Abstract
Background
Scholars acknowledge that both biologically-founded child temperament and environmental factors are influential in determining the quality of infant–mother attachment. Wepresent evidence for gene by environment (G × E) interaction in the organization of attachment.
Methods
Participants were 88 typically developing infants and their mothers. Molecular genetic measures of the infants focused on the polymorphism in the serotonin transporter gene (5-HTTLPR, ss/sl vs. ll genotype). Mothers’ responsiveness to their infants at 7 months was observed in lengthy naturalistic interactions, and was conceptualized as the environmental influence.
Results
For infants with a short allele (ss/sl ), variation in mothers’ responsiveness was significantly associated with attachment security, assessed at 15 months in the Strange Situation. For those infants, low responsiveness predicted particularly high risk for insecure attachment, and high responsiveness offset that risk. For infants homozygous for the long allele (ll ), there was no association between responsiveness and attachment organization.
Conclusions
The findings show that the quality of early care serves to amplify or offset the risk conferred by genotype.
Keywords: G × E interaction, attachment, maternal responsiveness, 5-HTTLPR, parent, child relationships
Because of its universal nature, attachment between infants and their caregivers has been a perennial research topic (Bowlby, 1969/1982; Cassidy & Shaver, 1999). Intense controversies ensued regarding its assessment, determinants, and implications, particularly the roles of biologically-founded child temperament (typically assessed behaviorally or through parental reports) versus qualities of early care. Although scholars continue to differ in how much emphasis they place on one or the other set of factors, the consensus in the field has largely coalesced around acknowledging both sets of influences (Goldsmith & Harman, 1994; Kochanska, 1998; Rothbart & Bates, 2006; Thompson, 2006; Vaughn & Bost, 1999).
That consensus reflects a more general shared assumption in behavioral sciences that a comprehensive understanding of adaptive and maladaptive development requires an integration of both genetic and environmental influences (Kendler & Prescott, 2006; Rutter, 2002, 2006, 2007). Advances in molecular genetics and the subsequent rise in interdisciplinary collaborations between geneticists and behavioral scientists have robustly invigorated that approach. Analyses of how genes and environments interact – Genotype × Environment (G × E) interactions – have been particularly fruitful (Rutter, Moffitt, & Caspi, 2006). A G × E interaction occurs when environmental experience moderates the effect of a person’s genotype on physical or mental health outcomes, or when a genotype moderates an environmental effect (Moffitt, Caspi, & Rutter, 2005).
In socio-emotional development, the 5-HTTLPR polymorphism, linked to the serotonergic system, has been among the most often studied. Serotonin is an inhibitory neurotransmitter in the central nervous system that has been linked with the regulation of mood and emotions. The 5-HTTLPR polymorphism has two common alleles, the short (s) and the long (l ). The short allele (s) has been linked to reduced 5-HTT transcription efficiency, lower transporter levels, and diminished serotonin uptake compared to individuals with the long (l) allele. Individuals who are either homozygous for the short allele (ss) or heterozygous (sl) have been found to be at risk for a range of emotional and behavioral maladaptive outcomes. Dysfunctions in the serotonergic system have been strongly implicated in broadly ranging psychopathology, including under-regulated, impulsive, excessively and inappropriately aggressive, risk-taking behavior, deficits of executive functions, alcohol use, as well as depressive or anxious disorders (Brown & Hariri, 2006; Lesch et al., 1996; Lucki, 1998; Posner, Rothbart, & Sheese, 2007; Sourbrie, 1986; van Goozen, Fairchild, Snoek, & Harold, 2007; Westernberg, Murphy & den Boer, 1996).
Perhaps most importantly, animal and human research has produced converging evidence of interactions between the genetic risk associated with 5-HTTLPR polymorphism (having a short allele) and environmental influences. Generally, those studies have shown that the link between the genetic risk and maladaptive outcomes is moderated by qualities of the environment. Specifically, individuals with a short allele develop significant problems when they are also exposed to sub-optimal or stressful environmental conditions. Monkeys with a short allele show multiple behavioral problems, but only if they have been peer raised, and not if they have been reared in natural, supportive mother–infant relationships (Champoux et al., 2002; Suomi, 2004, 2005, 2006). Adults with a short allele are likely to develop depression, but only if they also experienced multiple stressful life events (Caspi et al., 2003). Children with a short allele are at risk for depression, but only if they have been exposed to maltreatment, abuse, or neglect (Kaufman et al., 2004, 2006); they are also at risk for fearfulness, but only if they grew up in a family with poor social support (Fox et al., 2005).
Very few studies, however, have addressed G × E interactions in the development of infant attachment organization (security vs. insecurity) using molecular genetic measures of 5-HTTLPR and robust observational assessments of the environment. Most of the extant research has addressed specifically attachment disorganization; furthermore, that work has focused mostly on the genes related to the dopamine system.
Genotypes associated with the dopamine system (7-repeat allele of the dopamine D4 receptor, DRD4, Lakatos et al., 2000; DRD4 and the -521 C/T promoter gene, Gervai et al., 2005; Lakatos et al., 2002) have been linked to disorganized attachment, but that link has not been consistently replicated (Bakermans-Kranenburg & van IJzendoorn, 2004). Additionally, a previous study failed to find a main effect of the 5-HTTLPR polymorphism, or a significant interaction between 5HTTLPR and DRD4 genotypes in predicting infant disorganized attachment (Lakatos et al., 2003).
Three studies have reported a G × E interaction in the context of disorganized attachment. Van IJzendoorn and Bakermans-Kranenburg (2006) found that children with the 7-repeat DRD4 allele and whose mothers had an unresolved past trauma or loss (considered an environmental risk factor) were at almost 19-fold increased risk for disorganized attachment. By contrast, Gervai et al. (2007) found that maternal disrupted communication moderated risk of disorganized attachment for children without the 7-repeat DRD4 allele but did not moderate risk for children with the 7-repeat DRD4 allele. Finally, Spangler and Zimmermann (2007) examined 5-HTTLPR polymorphism along with variation in environmental influences in the development of disorganized attachment: Children homozygous for the short allele were significantly more likely to be classified as disorganized if their mothers also demonstrated low maternal responsiveness compared to children in the other groups.
To our knowledge, however, no studies have examined the role of the 5-HTTLPR polymorphism along with variation in environmental influences in the development of attachment security versus insecurity, although Bakermans-Kranenburg and van IJzendoorn (in press) specifically called for such investigation. To do so was the goal of this study.
We examined a G × E interaction in the development of attachment organization, assessed using the established classic Strange Situation paradigm conducted at the end of the first year of life, in a community sample of typically developing children. Children’s genotypes (5-HTTLPR polymorphism, ss/sl versus ll ) were assessed using molecular genetic measures, and the environmental influence – maternal responsiveness – was observed in naturalistic mother–child interactions in infancy.
Responsiveness has long been conceptualized as the core aspect of early care in the attachment literature. Mothers who respond more sensitively to their children are more likely to have securely attached infants, although the size of the effect tends to be modest (Ainsworth, Bell, & Stayton, 1971; for meta analyses, see Goldsmith & Alansky, 1987; de Wolff & van IJzendoorn, 1997; for a review, see Thompson, 1998).
Consistent with the extant research on G × E interactions, we expected that infants with a short allele (ss/sl) would be at an increased risk for insecure attachment, but only if they also experienced relatively unresponsive maternal care, and not if they received responsive care. In other words, we expected that mothers’ responsiveness to their infants, observed in infancy (at 7 months), would moderate the effect of the infants’ 5-HTTLPR genotypes on the organization of future attachment (at 15 months).
Following recent work by Belsky, and Bakermans-Kranenburg and van IJzendoorn (Belsky, 2005, 1997; Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Bakermans-Kranenburg & van IJzendoorn, in press), we further explored whether the G × E interaction would be consistent with the differential susceptibility model, or with the genetic vulnerability model (diathesis-stress). The former model assumes a form of G × E interactions such that children with certain biological predispositions (high negative reactivity, high genetic risk, including a short 5-HTT allele) are more susceptible than other children to variations in the environment, and respond more strongly than other children to both negative and positive environmental conditions. For those children, variations in experience may lead to worse or better outcomes compared to children without such predispositions. The latter model poses that for children with high genetic risk, negative environmental influences lead to particularly negative developmental outcomes, and positive environmental influences can buffer those children from the elevated risk.
In this study, both models would predict that, compared to infants homozygous for the long allele, infants with a short allele and unresponsive mothers would be at a greater risk of developing insecure attachment. However, the differential susceptibility model would further predict that the variation in mothers’ responsiveness would have a greater impact – for better or worse – on infants with a short allele than on those who were homozygous for the long allele (and thus, that ss/sl infants with responsive mothers would be more likely to develop a secure attachment than ll infants). In contrast, the genetic vulnerability model would predict that whereas high maternal responsiveness might offset the genetic risk for ss/sl infants, it is unlikely to lead to better outcomes than it has for children homozygous for the long allele (ll ).
Method
Participants
Two-parent families with normally developing infants volunteered for a longitudinal study. The families represented a relatively broad range of education and income (approximately 30% of parents had high school education, 20% had post-college education; 43% had annual income below, and 57% above, $50,000). Among mothers, 90% were White, 3% Hispanic, 2% African American, 1% each Asian and Pacific Islander, 3% ‘other’; among fathers, 84% were White, 8% Hispanic, 3% African American, 3% Asian, 2% ‘other’. In 20% of families, one or both parents were non-White.
Overview
All procedures were approved by the IRB at the University of Iowa. Informed consent was obtained from parents prior to data collection. Data in this report include maternal responsiveness observed during lengthy home sessions when children were 7 months (N = 102, M = 7.21, SD = .43, 51 girls), children’s attachment organization assessed in the Strange Situation in the laboratory at 15 months (N = 101, M = 15.13, SD = .42, 51 girls), and children’s 5-HTTLPR genotype, determined when they returned at 52 months (N = 99, M = 52.52, SD = 1.10, 49 girls; parents of 89 children consented to the genetic testing).
All behavioral coding was done from the videotapes by independent teams; attachment organization was coded by professional coders at another university, blind to all information about the children. Typically, 15–20% of cases were used for reliability, with more used for rare codes. The coders also realigned periodically to prevent drift.
Assessment of mothers’ responsiveness to children, 7 months
Responsiveness was assessed during 45 min of mother–child naturalistic interactions that encompassed many typical daily contexts, such as preparing and having a snack with the baby, free play, playing with toys, bathing and dressing the child, free time, and other routine activities. Two coding systems were used: macroscopic (global ratings) and microscopic, where mothers’ responses to all discrete bids by the child were coded (for details, see Kochanska & Aksan, 2004). The measures were aggregated at multiple levels to assure their robustness (Rushton, Brainerd, & Pressley, 1983).
Macroscopic coding
This system was adapted from Ainsworth’s (Ainsworth et al., 1971) coding of responsiveness. Each observed context (e.g., play, snack) was rated separately, to increase the robustness of the final measure. Because our past research had reliably shown that sensitivity, acceptance, and cooperation judgments were very strongly inter-correlated, they were combined into one responsiveness rating (from 1, highly unresponsive, to 7, highly responsive). Each anchor point was carefully described. Reliability, alpha, was .91. The scores were then aggregated across all contexts into an overall macroscopic responsiveness score, M = 4.82, SD = .73.
Microscopic coding
This coding combined a time-sampled and event-triggered approach. There were two passes through a videotape, each using 60-s intervals. During the first pass, coders decided whether or not the child directed one or more bids or signals toward the mother that called for a response (reliability, kappa, .82). If so, each bid was coded as a negative/distress signal or bid (e.g., crying, whimpering), neutral/positive social bid, and physical bid (e.g., sneezing, coughing). Kappa was .77.
During the second pass, coders evaluated the mother’s response to each child’s bid as poor, fair, good, or exceptional (kappas .79–.80). In doing so, they considered promptness, engagement, sincerity, sensitivity, acceptance, cooperation, emotional availability, following child lead and/or focus of attention, and adjusting stimulation to child state (de Wolff & van IJzendoorn, 1997; Thompson, 1998), defined with respect to the type of the child’s bid (for example, empathy, warmth, and comfort were critical when responding to child distress, and enthusiasm, shared attention, and desire for interaction – when responding to a positive social bid).
Data reduction
We tallied all the instances when the mother responded poorly, fairly, well, or exceptionally to the child’s bids in each of the three categories. Each tally was then divided by the total number of the bids in that category (e.g., the proportions of all instances of child distress to which the mother responded poorly, fairly, well, or exceptionally). Next, we created four broader composites: of poor, fair, good, and exceptional response. Each of the above was the result of the relevant proportional response scores averaged across all three categories of child bids (e.g., the poor response composite was the average of the poor responses to distress, poor response to positive bids, and poor responses to physical signals). Finally, we created the final maternal microscopic responsiveness score by (a) weighing the poor response composite by −2, the fair response composite by −1, the good response composite by +1, and the exceptional response composite by +2, and (b) summing these scores, M = .40, SD = .38.
Overall responsiveness scores
The macro- and microscopic scores correlated, r(102) = .48, p < .001 and were standardized and averaged into one maternal responsiveness score at 7 months of age, M = .00, SD = .86.
Assessment of child attachment security, 15 months
The standard Strange Situation was conducted at the beginning of laboratory sessions and coded by professional attachment coders. Reliability, kappa, for the four attachment categories (avoidant, A, secure, B, resistant, C, and disorganized/unclassifiable, D/U) was .78. Kappa for the coding of secure versus insecure attachment was .85. All cases coded with low confidence by one coder and all D/U cases were double-coded and adjudicated. There were 56 secure (B) and 45 insecure children (12 avoidant, 19 resistant, and 14 disorganized/unclassifiable). There were no significant differences in the distribution of security versus insecurity in girls and boys, Pearson Chi-square (1) = 2.22, ns.
In addition to the categorical scores, we also generated continuous scores of attachment security, following Richters, Waters, and Vaughn (1988, p. 517). We standardized the children’s scores on the social-interactive behaviors (proximity-contact seeking, proximity maintaining, contact resistance, and avoidance), and crying in episodes 5 and 8 (reunions), multiplied each by the respective weights, summed, and reversed, M = −.01, SD = 1.16. Higher scores denote higher security. As expected, secure children had significantly higher scores, M = .71, SD = .82, than insecure children, M = −.92, SD = .85, t(98) = −9.74, p < .001 (one child did not receive a continuous score, but did receive a categorical score).
Assessment of genotypes (5-HTTLPR polymorphism, 52 months)
DNA was obtained using buccal swabs and genotyped using the method previously described (Philibert et al., 2007). Eighty-eight of 89 samples were successfully genotyped. There were 60 children with a short allele (13 ss, 47 sl; 26 girls, 34 boys), and 28 ll homozygotes (18 girls, 10 boys). Hardy Weinberg equilibrium testing was non-significant (p < .66). Descriptive data for all measures are in Table 1.
Table 1.
Descriptive statistics for all measures
| Children’s genotype
|
||||
|---|---|---|---|---|
|
ss/sl |
ll |
|||
|
n = 60
|
n = 28
|
|||
| M | SD | M | SD | |
| Maternal responsiveness | ||||
| Macroscopic (ratings) | 4.76 | .76 | 4.95 | .73 |
| Microscopic to: | ||||
| Negative (distress) bids | .34 | .56 | .58 | .45 |
| Positive bids | .52 | .68 | .62 | .57 |
| Physical bids | .20 | .46 | .18 | .68 |
| Microscopic across bids | .35 | .35 | .46 | .41 |
| Overall (micro-/macroscopic)a | −.10 | .80 | .17 | .93 |
| Continuous security score | −.19 | 1.12 | .25 | 1.11 |
Composite of standardized overall macroscopic and microscopic scores.
Results
Preliminary analyses
In the analyses, N varied from 87 to 100. We first examined bivariate associations among mothers’ responsiveness at 7 months, infants’ attachment at 15 months, and infants’ genotypes. Mothers’ responsiveness was unrelated to child genotype, t(86) < 1 (and remained unrelated in additional analyses that examined responsiveness to negative, positive, and physical bids, captured in microscopic coding). Mothers’ responsiveness was also unrelated to child attachment organization, assessed either as secure vs. insecure, t(99) < 1, or continuously, r(100) = .07. Infant attachment (secure vs. insecure) was significantly associated with genotype: for the ss/sl children, 33 were insecure and 27 were secure, and for ll children, 7 were insecure and 21 were secure (Pearson’s chi-square 9.37, p < .01). The continuous measure of security was marginally associated with child genotype, t(85) = 1.90, p <.10.
Prediction of children’s secure vs. insecure attachment status
Prior to the regression analyses, data were screened for outliers, and three cases were excluded. Hierarchical logistic regression was used to estimate the main and interaction effects of the genotype (homo- and heterozygotes for the short allele, ss/sl, coded as 0, versus homozygotes for the long allele, ll, coded as 1) and mother responsiveness as the predictors of the child’s attachment organization (insecure, coded as 0, and secure, coded as 1).
When the main effects of mother responsiveness and 5-HTTLPR genotype were entered into a logistic regression predicting infant security, only 5-HTTLPR genotype was significant, b = .35, SE = .27, ns, and b = 1.54, SE = .54, p < .01 respectively. In the final equation, when the interaction term was entered, both main effects were significant, b =.83, SE = .38, p < .05, and b = 1.85, SE = .66, p < .01. They were, however, qualified by the significant interaction of 5-HTTLPR genotype with mother responsiveness, b = −1.76, SE = .90 p < .05, and thus should not be interpreted separately.
To interpret the meaning of the significant interaction effect, we conducted follow-up logistic regressions separately for the children who had the short allele (ss/sl ) and those homozygous for the long allele (ll ), regressing mother responsiveness on attachment organization. For the ss/sl infants, mothers’ responsiveness at 7 months significantly positively predicted infants’ attachment security (odds ratio = 2.46, p < .01). For the ll infants, mothers’ responsiveness was not significantly associated with attachment organization (odds ratio = .40, ns). Thus, the effect of maternal responsiveness on attachment security was only evident for infants with the ss/sl genotype.
Prediction of children’s continuous security scores
In a hierarchical multiple regression, the children’s continuous security scores were predicted from maternal responsiveness, their genotypes, and their interaction. As in the logistic regression, responsiveness was not significant when first entered, but became significant in the final equation, Beta = .32, p < .025; the effect of genotype was also significant, Beta = .22, p < .05. Those main effects should not be interpreted, however, because they were again qualified by a significant interaction between genotype and responsiveness, Beta = −.31, p < .05.
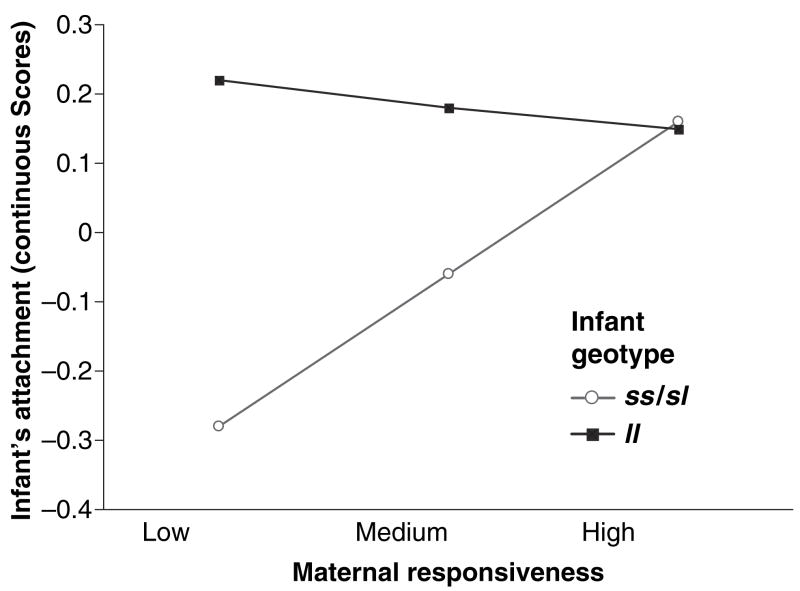
That interaction was further probed using a simple slopes test (Aiken & West, 1991). High maternal responsiveness was represented by scores one standard deviation above the mean, and low responsiveness was represented by scores one standard deviation below the mean (Aiken & West, 1991). The graphed results are in Figure 1. The simple slope for the ss/sl infants was significant, b = .27, SE = .12, p < .05, but for the ll infants it was not, b = −.04, SE = .58, ns. These results are consistent with the genetic vulnerability model rather than a differential susceptibility model. Children with ss/sl genotype who had responsive mothers were as secure – but not more secure – than children with the ll genotype who had responsive mothers.
Figure 1.
Maternal responsiveness moderates the effect of infants’ 5-HTTLPR genotypes on the continuous measure of their attachment security (simple slopes of maternal responsiveness on ss/sl and ll infants’ security)
Discussion
The findings were clear and straightforward, and they converged across the categorical and continuous measures of attachment security. The genetic risk for attachment insecurity conferred by 5-HTTLPR genotype (having a short allele, ss/sl ) was moderated by early maternal responsiveness. Infants who had greater genetic risk due to the short 5-HTT allele (ss/sl), but whose mothers had been relatively responsive to their signals and bids for care at 7 months were more likely to be secure, as compared to infants with the short allele whose mothers were relatively unresponsive. For infants at low genetic risk (ll), the variation in mothers’ responsiveness at 7 months was not significantly associated with subsequent attachment organization.
These results are consistent with both animal and human studies demonstrating G × E interactions between the genetic risk associated with 5-HTTLPR polymorphism and environmental or experiential factors (e.g., Caspi et al., 2003; Champoux et al., 2002; Fox et al., 2005; Kaufman et al., 2004, 2006; Suomi, 2004, 2005, 2006). This study extends that body of research to the analysis of human attachment security. The link between the genetic risk and maladaptive outcomes is moderated by environmental risk, such that individuals with a short allele develop insecure attachment, but only when they are also exposed to sub-optimal rearing conditions.
We further explored whether the G × E interaction effect would fit the differential susceptibility model or the genetic vulnerability model (Bakermans-Kranenburg & van IJzendoorn, in press; Belsky, 2005, 1997; Belsky et al., 2007). It appears that our findings are consistent with the genetic vulnerability model, sometimes also referred to as ‘dual-risk’ or ‘diathesis-stress’ model. Negative early experience amplified the risk conferred by the short 5-HTT allele, whereas positive early experience, while it served to buffer that risk, did not appear to lead to better outcomes than outcomes for children without the genetic risk (i.e., those who were homozygous for the long allele).
Given the sample size, we opted for the analyses where infants were examined as secure or insecure (or as having varying security scores), and we did not consider the sub-classifications. However, it is notable and intriguing that all disorganized/unclassifiable infants (D/U, n = 12) carried the short allele (ss/sl ). These results suggest a role of 5-HTTLPR in attachment disorganization in addition to previous research demonstrating G × E interactions focusing on DRD4. Previous research has not found a main effect of 5-HTTLPR (Lakatos et al., 2003), but has found an interaction of 5-HTTLPR genotype with maternal responsiveness in predicting disorganization (Spangler & Zimmermann, 2007).
In research on interplay between genetics and environment, it is often possible that rather than the child’s genotype interacting with his or her environment, the environment may have adapted to the child’s genotype. Although this effect may be represented conceptually in a number of ways, it has been referred to as evocative gene by environment correlation, rGE (Rutter, 2007; Rutter, Moffitt, & Caspi, 2006). In this scenario, infants with certain genetic qualities, for example a short 5-HTT allele, would behave in a way that elicits lesser or greater maternal responsiveness. Thus, maternal responsiveness would vary as a function of the infant’s genotype. Our data, however, are not consistent with such interpretation, because mothers of ss/sl and ll children did not significantly differ in their responsiveness, whether assessed using a composite score or examined separately to different types of child bids.
To our knowledge, this is the first study to examine the interaction between child 5-HTTLPR genotype and maternal responsiveness in infancy as predicting attachment security versus insecurity. Most previous research of G × E interactions in the development of attachment has focused on DRD4, and on disorganization. Our study had several methodological strengths. We examined a G × E interaction in the development of infant attachment organization using molecular measures of the 5-HTTLPR genotype. Additionally, we utilized a longitudinal design assessing the quality of children’s early care and subsequent development of their attachment using robust and well-established observational measures for both sets of constructs.
One limitation of the current study is the relatively small sample size. Consequently, we opted for combining the ss and sl children, rather than examining them separately, particularly given that we had established that the studied relations were similar in those two groups. Although this is an accepted strategy (e.g., Caspi et al., 2003; Lesch et al., 1996), differences between the two groups have been occasionally reported (e.g., Williams et al., 2003).
Another limitation involves a relatively ethnically homogenous population (although note that 20% of families had at least one non-White parent). Consequently, the findings, although theoretically meaningful, consistent with the literature, and potentially relevant to prevention and intervention, should be interpreted with caution until the results are replicated.
Finally, the absence of an overall link between maternal responsiveness and children’s attachment organization is puzzling. The link was found for ss/sl children only. To explain that pattern of findings, we may consider that although most studies have reported the main effect of responsiveness, those effects have been often modest in size. It is theoretically possible that the average modest effect size is due to the fact that to date, possible moderators of the relation have been often ignored (Belsky, 1997). Consequently, the effect has been ‘diluted’. With moderators considered statistically, the effect may very well be stronger. The child’s genotype may be one example of such a moderator. In samples similar to ours, 60–70% of children have at least one short 5-HTT allele (ss/sl); therefore, the relation between responsiveness and security is usually obtained for the entire sample, but it may actually be due only to children with that genotype. Although consistent with our findings, this possibility should be tested again in future studies.
Although the current findings are meaningful, and infant attachment organization at the end of the first year of life is an important predictor of child developmental outcomes (Thompson, 2006), children as well as their environments continue to change and evolve. The genetic risk and/or susceptibility conferred by 5-HTTLPR genotype may continue to interact with the child’s environment, as well as other genetic, physiological, behavioral, and relationship factors in development. Longitudinal studies are needed to map in more depth the long-term predictive value of the very early interplay of genotype and social relationships.
It is now broadly accepted that children’s development is determined by complex interactions of factors at multiple levels, ‘from neurons to neighborhoods’ (Shonkoff & Phillips, 2000), including genetics and biology along with early meaningful social relationships (Collins, Maccoby, Steinberg, Hetherington, & Bornstein, 2000). This study is a promising step toward elucidating those intricate processes.
Acknowledgments
This research has been funded by the grants from NIMH, RO1 MH63096 and KO2 MH01446, to Grazyna Kochanska, who was also supported by the Stuit Professorship, and by RO1 DA015789 to Robert A. Philibert. The authors wish to thank Dr. Jay Belsky for his helpful comments on an earlier draft, the many Family Study students and staff, including Nazan Aksan, Jennifer Carlson, Amanda Friesenborg, Lindsey Lange, and Ryan Brink for all their efforts, Dianna Edwards for help with genotyping, and the participants in the Family Study for their enthusiastic commitment to this research.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury, CA: Sage; 1991. [Google Scholar]
- Ainsworth MDS, Bell SM, Stayton D. Individual differences in Strange Situation behavior of one-year-olds. In: Schaffer HR, editor. The origins of human social relations. London: Academic Press; 1971. pp. 17–57. [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. No association of the dopamine D4 receptor (DRD4) and -521 C/T promoter polymorphisms with infant attachment disorganization. Attachment and Human Development. 2004;6:211–218. doi: 10.1080/14616730412331281584. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Research review: Genetic vulnerability or differential susceptibility in child development: The case of attachment. Journal of Child Psychology and Psychiatry. doi: 10.1111/j.1469-7610.2007.01801.x. (in press) [DOI] [PubMed] [Google Scholar]
- Belsky J. Theory testing, effect-size evaluation and differential susceptibility to rearing influence: The case of mothering and attachment. Child Development. 1997;64:598–600. [PubMed] [Google Scholar]
- Belsky J. Differential susceptibility to rearing influence: An evolutionary hypothesis and some evidence. In: Ellis BJ, Borklund DF, editors. Origins of the social mind: Evolutionary psychology and child development. New York: Guilford; 2005. pp. 139–163. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Bowlby J. Attachment and loss: Vol. 1. Attachment. 2. New York: Basic; 19691982. [Google Scholar]
- Brown SM, Hariri AR. Neuroimaging studies of serotonin gene polymorphisms: Exploring the interplay of genes, brain, and behavior. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:44–2. doi: 10.3758/cabn.6.1.44. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. New York: Guilford; 1999. [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Collins WA, Maccoby EE, Steinberg L, Hetherington EM, Bornstein MH. Contemporary research on parenting: The case for nature and nurture. American Psychologist. 2000;55:218–232. [PubMed] [Google Scholar]
- de Wolff M, van IJzendoorn MH. Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Development. 1997;68:571–591. [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, et al. Evidence for a gene–environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Gervai J, Nemoda Z, Lakatos K, Ronai Z, Toth I, Ney K, et al. Transmission disequilibrium tests confirm the link between DRD4 gene polymorphism and infant attachment. American Journal of Medical Genetics. 2005;132B:126–130. doi: 10.1002/ajmg.b.30102. [DOI] [PubMed] [Google Scholar]
- Gervai J, Novak A, Lakatos K, Toth I, Danis I, Ronai Z, et al. Infant genotype may moderate sensitivity to maternal affective communications: Attachment disorganization, quality of care, and the DRD4 polymorphism. Social Neuroscience. 2007;2:307–319. doi: 10.1080/17470910701391893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Alansky JA. Maternal and infant predictors of attachment: A meta-analytic review. Journal of Consulting and Clinical Psychology. 1987;55:805–816. doi: 10.1037//0022-006x.55.6.805. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Harman C. Temperament and attachment: Individuals and relationships. Current Directions in Psychological Science. 1994;3:53–57. [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, et al. Brain-derived neurotrophic factor–5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment and psychopathology: Understanding the causes of psychiatric and substance use disorders. Boston, MA: Guilford; 2006. [Google Scholar]
- Kochanska G. Mother–child relationship, child fearfulness, and emerging attachment: A short-term longitudinal study. Developmental Psychology. 1998;34:480–490. doi: 10.1037//0012-1649.34.3.480. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Aksan N. Development of mutual responsiveness between parents and their young children. Child Development. 2004;75:1657–1676. doi: 10.1111/j.1467-8624.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Birkas E, Ronai Z, Kovacs E, Ney K, et al. Association of D4 dopamine receptor gene and serotonin transporter promoter polymorphisms with infants’ response to novelty. Molecular Psychiatry. 2003;8:90–97. doi: 10.1038/sj.mp.4001212. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Toth I, Ronai Z, Ney K, Sasvari-Szekely M, et al. Further evidence for the role of the dopamine D4 receptor gene (DRD4) in attachment disorganization: Interaction of the III exon 48 bp repeat and the -521 C/T promoter polymorphisms. Molecular Psychiatry. 2002;7:27–31. doi: 10.1038/sj.mp.4000986. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Toth I, Nemoda Z, Ney K, Sasvari- Szekely M, Gervai J. Dopamine D4 receptor (DRD4) polymorphism is associated with attachment disorganization in infants. Molecular Psychiatry. 2000;5:633–637. doi: 10.1038/sj.mp.4000773. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biological Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Madan A, Anderson A, Cadoret R, Packer H, Sandhu HK. Regulation of serotonin transporter mRNA levels by an upstream CpG island. American Journal of Medical Genetics. 2007;144:101–105. doi: 10.1002/ajmg.b.30414. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE. Attention genes. Developmental Science. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Richters JE, Waters E, Vaughn BE. Empirical classification of infant–mother relationships from interactive behavior and crying during reunion. Child Development. 1988;59:512–522. [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner RM, editors. Handbook of child psychology, N. Eisenberg (Volume Ed.), Social, emotional, and personality development. New York: Wiley; 2006. pp. 99–166. [Google Scholar]
- Rushton JP, Brainerd CJ, Pressley M. Behavioral development and construct validity: The principle of aggregation. Psychological Bulletin. 1983;94:18–38. [Google Scholar]
- Rutter M. The interplay of nature, nurture, and developmental influences: The challenge ahead for mental health. Archives of General Psychiatry. 2002;59:996–1000. doi: 10.1001/archpsyc.59.11.996. [DOI] [PubMed] [Google Scholar]
- Rutter M. Genes and behavior: Nature–nurture interplay explained. Malden, MA: Blackwell; 2006. [Google Scholar]
- Rutter M. Gene–environment interdependence. Developmental Science. 2007;10:12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene– environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Phillips DA. From neurons to neighborhoods: The science of early childhood development. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- Sourbrie P. Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral and Brain Sciences. 1986;9:319–335. [Google Scholar]
- Spangler G, Zimmermann P. Genetic contribution to attachment disorganization and temperament. Paper presented at the 2007 meeting of the Society of Research in Child Development.2007. Mar 30, [Google Scholar]
- Suomi SJ. How gene–environment interactions shape biobehavioral development: Lessons from studies with rhesus monkeys. Research in Human Development. 2004;1:205–222. [Google Scholar]
- Suomi SJ. Genetic and environmental factors influencing the expression of impulsive aggression and serotonergic functioning in rhesus monkeys. In: Tremblay RE, Hartup WW, Archer J, editors. Developmental origins of aggression. New York: Guilford; 2005. pp. 63–82. [Google Scholar]
- Suomi SJ. Risk, resilience, and gene × environment interactions in rhesus monkeys. Annals of New York Academy of Sciences. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Early sociopersonality development. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 5. Hoboken, NJ: Wiley; 1998. pp. 25–104. [Google Scholar]
- Thompson RA. The development of the person: Social understanding, relationships, conscience, self. In: Eisenberg N, Damon W, Lerner M, editors. Handbook of child psychology: Vol. 3, Social, emotional, and personality development. 6. Hoboken, NJ: Wiley; 2006. pp. 24–98. [Google Scholar]
- van Goozen SHM, Fairchild G, Snoek H, Harold GT. Theevidence for aneurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Bakermans-Kranenburg MJ. DRD47-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attachment and Human Development. 2006;8:291–307. doi: 10.1080/14616730601048159. [DOI] [PubMed] [Google Scholar]
- Vaughn BE, Bost KK. Attachment and temperament: Redundant, independent, or interacting influences on interpersonal adaptation and personality development? In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. New York: Guilford; 1999. pp. 198–225. [Google Scholar]
- Westernberg HG, Murphy DL, den Boer JA, editors. Advances in the neurobiology of anxiety disorders. New York: Wiley; 1996. [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]