science. 1994;265:1454–8. doi: 10.1126/science.8073290. [DOI] [PubMed] [Google Scholar]
- 15.Naviglio S, Pagano M, Romano M, et al. Adenylate cyclase regulation via proteasome-mediated modulation of Gα levels. Cell Signal. 2004;16:1229–37. doi: 10.1016/j.cellsig.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Shenoy SK, McDonald PH, Kohout TA, et al. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–13. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 17.Girnita L, Girnita A, Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci U S A. 2003;100:8247–52. doi: 10.1073/pnas.1431613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salcedo A, Mayor F, Jr, Penela P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J. 2006;25:4752–62. doi: 10.1038/sj.emboj.7601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao M, Karin M. Regulating the regulators: control of protein ubiquitination and ubiquitin-like modifications by extracellular stimuli. Mol Cell. 2005;19:581–93. doi: 10.1016/j.molcel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Feng J, Tamaskovic R, Yang Z, et al. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J Biol Chem. 2004;279:35510–7. doi: 10.1074/jbc.M404936200. [DOI] [PubMed] [Google Scholar]
- 21.Wettschureck N, Rutten H, Zywietz A, et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat Med. 2001;7:1236–40. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 22.Esposito G, Rapacciuolo A, Naga Prasad SV, et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 23.Liggett SB, Kelly RJ, Parekh RR, et al. A functional polymorphism of the Gαq (GNAQ) gene is associated with accelerated mortality in African-American heart failure. Hum Mol Genet. 2007;16:2740–50. doi: 10.1093/hmg/ddm229. [DOI] [PubMed] [Google Scholar]
- 24.Frey UH, Lieb W, Erdmann J, et al. Characterization of the GNAQ promoter and association of increased Gq expression with cardiac hypertrophy in humans. Eur Heart J. 2008;29:888–97. doi: 10.1093/eurheartj/ehm618. [DOI] [PubMed] [Google Scholar]