Abstract
Medial temporal lobe (MTL) atrophy is associated with increased risk for conversion to Alzheimer's disease (AD), but manual tracing techniques and even semi-automated techniques for volumetric assessment are not practical in the clinical setting. In addition, most studies that examined MTL atrophy in AD have focused only on the hippocampus. It is unknown the extent to which volumes of amygdala and temporal horn of the lateral ventricle predict subsequent clinical decline. This study examined whether measures of hippocampus, amygdala, and temporal horn volume predict clinical decline over the following 6-month period in patients with mild cognitive impairment (MCI). Fully-automated volume measurements were performed in 269 MCI patients. Baseline volumes of the hippocampus, amygdala, and temporal horn were evaluated as predictors of change in Mini-mental State Exam (MMSE) and Clinical Dementia Rating Sum of Boxes (CDR SB) over a 6-month interval. Fully-automated measurements of baseline hippocampus and amygdala volumes correlated with baseline delayed recall scores. Patients with smaller baseline volumes of the hippocampus and amygdala or larger baseline volumes of the temporal horn had more rapid subsequent clinical decline on MMSE and CDR SB. Fully-automated and rapid measurement of segmental MTL volumes may help clinicians predict clinical decline in MCI patients.
Introduction
Mild cognitive impairment (MCI) is considered a transitional stage between normal aging and Alzheimer's disease (AD), yet a certain proportion of patients do not appear to progress to AD, at least with the follow-up periods published thus far.1 Such patients are not likely to be appropriate for the treatments targeted to prevent or slow down degenerative processes specific for AD. Therefore, robust and objective measures are necessary to distinguish patients with MCI who will clinically decline, particularly those who exhibit rapid decline, from those who will remain stable.2 One such measure is atrophy of medial temporal lobe (MTL) structures.
Assessment of MTL atrophy has previously required input from skilled personnel, and manual tracing of structures or editing of semi-automated segmentations, making such techniques impractical for the clinical setting. Advancements in automated volumetric segmentation have minimized the requirement for human intervention3 allowing fully-automated quantitative analysis to be performed. Such fully-automated techniques are faster, less subjective, and less expensive than manual or semi-automated techniques.4
In this study, fully-automated volumetric methods were applied to the baseline MR images from the ADNI dataset of MCI patients (n = 269). The goal was to examine whether volumes of hippocampus, amygdala, and temporal horn of the lateral ventricle would predict clinical decline over the next 6-month interval. Although atrophy in hippocampal volume is a well-established predictor of conversion from MCI to AD, much less is known about the value of temporal horn of the lateral ventricle and amygdala volume in predicting clinical decline, especially over a short time interval. We also investigated whether treatment with acetylcholinesterase inhibitors (AChE-I) would modify prediction of decline. We hypothesized that the baseline MTL volumes would predict rate of clinical decline over a 6-month interval.
Methods
ADNI protocol
Data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu/ADNI). The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and nonprofit organizations. The principle investigator of this initiative is Michael W. Weiner, M.D., VA Medical Center and University of California – San Francisco. ADNI is the result of efforts of many co-investigators from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research – approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years, and 200 people with early AD to be followed for 2 years. For up-to-date information see www.adni-info.org. The ADNI study has undergone institutional review board evaluation and approval at each participating site and informed consent was obtained from all participants in the study.
Participants
Enrolled patients were in good general health and had no MR scanning contraindications, had completed at least 6 years of education (or had a good work history sufficient to exclude mental retardation), and had adequate visual and auditory acuity to allow neuropsychological testing. In addition, each patient had an informant who had a frequent contact with the patient and was able to provide an independent evaluation of functioning.
To be included in the ADNI study in the MCI category, patients were required to have Mini-mental State Exam (MMSE)5 scores between 23-30 (inclusive), a memory complaint, objective memory loss measured by education-adjusted scores on Wechsler Memory Scale-Revised (WMS-R) Logical Memory II6, a Clinical Dementia Rating (CDR)7 score of 0.5, absence of significant enough levels of impairment in other cognitive domains so that criteria for dementia are not met, largely preserved activities of daily living, and an absence of dementia.1 In addition, Geriatric Depression Scale8 score less than 6 and vascular etiology modified Hachinski9 score less than 4 was required. Both subtypes of amnestic MCI, single-domain and multiple-domain, were enrolled in ADNI protocol, since both subtypes have a high rate of conversion to AD10.
Exclusion criteria included any significant neurologic disease other than suspected incipient Alzheimer's disease. Patients with screening/baseline MR scans with evidence of infection, large infarction, or other focal lesions, or multiple lacunes or lacunes in a critical memory structure were excluded from the study. In addition, exclusion criteria were major depression or bipolar disorder within the past 1 year of baseline visit, history of schizophrenia, or history of alcohol or substance abuse.
Demographic characteristics of MCI subjects are presented in Table 1. ADNI is an ongoing project and longitudinal data are still being collected. The present study included all MCI patients from the ADNI dataset who received 6-month follow-up clinical evaluations at the time of download (July 24, 2007). Out of 269 subjects, 154 subjects had APOE-ε4 genotype (ε3/ε4 [n = 114], ε4/ε4 [n = 32], or ε2/ε4 [n = 8]), while 115 subjects did not (ε3/ε3 [n = 106], ε2/ε3 [n = 9]). Nearly half of the MCI patients, 120 subjects (45%), received AChE-I treatment for the duration of the follow-up or longer.
Table 1.
Demographic characteristics of the MCI cohort.
| Characteristic | |
|---|---|
| Number of subjects | 269 |
| Females | 87 (32%) |
| APOE-ε4 allele present | 154 (57%) |
| Treated with AChE-I | 120 (45%) |
| Age (years) | 74.6 ± 7.3 |
| Range | 55.4, 89.7 |
| Education (years) | 15.7 ± 3.0 |
| Range | 6, 20 |
| MMSE at baseline | 26.9 ± 1.8 |
| Range | 23, 30 |
| CDR SB at baseline | 1.5 ± 0.9 |
| Range | 0.5, 4.5 |
| HC volume (% WBV) at baseline | 0.39 ± 0.06 |
| AMG volume (%WBV) at baseline | 0.19 ± 0.03 |
| TH volume (%WBV) at baseline | 0.15 ± 0.09 |
| ΔMMSE in 6 months | −1 ± 2 |
| Range | −8, 5 |
| ΔCDR SB in 6 months | 0 ± 1 |
| Range | −2.5, 5 |
Mean and standard deviation are presented unless indicated otherwise. Abbreviations: HC – hippocampus, AMG – amygdala, TH – temporal horn of the lateral ventricle, WBV – whole-brain volume.
Cognitive Assessment
A number of tests were given to the subjects. Of particular interest in this report were results from the MMSE and CDR, because these tests, especially MMSE, are widely used in clinical practice to assess global cognitive functioning. Although subjects participated in a detailed neuropsychological testing, Logical Memory (LM) Test (Delayed Paragraph Recall) and Rey Auditory Verbal Learning Test (AVLT)11 were selected as memory tests especially relevant to atrophy of MTL structures. In addition, Clock Drawing Test 12 Copy score was used to assess visuospatial abilities, Boston Naming Test 13 was used to assess language abilities, and Trail Making Test 14 difference score (B - A) was used to assess attention and executive function.
MR image analysis
For each subject a volume of T1-weighted anatomical images obtained using various 1.5 T scanner systems (Siemens, Philips, General Electric) was downloaded from the ADNI image depository at the Laboratory of Neuroimaging at the University of California, Los Angeles, CA. A detailed description of the sequences used on different participating sites with various scanner systems can be found on http://www.loni.ucla.edu/ADNI/Research/Cores/.
Images from an MPRAGE sequence were processed by the NeuroQuant™ software package (CorTechs Labs Inc, La Jolla, CA). This recently released software package provides a full volume segmentation of 20 subcortical brain regions in each hemisphere and reports volumes for each. This procedure was compared to manual segmentation and based on those studies, received FDA 510K approval for clinical use in measuring volumes of brain structures in MR images. The algorithm used by this software package includes 1) a quality checking step to determine that the MR imaging sequence conforms to the specifications required to perform automated segmentations (i.e. it consists of a 3-dimensional T1-weighted imaging sequence, such as MPRAGE or IRSPGR, with adequate contrast to differentiate structures of interest from surrounding structures), 2) correction for gradient nonlinearity and B1 field inhomogeneity, and 3) automated subcortical segmentation of structures as defined in a probabilistic brain atlas. Of interest to this study of MTL structures were volumes of the hippocampus, amygdala, and temporal horn of the lateral ventricle. These automated methods are similar to widely used semi-automated methods15-18, but the probabilistic atlas employed by NeuroQuant was designed to better represent the aged population. The volumes reported in the present study are numerically consistent with previously published results in MCI using manual or semi-automated methods.19-21 The processing procedure takes about 8 minutes to complete on a conventional desktop computer and does not require any user input aside from selecting the study to be segmented. A coronal slice from a full-volume segmentation of an MCI patient's brain is provided in Figure 1, and an entire full-volume brain segmentation can be viewed in data supplement Movie 1.
Figure 1.
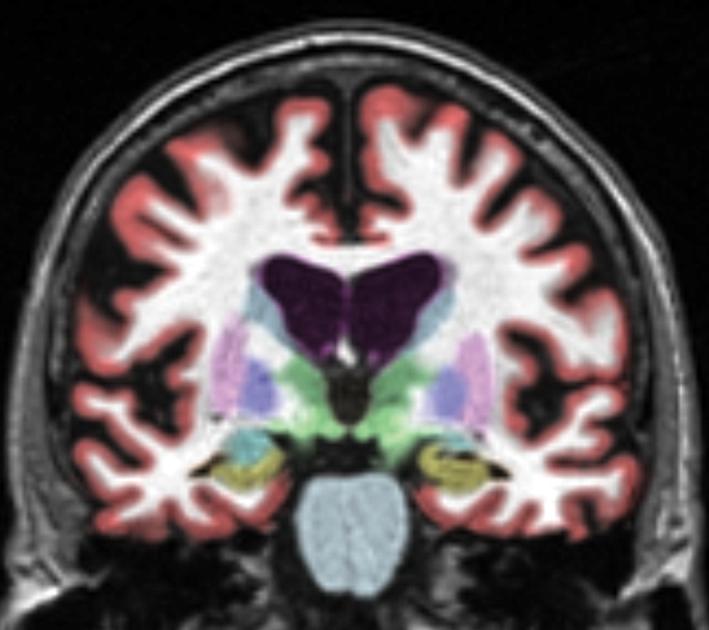
Segmentation example for a representative MCI subject. Hippocampus is colored in gold. Amygdala is the adjacent structure colored in light blue.
Fully-automated volumetric measures of MTL structures were validated against volumetric measurements based on expert manual tracing methods in a separate group of 40 subjects randomly selected from the Open Access Series of Imaging Studies.22 Half had been diagnosed with mild probable AD (CDR of .5 or 1), and half were healthy controls (CDR=0), balanced for gender and age (average age 77 years in each group). The characteristics of these subjects generally match with the ADNI subjects. Fully-automated measurements were validated against manual methods performed by experts blinded to the results of automated segmentation using intraclass correlation coefficients (ICC).23 Tracing was performed on all structures of the atlas described by Desikan et al.24 Contour drawing and editing was performed by an expert neuroanatomist who used structure boundary definitions as described by the Center for Morphometric Analysis (http://www.cma.mgh.harvard.edu/). The results of this analysis suggested good agreement between the two methods, especially for hippocampus and temporal horn (ICC = 0.946 for the hippocampus, ICC = .779 for the amygdala, and ICC = 0.942 for the temporal horn). Additionally, fully-automated measurements of total hippocampal volume showed excellent agreement with publicly available semi-automated (FreeSurfer15-18) measurements of hippocampal volumes in all 136 ADNI MCI subjects for which such data were available (ICC = .965, data shown in data supplement Figure 1). FreeSurfer analysis was performed by Dr. Anders Dale's laboratory. The demographic characteristics of this subset (29 % females, mean age 75.1 years) are similar to the entire set of subjects included in this study (Table 1).
Statistical Analysis
To correct for variability related to overall brain size, hippocampus, amygdala, and temporal horn of the lateral ventricle were expressed as a percentage of whole-brain volume. Whole-brain volume was defined as the summed volume of all brain and ventricular structures, excluding sulcal CSF. Pearson correlation coefficient was used to describe the relationships between volumetric and all other variables from the baseline visit.
Clinical decline was measured as an absolute change in MMSE or CDR Sum of Boxes (SB) score at the 6-month follow-up visit relative to the baseline visit. Multiple linear regression models were used to examine association of MTL volumes on clinical decline separately for hippocampus, amygdala, and temporal horn with each of the clinical change variables, while controlling for age, education, APOE-ε4 status, and respective baseline clinical scores. Since treatment with AChE-I might have introduced variability in clinical scores on the follow-up visit, presence or absence of treatment with AChE-I was tested as a predictor of decline. Linear regression assumptions were tested for each final model formally (i.e. Kolmogorov-Smirnov test for normality of residuals) and by graphical means (i.e. evaluating residual plots for deviations from normality and linearity). Linear regression models were used and later checked using nonparametric models. The results did not differ across the different models used for analysis. Results from linear regression models are presented here.
Results
Association of MRI Volumes with Baseline Characteristics
Hippocampus, amygdala and temporal horn volumes were correlated with age and neuropsychological measures of memory (Table 2). Specifically, larger hippocampus and amygdala volumes correlated with higher baseline delayed recall memory scores. Correlations between hippocampus volume and AVLT learning and delayed recall, as well as logical memory delayed recall, were stronger (r > 0.22, p < 0.001). Larger amygdala volumes correlated significantly with higher baseline AVLT delayed recall and logical memory delayed recall (r > 0.23, p < 0.001). In addition, larger temporal horn volumes correlated with lower AVLT learning and delayed recall (r < −0.20, p < 0.001). Controlling for age and education resulted in qualitatively similar correlation values. Hippocampus, amygdala, and temporal horn volumes were also correlated with each other, with particularly strong correlation between hippocampus and amygdala and between hippocampus and temporal horn volumes (HC-AMG r = 0.67; HC-TH r = −0.60; TH-AMG r = −0.36; all p < 0.0001).
Table 2.
Correlation between MTL volumes and demographic variables and baseline clinical and neuropsychological scores in MCI patients.
| HC | AMG | TH | WBV | |
|---|---|---|---|---|
| Age | −0.37**** | −0.27**** | 0.44**** | −0.15† |
| Education | −0.05 | 0.02 | 0.08 | 0.07 |
| MMSE | 0.14† | 0.12 | −0.13† | −0.007 |
| CDR SB | −0.14† | −0.17* | 0.14† | −0.05 |
| AVLT Learning | 0.24*** | 0.17* | −0.29**** | −0.06 |
| AVLT Delayed Recall | 0.34**** | 0.33**** | −0.20** | 0.02 |
| LM Immediate Recall | 0.08 | 0.04 | −0.13† | 0.04 |
| LM Delayed Recall | 0.22** | 0.23** | −0.09 | 0.11 |
| Clock Copy | −0.04 | −0.05 | −0.12 | 0.08 |
| Boston Naming Test | 0.01 | 0.16* | −0.12† | 0.14† |
| Trails B – Trails A | −0.08 | −0.10 | 0.16* | 0.03 |
Pearson r and two-tailed significance level are indicated:
p < .05
p < .01
p < .001
p < .0001
p < .00001.
Abbreviations: HC – hippocampus, AMG – amygdala, TH – temporal horn of the lateral ventricle, WBV – whole-brain volume.
Association of MRI Volumes with Longitudinal Change in MMSE and CDR SB Scores
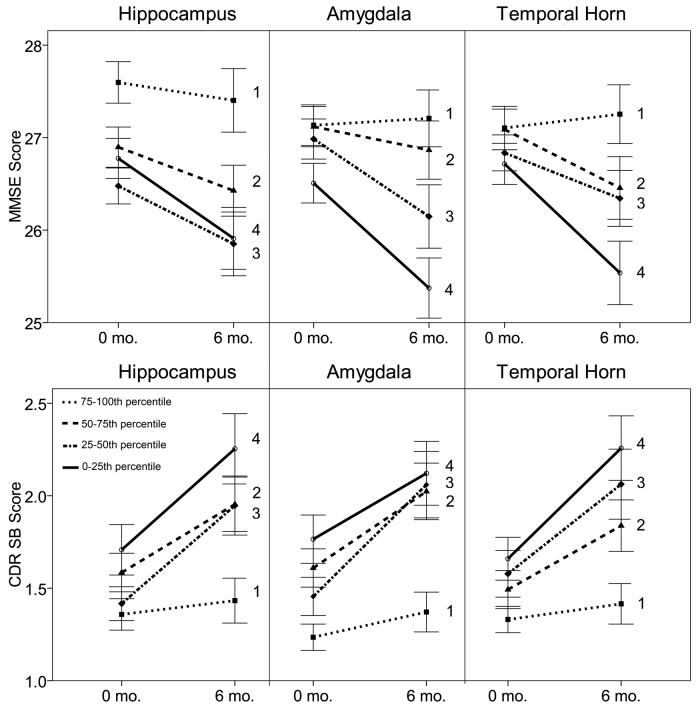
Baseline hippocampus, amygdala, and temporal horn volumes correlated with change in MMSE and CDR SB scores over the following 6 months (Table 3). Smaller baseline hippocampus, smaller baseline amygdala, or larger baseline temporal horn volumes predicted greater subsequent decline in MMSE, indicating worsening global cognitive function, and predicted greater subsequent increase in the CDR SB, indicating a decline in cognition and activities of daily living (ADL). Although each MTL volume was analyzed as a continuous variable, Figure 2 graphically illustrates the results using quartiles based on the volumes of each MTL structure. MMSE scores at the baseline and the 6-month follow-up were plotted for each quartile group. This plot demonstrates that, in general, patients with smaller hippocampus, smaller amygdala, or larger temporal horn show the greatest decline on the 6-month follow-up visit, as measured by MMSE and CDR SB.
Table 3.
Correlation between change in clinical scores and demographic, baseline volumetric, and cognitive variables in MCI subjects.
| ΔMMSE | ΔCDRSB | |
|---|---|---|
| Age | −0.03 | 0.05 |
| Education | 0.002 | 0.05 |
| Hippocampus | 0.12† | −0.17* |
| Amygdala | 0.18* | −0.12† |
| Temporal Horn | −0.15† | 0.15† |
| Whole Brain | 0.09 | 0.03 |
| MMSE | −0.14† | −0.19* |
| CDR SB | −0.10 | −0.14† |
| AVLT Learning | 0.30**** | −0.25*** |
| AVLT Delayed Recall | 0.30**** | −0.17* |
| LM Immediate Recall | 0.26*** | −0.22** |
| LM Delayed Recall | 0.27**** | −0.22** |
Pearson r and two-tailed significance level are indicated:
p < .05
p < .01
p < .001
p < .0001
p < .00001.
Figure 2.
Clinical scores at 0- and 6-month time-points for MCI patients in quartiles based on WBV-normalized MTL volumes. Means and standard errors are shown for each of the quartiles ranging from least (1) to greatest (4) suggested atrophy. For hippocampus and amygdala, the first quartile represents the group with the largest volumes. For temporal horn, the first quartile represents the group with the smallest volumes.
Correlation between baseline neuropsychological measures of memory and subsequent clinical decline was even stronger (Table 3). Therefore, multiple regression analyses were performed to determine if automated measures of MTL atrophy provide independent predictive information beyond that of demographics and baseline clinical and neuropsychological measures.
In the multiple regression analysis, after adjusting for age and education, smaller baseline hippocampus volume was associated with greater decline on MMSE (β = 0.13, p = 0.048) and CDR SB (β = −0.17, p = 0.01). Smaller baseline amygdala volume was associated with greater decline on MMSE (β = 0.19, p = 0.003) and showed a trend toward association with CDR SB (β = −0.12, p = 0.07). Larger baseline temporal horn volume was associated with greater decline on MMSE (β = −0.17, p = 0.02) and CDR SB (β = 0.15, p = 0.03).
MTL volumes were associated with decline on MMSE and CDR SB (Table 4) after adjusting for age, education, APOE- ε4 status, and the baseline clinical scores. Smaller baseline hippocampus volume was associated with greater decline on MMSE (β = 0.14, p = 0.04) and CDR SB (β = −0.19, p = 0.005). Smaller baseline amygdala volume was associated with greater decline on MMSE (β = 0.18, p = 0.004) and CDR SB (β = −0.12, p = 0.06, trend). Larger baseline temporal horn volume was associated with greater decline on MMSE (β = −0.20, p = 0.003) and CDR SB (β = 0.20, p = 0.005). Treatment status did not influence the ability of automated MTL measures to predict clinical decline as measured by MMSE and CDR SB scores.
Table 4.
MTL volumes predict decline on clinical tests after adjustment for age, education, baseline clinical scores, and APOE- ε4 status in MCI subjects.
| ΔMMSE | ΔCDRSB | |||||
|---|---|---|---|---|---|---|
| Model | Predictor | β | p | Predictor | β | p |
| HC | Age | −0.02 | 0.7 | Age | −0.02 | 0.7 |
| Education | 0.03 | 0.6 | Education | 0.03 | 0.6 | |
| MMSE | −0.19 | 0.003 | CDR SB | −0.17 | 0.006 | |
| APOE- ε4 | −0.15 | 0.02 | APOE- ε4 | 0.04 | 0.5 | |
| HC | 0.14 | 0.04 | HC | −0.19 | 0.005 | |
| AMG | Age | 0.02 | 0.7 | Age | 0.02 | 0.8 |
| Education | 0.03 | 0.6 | Education | 0.04 | 0.5 | |
| MMSE | −0.19 | 0.003 | CDR SB | −0.17 | 0.009 | |
| APOE- ε4 | −0.14 | 0.02 | APOE- ε4 | 0.05 | 0.4 | |
| AMG | 0.18 | 0.004 | AMG | −0.12 | 0.06 | |
| TH | Age | 0.02 | 0.8 | Age | −0.04 | 0.6 |
| Education | 0.05 | 0.4 | Education | 0.02 | 0.7 | |
| MMSE | −0.19 | 0.002 | CDR SB | −0.18 | 0.005 | |
| APOE- ε4 | −0.15 | 0.01 | APOE- ε4 | 0.06 | 0.3 | |
| TH | −0.20 | 0.003 | TH | 0.20 | 0.005 |
Abbreviations: HC – hippocampus, AMG – amygdala, TH – temporal horn of the lateral ventricle; MMSE – MMSE baseline score, CDR SB – CDR SB baseline score.
When the model included baseline neuropsychological measures of memory, baseline MTL volumes did not add significantly to prediction of clinical decline, partially due to the high correlation between baseline MTL volumes and the memory scores.
Discussion
Volume measurements of the MTL are associated with the rate of clinical decline in a large cohort of MCI patients. This study, using fully-automated segmentation, demonstrates the novel finding that in addition to smaller baseline hippocampus, patients with smaller amygdala, or larger temporal horn declined more rapidly on two widely available clinical assessments of general cognitive and functional ability. Measures of MTL atrophy contributed to prediction beyond that provided by age, education, APOE-ε4 and baseline clinical scores. In addition to associations between hippocampal volume and baseline learning and delayed recall scores25, 26, measures of amygdala volume and temporal horn volume were also related to memory performance.
In this study, we used fully-automated brain MR image segmentation to examine MTL volume in MCI patients, and identified an association between MTL volume and clinical decline within a 6-month interval. Previous studies using manual or semi-automated measures of segmental MTL volumes with longer follow-up times (1 year or more) have shown a significant association between rate of clinical decline and extent of MTL atrophy in elderly patients.27, 28 AD is associated with early MTL atrophy29, and prodromal AD may underlie the memory complaints of most, but not all, amnestic MCI patients.30, 31 MCI patients with MTL atrophy may be more likely to have prodromal AD and a more rapid clinical decline. Furthermore, the extent of MTL atrophy may reflect disease severity, which also contributes to a rapid decline. Further studies with attention to long-term clinical course and pathological findings will be essential to understanding the observed relationship between MTL atrophy in MCI patients and subsequent development of AD.
Both subtypes of amnestic MCI, single-domain and multiple-domain, are known to have a high rate of conversion to AD, and were studied in the ADNI cohort. As expected, a number of MCI patients (20 out of 269, 7 %) converted to AD within the 6-month period examined at the time of this study. Interestingly, conversion rates from MCI to AD have been reported to be approximately 16 % per year31, 32 and our analysis of the 6-month ADNI data supports this finding. Prior studies have compared baseline MR image volumetry in patients who progress to AD versus those who do not.33-35 Such studies have suggested that MTL atrophy is associated with subsequent conversion to AD. The current study assessed whether MTL atrophy predicts rate of clinical decline, which may be associated with earlier conversion from MCI to AD. The results from this large multi-center sample of MCI patients are consistent with the findings reported in previous studies that typically used smaller samples and manual tracing or visual assessment techniques in evaluating hippocampal or entorhinal atrophy in prediction of conversion from MCI to AD.33-42
Few studies have evaluated amygdala atrophy and temporal horn enlargement in prediction of disease progression in MCI subjects.43-46 Neuropathological studies have implicated involvement of amygdala in AD progression.47 However, findings from previous manual volumetric studies of amygdala have been inconsistent.48 In the current study, amygdala atrophy was a significant predictor of decline as measured by MMSE and CDR SB, and remained a significant predictor after adjustment for baseline clinical scores.
Fully-automated temporal horn measurements were also significant predictors of decline as measured by MMSE and CDR SB even after accounting for age, education, and baseline clinical scores. Enlargement of the temporal horn of the lateral ventricle reflects atrophy of the hippocampus as well as atrophy of the surrounding tissue, and thus may gauge regional atrophy beyond the MTL. It remains to be seen whether such fully-automated measures are able to predict clinical decline in patients with more severe disease, such as when the pathology of AD extends beyond MTL structures. Temporal horn measures may complement hippocampal measures in predicting cognitive decline in such a group of patients. The current results suggest that temporal horn measures may provide information that is supportive to hippocampal measures in predicting subsequent decline when a memory deficit is noted, but overall level of functional impairment is mild.
In this study, treatment with AChE-I was allowed, and 45% of subjects received treatment for the duration of the follow-up or longer. Given that modest treatment effects on behavioral performance have been reported in AChE-I trials, to control for treatment effects on prediction of clinical decline, treatment status was included in the analysis. Controlling for treatment status in prediction of decline did not change qualitatively the association between MTL volumes and decline on MMSE and CDR SB. Beneficial effects of treatment could have been obscured in this study because selection of patients for treatment could have been biased towards more severe cases. In fact, the treated group had more MTL atrophy and lower baseline clinical and memory scores. However, the goal was not to study treatment effects per se, but to control for them if they exist. It is important that future studies control for AChE-I medication effects as many MCI patients receive such treatment off label.
Some limitations of this study warrant mentioning. Due to the brief follow-up period, the cohort included very few converters to clinical AD and, therefore, relating baseline volumetry to clinical conversion was not possible. In addition, the outcome measures, MMSE and CDR SB, widely used to define clinical decline, are relatively insensitive to clinical decline over a 6-month period. On the other hand, the more specialized neuropsychological batteries used to assess memory in these patients are time-consuming (approximately 3 hours) and not as widely available in all clinical settings. Though these measures have been shown to have a higher predictive value in relation to cognitive decline in some studies49, the use of additional brain regions in the analysis44, 50 may prove to be superior when compared to these specialized tests.
This study demonstrates that fully-automated MTL volume measurement can be performed rapidly and without manual input, yielding measures that are consistent with manual tracing and that are associated with the rate of clinical decline in a large cohort of MCI patients. Association of baseline atrophy with rapid decline was noted even with the short follow-up time of 6 months. Quantitative and objective measures, such as these, may improve selection of patients appropriate for early intervention against AD, and will be especially valuable as new medications that target AD pathophysiology are developed. Given that MR imaging is widely available and is already performed on patients with cognitive impairment, fully-automated methods for subregional volume assessment may provide additional clinically relevant information at little added cost. The findings of the current study suggest that this capability may help clinicians identify which MCI patients are at greatest risk for rapid clinical decline.
Supplementary Material
Acknowledgements
Data collection and sharing for this project was funded by the ADNI (Principal Investigator: Michael Weiner; NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and through generous contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer's Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the U.S. Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Authors report no conflicts with Cortechs, manufacturers of NeuroQuant software used for automated segmentations. Cortechs provided authors with free access to their proprietary software to analyze the data for the purposes of this paper, and had no input in the data analysis or the preparation of this manuscript.
Supported by NINDS K23 NS050305
References
- 1.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer's disease: The Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes J, Boyes RG, Lewis EB, et al. Automatic calculation of hippocampal atrophy rates using a hippocampal template and the boundary shift integral. Neurobiol Aging. 2007;28:1657–1663. doi: 10.1016/j.neurobiolaging.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Crum WR, Scahill RI, Fox NC. Automated hippocampal segmentation by regional fluid registration of serial MRI: validation and application in Alzheimer's disease. Neuroimage. 2001;13:847–855. doi: 10.1006/nimg.2001.0744. [DOI] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 6.Wechsler D. WMS-R Wechsler Memory Scale - Revised Manual. The Psychological Corporation, Harcourt Brace Jovanovich, Inc.; New York: 1987. [Google Scholar]
- 7.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 8.Yesavage J, Brink T. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 9.Rosen WG, Terry RD, Fuld PA, et al. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC. Mild cognitive impairment clinical trials. Nat Rev Drug Discov. 2003;2:646–653. doi: 10.1038/nrd1155. [DOI] [PubMed] [Google Scholar]
- 11.Rey A. L'examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- 12.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 13.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 14.Spreen O, Strauss E. A compendium of neuropsychological tests. Oxford University Press; New York: 1998. [Google Scholar]
- 15.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 16.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 17.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Fleisher A, Grundman M, Jack CR, Jr., et al. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953–957. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- 20.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 21.Wang PN, Lirng JF, Lin KN, et al. Prediction of Alzheimer's disease in mild cognitive impairment: a prospective study in Taiwan. Neurobiol Aging. 2006;27:1797–1806. doi: 10.1016/j.neurobiolaging.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Marcus DS, Wang TH, Parker J, et al. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19:1498–1507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- 23.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- 24.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Lye TC, Grayson DA, Creasey H, et al. Predicting memory performance in normal ageing using different measures of hippocampal size. Neuroradiology. 2006;48:90–99. doi: 10.1007/s00234-005-0032-5. [DOI] [PubMed] [Google Scholar]
- 26.Grundman M, Jack CR, Jr., Petersen RC, et al. Hippocampal volume is associated with memory but not monmemory cognitive performance in patients with mild cognitive impairment. J Mol Neurosci. 2003;20:241–248. doi: 10.1385/jmn:20:3:241. [DOI] [PubMed] [Google Scholar]
- 27.Mungas D, Reed BR, Jagust WJ, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- 29.Jack CR, Jr., Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease? Lancet Neurol. 2004;3:246–248. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- 31.Yaffe K, Petersen RC, Lindquist K, et al. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 33.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 34.Convit A, de Asis J, de Leon MJ, et al. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer's disease. Neurobiol Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 35.Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 36.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 37.Jack CR, Jr., Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeCarli C, Frisoni GB, Clark CM, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108–115. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 39.Korf ES, Wahlund LO, Visser PJ, et al. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 40.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 41.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 42.Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 43.Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karas G, Sluimer J, Goekoop R, et al. Amnestic mild cognitive impairment: structural MR imaging findings predictive of conversion to Alzheimer disease. AJNR Am J Neuroradiol. 2008 doi: 10.3174/ajnr.A0949. published online on Feb 22, 2008 as doi:2010.3174/ajnr.A0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giesel FL, Hahn HK, Thomann PA, et al. Temporal horn index and volume of medial temporal lobe atrophy using a new semiautomated method for rapid and precise assessment. AJNR Am J Neuroradiol. 2006;27:1454–1458. [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrarini L, Palm WM, Olofsen H, et al. MMSE scores correlate with local ventricular enlargement in the spectrum from cognitively normal to Alzheimer disease. Neuroimage. 2008;39:1832–1838. doi: 10.1016/j.neuroimage.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Braak H, Braak E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol (Berl) 1996;92:197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- 48.Atiya M, Hyman BT, Albert MS, et al. Structural magnetic resonance imaging in established and prodromal Alzheimer disease: a review. Alzheimer Dis Assoc Disord. 2003;17:177–195. doi: 10.1097/00002093-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Fleisher AS, Sun S, Taylor C, et al. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology. 2008;70:191–199. doi: 10.1212/01.wnl.0000287091.57376.65. [DOI] [PubMed] [Google Scholar]
- 50.Fan Y, Batmanghelich N, Clark CM, et al. Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage. 2008;39:1731–1743. doi: 10.1016/j.neuroimage.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.