Summary
In this issue of Clinical Cancer Research Conti et al. (2008) identify a crucial cell adhesion and signaling axis that promotes the proliferation, survival, and drug-resistant properties of metastatic colorectal cancer cells in the liver. The components of this pathway may be effective targets for therapeutically treating established metastatic tumors in colorectal adenocarcinomas.
The extracellular matrix (ECM) is an insoluble network of proteins that provides structural support to nearly all multicellular tissues and organs as well as solid malignancies (1). Most metazoan cells dynamically interact with ECM components via integrins, which are heterodimeric transmembrane proteins comprised of α and β subunits (2). Most integrins expressed on the cell surface are present in inactive conformations, and their adhesion to ECM ligands must be precisely regulated via ‘inside-out’ activation mechanisms. Such regulatory mechanisms occur after extracellular stimuli, e.g., growth factors or cytokines, alter intracellular effector proteins that in turn bind to integrin cytoplasmic regions and induce conformational changes in the integrin extracellular domains (2). Following activation and engagement with ECM ligands, integrins regulate cytoskeletal dynamics as well as intracellular signal transduction cascades that lead to a wide variety of cellular responses, including proliferation, differentiation, and survival. Pathological regulation of integrin-mediated adhesion and signaling is linked to many human diseases, particularly cancer. Indeed, many primary and metastatic cancer cells display altered integrin expression levels and/or activation states, leading to adhesion-independent cell growth and survival, which are pathological hallmarks of cancer.
Stromal cells within an tumor microenvironment also play important roles in tumorigenesis and metastases, and many integrins are expressed in tumor-associated stromal components, including fibroblasts, vascular endothelial cells, and inflammatory cells. Surprisingly very little is understood about the mechanisms by which tumor cells alter the ECM composition of their microenvironment; furthermore, how altered integrin-ECM interactions then promote tumor cell growth and survival remains elusive. In this issue of Clinical Cancer Research, Conti and colleagues make an important step toward deciphering how metastatic tumor cells manipulate their repertoire of integrins in response to altered ECM composition of the malignant organ to promote their growth and survival (3). Specifically, the authors have analyzed how metastatic colorectal adenocarcinoma cells effectively colonize and thrive within the hepatic microenvironment. Preferential metastasis to the liver is a particularly deadly characteristic of colorectal adenocarcinomas; indeed, nearly 70% of patients with late-stage colorectal adenocarcinomas develop liver metastases, accounting for approximately 50,000 deaths per year in the United States (4).
The molecular mechanisms by which colorectal cancer cells exploit the hepatic microenvironment for selective growth and survival remain obscure. The report by Conti et al. has now identified essential functions for αv integrins in promoting metastatic colorectal adenocarcinoma cell growth and survival in the liver. There are five members of the αv sub-family of integrins: αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8. These various integrins recognize argine-glycine-aspartic acid (RGD) peptide sequences found in a many ECM proteins. With the exception of the central nervous system, αv integrins are largely dispensable for organogenesis (5); however, they contribute essential, yet complex, roles during tumorigenesis (6, 7). For example, genetic ablation of the αv integrin gene in epithelial cells of the murine skin leads to development of squamous cell carcinomas (6), revealing tumor suppressor-like functions for αv integrins during epithelial cell homeostasis. In contrast, elevated αvβ6 integrin protein expression is associated with advanced stages of human squamous cell carcinomas (7). Collectively, these data suggest that in certain forms of cancer, αv integrins provide differential functions in tumor initiation versus tumor progression.
Adhesion and signaling functions for αv integrins in regulating metastatic tumor cell growth and survival are not well understood. Conti et al. address this important topic by analyzing resected liver metastases derived from primary colorectal adenocarcinomas, and show that sub-populations of metastatic tumor cells express elevated levels of αvβ3 and αvβ5 integrins. Furthermore, they demonstrate that tumor cells overexpressing these integrins preferentially reside near regions of tumor-induced fibrosis, or desmoplastic reactions. The authors proceed to characterize the ECM composition of desmoplastic reactions associated with liver metastases and show dramatically increased levels of Collagen I and decreased amounts of Collagen IV. In its intact form, Collagen I is a poor physiological ligand for αvβ3 and αvβ5 integrins; however, protease-mediated degradation of Collagen I exposes ‘cryptic’ RGD peptide sequences that are recognized by αv integrins. Conti et al. also show in vitro tumor cell adhesion to denatured Collagen I, but not to a protease-resistant form of Collagen I, promotes metastatic tumor cell proliferation, survival, and resistance to 5-fluorouracil. Antibodies directed against αvβ3 or αvβ5 integrins block these responses. These novel findings raise many intriguing questions about how metastatic tumor cells exploit the liver microenvironment for selective growth advantages. Since Collagen I is highly expressed in desmoplastic reactions of other malignancies (8), these findings suggest similar functions for integrins in other types of cancer.
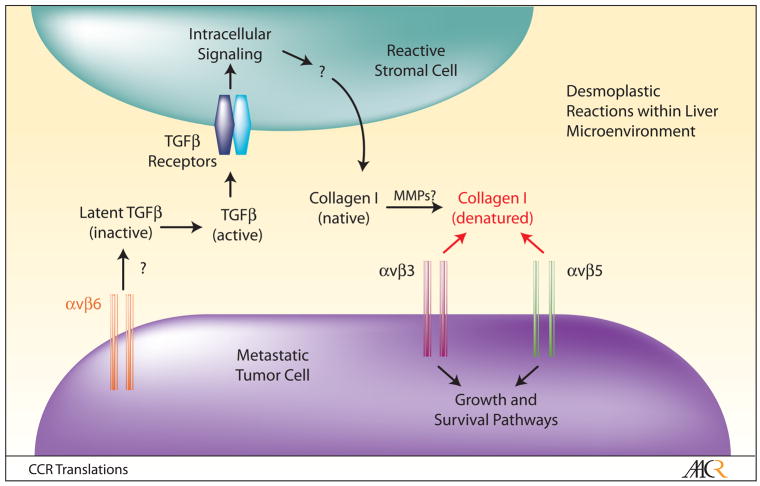
One remarkable finding from the work by Conti et al. involves the striking degree of stromal cell plasticity in response to tumor cell growth in the liver. Desmoplastic reactions in liver metastases are associated with robust differentiation of α-smooth muscle actin (α-SMA)-expressing stromal cells, which are the primary sources of Collagen I in desmoplastic reactions. Expression of α-SMA suggests that they may be reactive myofibroblasts that differentiate near the metastatic site; however, the exact identities of the stromal components remains unclear, and necessitates further studies to definitively characterize desmoplastic reaction-associated cell types using additional molecular markers. Additionally, the hepatic cell of origin that gives rise to these cells is an important topic that warrants more investigation. There are several cell types within the liver parenchyma that retain multipotent properties, including stellate cells and blood vessel-associated smooth muscle cells (9). Thus, it is enticing to speculate that one or more of these resident progenitor cell types may be recruited to the metastatic lesion, where they give rise to Collagen I-expressing cells that comprise desmoplastic reactions. It will also be interesting to characterize the molecular mechanisms by which metastatic tumor cells induce the deposition of Collagen I within desmoplastic reactions. Collagen I deposition by reactive myofibroblasts and inflammatory cells in other pathophysiological conditions, e.g., wound repair and autoimmunity, is induced, in part, by transforming growth factor β (TGFβ) cytokines (10), which are produced by cells as inactive, ECM-bound latent complexes. αvβ6 and αvβ8 integrins adhere to RGD peptide sequences within latent TGFβ’s. Integrin-mediated TGFβ activation plays essential roles in tissue homeostasis, and these events are dysregulated in human diseases (11). Interestingly, a recent report showed that colorectal cancer cells express αvβ6 integrin, and utilize this integrin for growth in the hepatic microenvironment (12). Collectively, these results lead one to speculate that tumor cell-expressed αvβ6 integrin, possibly in combination with αvβ8 integrin, may activate TGFβ’s within the hepatic microenvironment, which in turn induces Collagen I deposition by reactive stromal cells within desmoplastic reactions (Figure 1). Upon protease-mediated degradation, integrins αvβ3 and αvβ5, also expressed in metastatic tumor cells, engage exposed RGD binding sites and activate intracellular pathways that promote cell growth and survival. If this model were correct, this would reveal a complex network of αv integrin-mediated adhesion and signaling pathways that collectively promote metastatic tumor cell growth in the liver. Obviously, it will be important to identify the extracellular proteases that degrade Collagen I within desmoplastic reactions, as well as the integrin-activated intracellular signaling pathways that promote metastatic tumor cell growth and survival.
Figure 1. A Model for αv Integrin-Mediated Adhesion and Signaling Events During Metastatic Tumor Cell Growth and Survival in the Liver.
Sub-populations of metastatic colorectal tumor cells within the hepatic microenvironment preferentially associate with desmoplastic reactions, in part, via increased expression of αv integrins. αvβ6 integrin, a receptor for latent forms of TGFβ’s, likely mediates activation of TGFβ signaling in reactive stromal cells, leading to increased extracellular deposition of native Collagen I. Members of the matrix metalloprotease (MMP) family then degrade Collagen I, leading to the exposure of cryptic RGD peptide sequences, which serve as binding motifs for αvβ3 and αvβ5 integrins expressed in tumor cells. Subsequently, ligation of αvβ3 and αvβ5 integrins activates intracellular signaling cascades that promote metastatic tumor cell growth, survival, and chemotherapeutic drug-resistance. Targeting the various components of this signaling axis may prove efficacious for treating established metastases in patients with advanced colorectal adenocarcinomas.
Lastly, inhibiting integrin functions has proven efficacious for some human diseases, and similar strategies may prove beneficial for treating human cancer. Tumor growth and metastasis are dependent on neovascularization, which has initiated efforts to develop angiogenesis inhibitors to block tumor progression; indeed, many of these strategies have targeted integrins (13). For example, αvβ3 and αvβ5 integrins are expressed in vascular endothelial cells as well as tumor cells, and have been intensely pursued as potential therapeutic targets. To date, anti-integrin antibodies and inhibitory peptides have yielded varied results in human clinical trials, although these reagents combined with standard chemotherapies may prove more efficacious (13). Based on the results of Conti et al., integrating similar integrin-based therapeutics may prove beneficial for treating established metastatic tumors in patients with advanced colorectal adenocarcinomas.
Acknowledgments
I thank Dr. Lee M. Ellis (MD Anderson Cancer Center) for helpful comments on the manuscript and discussions about the pathophysiology of colorectal adenocarcinoma metastases. Lastly, I apologize to various colleagues for not citing their published work. Due to space limitations, many relevant citations could not be listed in the references section.
References
- 1.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 3.Conti JA, Kendall TJ, Bateman A, et al. Kendell The desmoplastic reaction surrounding hepatic colorectal adenocarcinoma metastases aids tumour growth and survival via αv integrin ligation. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-0816. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad SA, Berman RS, Ellis LM. Biology of colorectal liver metastases. Surg Oncol Clin N Am. 2003;12:135–50. doi: 10.1016/s1055-3207(02)00078-9. [DOI] [PubMed] [Google Scholar]
- 5.McCarty JH, Lacy-Hulbert A, Charest A, et al. Selective ablation of {alpha}v integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2004 doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 6.McCarty JH, Barry M, Crowley D, Bronson RT, Lacy-Hulbert A, Hynes RO. Genetic ablation of alphav integrins in epithelial cells of the eyelid skin and conjunctiva leads to squamous cell carcinoma. Am J Pathol. 2008;172:1740–7. doi: 10.2353/ajpath.2008.070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Aarsen LA, Leone DR, Ho S, et al. Antibody-mediated blockade of integrin alpha v beta 6 inhibits tumor progression in vivo by a transforming growth factor-beta-regulated mechanism. Cancer Res. 2008;68:561–70. doi: 10.1158/0008-5472.CAN-07-2307. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–37. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 9.Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J. 2008;411:1–18. doi: 10.1042/BJ20071570. [DOI] [PubMed] [Google Scholar]
- 10.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheppard D. Roles of alphav integrins in vascular biology and pulmonary pathology. Curr Opin Cell Biol. 2004;16:552–7. doi: 10.1016/j.ceb.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Yang GY, Xu KS, Pan ZQ, et al. Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci. 2008;99:879–87. doi: 10.1111/j.1349-7006.2008.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth JA, Nakada MT, Trikha M, et al. Alpha-v integrins as therapeutic targets in oncology. Cancer Invest. 2007;25:632–46. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]