Summary
The oncogenic G12/13 subfamily of heterotrimeric G proteins transduces extracellular signals that regulate the actin cytoskeleton, cell cycle progression, and gene transcription. Previously, structural analyses of fully-functional Gα12/13 subunits have been hindered by insufficient amounts of homogeneous, functional protein. Herein we report that substitution of the N-terminal helix of Gαi1 for the corresponding region of Gα12 or Gα13 generated soluble chimeric subunits (Gαi/12 and Gαi/13) that could be purified in sufficient amounts for crystallographic studies. Each chimera bound guanine nucleotides, Gβγ subunits and effector proteins, and exhibited GAP responses to p115RhoGEF and leukemia-associated RhoGEF. Like their wild-type counterparts, Gαi/13, but not Gαi/12, stimulated the activity of p115RhoGEF. Crystal structures of the Gαi/12·GDP·AlF4− and Gαi/13·GDP complexes were determined using diffraction data extending to 2.9 and 2.0 Å, respectively. These structures reveal not only the native structural features of Gα12 and Gα13 subunits, which are expected to be important for their interactions with GPCRs and effectors such as Gα-regulated RhoGEFs, but also novel conformational changes that are likely coupled to GTP hydrolysis in the Gα12/13 class of heterotrimeric G proteins.
Heterotrimeric GTP-binding proteins (G proteins) receive inputs from G protein-coupled receptors (GPCRs) at the cell surface and elicit a wide variety of responses within the cell (1). Activation of GPCRs by extracellular stimuli induces the Gα subunit to release its bound GDP and bind GTP, and facilitates dissociation of Gα·GTP from the β and γ subunits (Gβγ). Both Gα·GTP and Gβγ subsequently bind to and regulate the activity of various effector molecules. A family known as regulator of G protein signaling (RGS) proteins can bind to activated Gα subunits and accelerate their rate of deactivation, thereby serving as GTPase-activating proteins (GAPs) (2). It was shown through biochemical and structural studies of RGS4 that its core helical domain, referred to herein as the RGS homology (RH) domain1, binds to all three switch regions of Gα and thereby stabilizes the transition state for GTP hydrolysis (3, 4).
Among the four subfamilies of Gα subunits (5), the members of the Gα12/13 subfamily are distinct in that they are potently oncogenic in cell-based assays (6, 7). Stimulation of Gα12/13-coupled receptors, such as those for thrombin or lysophosphatidic acid, transforms cells in a manner implicating the activation of Rho family GTPases (8, 9). Downstream effector targets of Gα12/13 subunits are diverse and include cadherin (10), protein phosphatase 5 (11) and many others (12). However, the best-characterized targets of Gα12/13 subunits are a family of Rho guanine nucleotide exchange factors (RhoGEFs), including p115RhoGEF, leukemia-associated RhoGEF (LARG), and PDZ-RhoGEF (13–15). Each of these RhoGEFs contains an RH domain (also known as the rgRGS or LH domain)1 that binds specifically to activated Gα12/13 subunits (16). Unlike RGS proteins, the RhoGEF RH domain binds to the effector-binding site of the Gα subunit rather than the site traditionally used by RGS proteins (17). A small, acidic domain N-terminal to the RH domain, which is essential for GAP activity but not for the binding of Gα13 (18, 19), occupies the binding site traditionally used by RGS proteins.
Biochemical and crystallographic characterization of Gα12/13 subunits and their effector complexes requires large amounts of homogeneous, fully-functional Gα12 and Gα13 subunits, which previously have been produced with exceptionally low yields (20, 21). Although Chen et al. were able to express a “Gα13/i-5” chimera, in which the switch regions and α-helical domain of Gα13 were swapped for those of Gαi1, the resulting chimera exhibited approximately a 10-fold higher nucleotide exchange rate and 5% of the GAP activity of wild-type Gα13 in response to p115RhoGEF. Even so, it could still be crystallized as a complex with an N-terminal fragment of p115RhoGEF that included its RH domain (17).
Herein we show that substitution of the N-terminal helix of Gαi1 for the corresponding region of Gα12 and Gα13 generated soluble Gαi/12 and Gαi/13 chimera that retained all wild-type biochemical properties while also being expressed at much greater levels than their wild-type counterparts. By determining the crystal structures of the Gαi/12·GDP·AlF4− (activated) and Gαi/13·GDP (deactivated) complexes, we not only show that these proteins are suitable for structural studies, but also provide the first look at the native atomic structures of these oncogenic G protein subunits and a novel conformational change upon deactivation of these subunits that we propose is specific to the Gα12/13 subfamily. Differences in the structures of Gα12 and Gα13 undoubtedly contribute to their differential ability to couple with receptors and to regulate downstream effectors.
Experimental Procedures
Expression constructs
The Gαi/13 chimera was created by ligating the N-terminal coding region of rat Gαi1 (residues 1–28) to Gα13 (residues 47–377) and subcloning the product into the baculovirus transfer vector pFastBacHTA (Invitrogen, Carlsbad, CA). A construct of Gαi/12 was created using the same strategy. Each construct therefore includes an N-terminal hexahistidine (His6) tag followed by a tobacco etch virus (TEV) protease recognition site. The expression construct for glutathione-S-transferase (GST)-p115-RH or GST-LARG-RH was generated by subcloning the coding region of p115RhoGEF (residues 1 to 252) or LARG (residues 319 to 598) into pGEX-KG, respectively.
Production of chimeric Gα subunits
All purification steps were conducted at 4°C using ice-cold buffers supplemented with protease inhibitors. Cells from 4 liters of Sf9 culture were harvested 48 h after infection with 15 ml/L of either Gαi/13 or Gαi/12 amplified baculovirus stock, resuspended in 400 ml of Lysis Buffer (20 mM HEPES pH 8.0, 0.1 mM EDTA, 10 mM 2-mercaptoethanol (βME), 1 mM MgCl2, 100 mM NaCl, and 10 µM GDP), and then lysed by nitrogen cavitation. Lysates were centrifuged at 100,000 × g for 30 min, after which the supernatants were diluted with 1600 ml of Buffer A (20 mM HEPES pH 8.0, 10 mM βME, 1 mM MgCl2, 100 mM NaCl, 10 µM GDP, and 12.5 mM imidazole, pH 8.0) and loaded onto a Ni-NTA agarose (Qiagen, Valencia, CA) column equilibrated with Buffer A. The column was washed with 20 volumes of Buffer B (Buffer A containing 0.4 M NaCl and 20 mM imidazole, pH 8.0), and the chimera was eluted in 10 fractions of 1 volume of Buffer C (Buffer A containing 150 mM imidazole, pH 8.0). Peak fractions were supplemented with 10% glycerol, and exchanged to Buffer D (20 mM HEPES pH 8.0, 10 mM βME, 1 mM MgCl2, 100 mM NaCl, 10 µM GDP, 10% glycerol) with Centricon Plus-20 PL-30 (Millipore, Billerica, MA).
Purification of RH proteins
GST-p115-RH was produced in JM109 harboring pGEX-KG/p115-RH and pT-Trx plasmids (22) for 3 hr after induction with 0.1 mM IPTG at 30°C. GST-LARG-RH was similarly expressed in BL21(DE3) CodonPlus RP (Stratagene, La Jolla, CA) harboring the pGEX-KG/LARG-RH plasmid. GST-RH fusions were purified by glutathione Sepharose 4B resin (Amersham Biosciences).
Interaction of chimeric Gα subunits with RH proteins
Gαi/13 or Gαi/12 (2 µg) was diluted in Buffer E (20 mM Tris-HCl, pH 8.0, 1 mM EDTA, 10 mM βME, 300 mM NaCl, 10 mM MgCl2, 30 µM GDP, 0.1% C12E10), either in the absence or presence of AlF4− (30 µM AlCl3 and 10 mM NaF), and mixed with 33 µg of GST-p115-RH or 38 µg of GST-LARG-RH in a final volume of 500 µl. After incubation on ice for 20 min, glutathione Sepharose 4B (30 µl resin) was added and samples were incubated with constant mixing for 1 h at 4°C. Beads were pelleted by centrifugation (600 × g, 1 min, 4°C) and washed four times with 400 µl of Buffer E (with or without AlF4−). Beads were mixed with 20 mM reduced glutathione and SDS-PAGE sample buffer and boiled for 10 min. Immunoblotting was performed with anti-Gα13 B860 (21) or anti-Gα12 J168 (20) antibody.
Rho nucleotide exchange assays
His6-RhoA was expressed in Sf9 cells and purified as described (23). Gα13, Gαi/13, or Gαi/12 (5 pmol) was first incubated in the presence of AMF (60 µM AlCl3, 5 mM MgCl2, and 20 mM NaF) for 15 min on ice, then incubated with His6-RhoA (25 pmol) and p115RhoGEF (0.25 pmol) in binding buffer (50 mM Tris-HCl, pH 7.5, 1 mM DTT, 0.5 mM EDTA, 50 mM NaCl, 5 mM MgCl2, 0.05% C12E10, and 10 µM GTPγS with ~500 cpm/pmol [35S]-GTPγS) in a final reaction volume of 50 µl. Reactions were terminated after incubation for 5 min at 30°C by addition of wash buffer, and GTPγS binding to RhoA was determined by filter binding as described (24).
Crystallization of Gαi/12·GDP·AlF4− and Gαi/13·GDP
Gαi/13 or Gαi/12 was first incubated with 1.5% (w/w) recombinant TEV protease for 2 hr at 25°C to remove the His6-tag, and then activated by AlF4− (20 µM AlCl3 and 10 mM NaF) for 15 min on ice. This mixture was loaded onto tandem Superdex 200 10/30 gel filtration columns (Amersham Biosciences, Piscataway, NJ) equilibrated in gel filtration buffer (20 mM HEPES pH 8.0, 1 mM EDTA, 2 mM DTT, 5 mM MgCl2, 50 mM NaCl, 10 µM GDP, 20 µM AlCl3, and 10 mM NaF). Both Gαi/13 and Gαi/12 eluted from the column as apparent monomers. Peak fractions were pooled and concentrated using a Centricon YM-30 (Millipore).
Gαi/12 was crystallized by mixing 1 µl of 22 mg/ml Gαi/12 with 1 µl well solution containing 100 mM sodium citrate pH 6.5, 50 mM NaCl, and 14% PEG 8000, and then suspending the drop over 1 ml of well solution at 4°C. Paper-thin, diamond shaped crystals appeared within one week. Gαi/13 was crystallized similarly, except using 19 mg/ml Gαi/13 and a well solution containing 100 mM sodium citrate (pH 4.8), 50 mM NaCl, and 10% PEG 2000. Tetragonal rod-shaped crystals appeared in 7 to 20 days. Both crystals were stabilized in a harvesting solution containing all the components of both the gel filtration buffer and their respective well solution, and were serially transferred, in 5% steps at a time, into a final concentration of 20% glycerol in harvesting solution. Crystals were then flash frozen in liquid nitrogen on nylon loops.
X-ray analysis and structure determination
Diffraction maxima were measured from crystals maintained at 100 K at the Advanced Photon Source beam line 17-ID using a Quantum 210 CCD detector. Gαi/12 crystals exhibited severely anisotropic diffraction with disorder in the maxima that could have been due to warping of the extremely thin crystals. These defects, along with lower resolution diffraction limits, are probably responsible for the relatively high R-factors in the resulting model of Gαi/12 compared to the Gαi/13 structure (Table I). Data were reduced using HKL2000 (25). The space group choice and phase problem for Gαi/13 was solved via molecular replacement with the program PHASER (26) using Gαi1·GDP·AlF4− (PDB code: 1AGR) as the search model. The Gαi/12 structure was determined using the partially refined Gαi/13 structure as the search model. Each model was refined with several rounds of simulated annealing in CNS_SOLVE (27) and then restrained-refinement in REFMAC5 (28). Two-fold non-crystallographic symmetry restraints were used during refinement of Gαi/12. Model building was performed with O (29). All reflections were used in the last round of refinement with final combined R-factors of 23.7% and 21.1% for the Gαi/12·GDP·AlF4− and Gαi/13·GDP structures, respectively. Coordinates and structure factors for the Gαi/12 and Gαi/13 structures are deposited at the PDB with accession codes 1ZCA and 1ZCB, respectively.
Table I.
Crystallographic data and refinement statistics
| X-ray Source: APS 17-ID | Gαi/12·GDP·AlF4− | Gαi/13·GDP |
|---|---|---|
| Wavelength (Å) | 1.000 | 1.000 |
| Dmin (Å) | 2.9a | 2.0 |
| Space group | P21 | P43212 |
| Cell constants (Å, °) | a = 57.5, b = 85.2, c = 82.9; β = 106.1, α = γ = 90 | a = b = 67.4, c = 175.2; α = β = γ = 90 |
| Unique reflections | 15263 (1140)c | 27297 (2761)c |
| Average redundancy | 2.5 (1.7) | 5.7 (4.0) |
| Rsym (%)b | 10.2 (29.6) | 4.9 (38.5) |
| Completeness (%) | 88.7 (67.4) | 96.7 (100) |
| <I>/<σI> | 6.9 (1.7) | 42.6 (3.4) |
| Refinement resolution (Å) | 29.1 – 2.9 | 28.5 – 2.0 |
| Total reflections used | 14,452 | 25,848 |
| Protein atoms | 5239 | 2618 |
| Non-protein atoms | 74 | 80 |
| RMSD bond lengths (Å) | 0.007 | 0.015 |
| RMSD bond angles (°) | 1.0 | 1.5 |
| Rworkd | 23.9 (35.1)e | 20.7 (24.4)e |
| Rfreef | 29.6 (44.3) | 25.2 (29.4) |
Diffraction from this crystal form was anisotropic, with maxima extending beyond 2.9 Å in the b* direction, and from 3.5–4.5Å in orthogonal directions.
Rsym = ∑h∑i |I(h)i - I(h)|/ ∑h∑i I(h)i, where I(h) is the mean intensity of i reflections after rejections. A −0.5 I/σI cutoff was applied to the Gαi/12 data set due to its anisotropic data.
Numbers in parentheses correspond to the highest resolution shell of data for each set, which was 3.0 – 2.9 Å for Gαi/12 and 2.07 – 2.00 Å for Gαi/13.
Rwork = ∑hkl‖Fobs(hkl)| -|Fcalc(hkl)‖/ ∑hkl |Fobs(hkl)|; no I/σ cutoff was used during refinement.
Numbers in parentheses correspond to the highest resolution shell of data for each set, which was 3.0 – 2.9 Å for Gαi/12 and 2.05 – 2.00 Å for Gαi/13.
5% of the truncated data set was excluded from refinement to calculate Rfree.
Miscellaneous procedures
GTPγS binding to Gαi/13 or Gα13 was measured by filter binding assays as described previously (23). Single-turnover GTP hydrolysis by Gαi/13 or Gαi/12 was analyzed in the absence (basal) or presence of 100 nM GST-p115-RH or GST-LARG-RH, as described previously for Gα13 or Gα12 (16). EE-tagged, full-length p115RhoGEF and Gα13 were purified as described (24). Atomic representations were created with the program PYMOL (30).
Results and Discussion
Soluble expression of functional Gαi/12 or Gαi/13 in insect cells
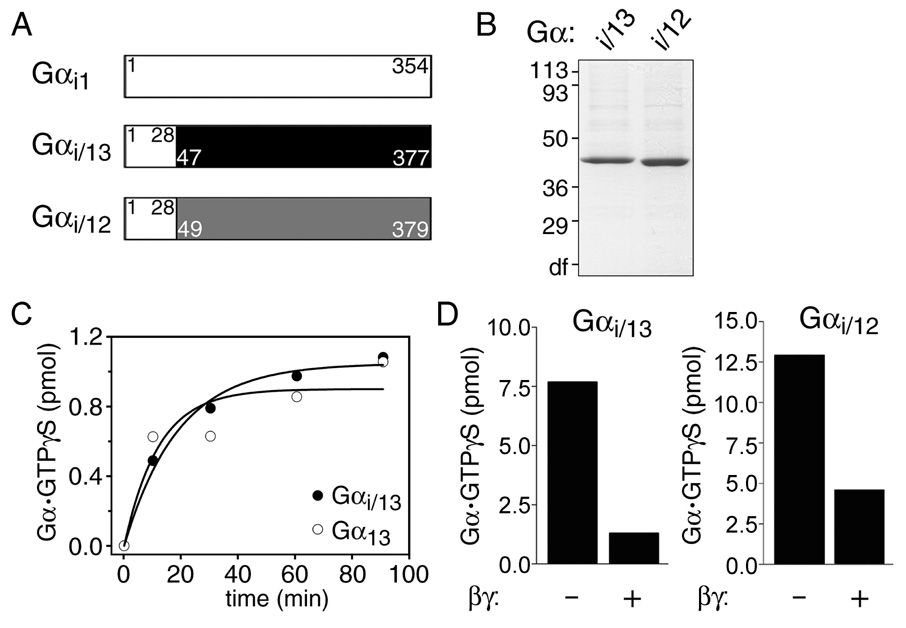
To increase yields of functional Gα12 or Gα13, we constructed the Gαi/12 and Gαi/13 chimera, in which the N-terminal helices of mouse Gα12 or Gα13 were swapped with that of rat Gαi1 (Fig. 1A and Figure S1). The Gαi/13 chimera partitioned almost equally to the soluble and crude membrane fractions of Sf9 cells (data not shown). The soluble Gαi/13 exhibited activation-dependent trypsin protection indicative of a functional Gα subunit (Fig. S2). Similar results were also obtained with Gαi/12 (data not shown). The yield of Gαi/13 was about 3.5 mg from 1 liter of Sf9 cell culture after Ni-NTA agarose chromatography (Fig. 1B), representing a vast improvement from previous methods (31). Similarly, the yield of Gαi/12 was about 4.0 mg from 1 liter of culture (Fig. 1B). The expression of analogous chimeric proteins in E. coli resulted in large yields of soluble protein, but these proteins could not bind guanine nucleotides (data not shown).
Figure 1.
Generation of Gαi/13 and Gαi/12 chimeric proteins. (A) Schematic representation of the Gαi/13 and Gαi/12 chimera. Residues of Gαi1 are shown in white, and numbers within the colored regions of the Gαi/13 or Gαi/12 chimera correspond to original numbering of residues contributed by Gα13 (black) or Gα12 (gray). (B) Purified His6-Gαi/13 or His6-Gαi/12 (3 µg) were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue. The position and apparent molecular weight of standard proteins (in kDa) is indicated. (C) GTPγS binding to Gαi/13 (●) or wild-type Gα13 (○) was measured at 30°C. Data are the mean of duplicate determinations. (D) Gαi/13 or Gαi/12 functionally interact with Gβγ. GTPγS binding to Gαi/13 or Gαi/12 (3 µg each) was measured either in the absence or presence of Gβγ (20 µg) as indicated. GTPγS binding was quantified after 90 min at 30°C. Data are the mean of duplicate determinations.
Gαi/12 and Gαi/13 subunits bind guanine nucleotides and Gβγ
Gα12/13 subunits possess biochemical properties that are distinct from those of most other Gα subunits. These include rates of nucleotide exchange (~0.01 min−1) and GTP hydrolysis (~0.2 min−1) that are roughly 3–10 fold slower than those of Gαs and Gαi subfamilies (20, 21, 32). The activity of purified Gαi/13 was evaluated by GTPγS binding assays. GTPγS binding to Gαi/13 was effectively reduced in the presence of AlF4−, which was similar to control samples containing purified Gαi1 (Fig. S2). Furthermore, purified Gαi/13 bound GTPγS with similar kinetics as wild-type Gα13 (Fig. 1C). Gαi/12 also bound GTPγS similarly to wild-type Gα12 (data not shown). Because the N-terminal helix of Gα subunits is known to interact with Gβγ (33–35), we tested whether its substitution affected association of Gαi/12 or Gαi/13 with Gβγ, as measured by the reduced binding of GTPγS to Gα in the presence of Gβγ (36). Assays performed in the presence of excess Gβγ showed marked inhibition of GTPγS binding to both chimeric proteins (Fig. 1D), indicating that Gαi/12 and Gαi/13 retain the ability to interact with Gβγ.
RhoGEFs exhibit GAP activity for Gαi/12 or Gαi/13
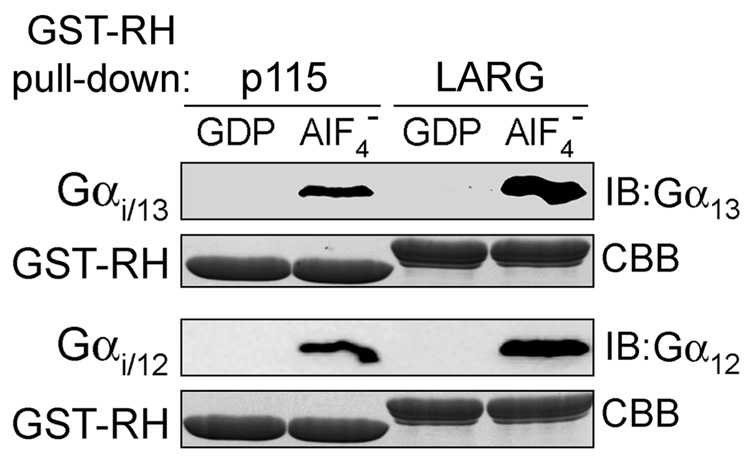
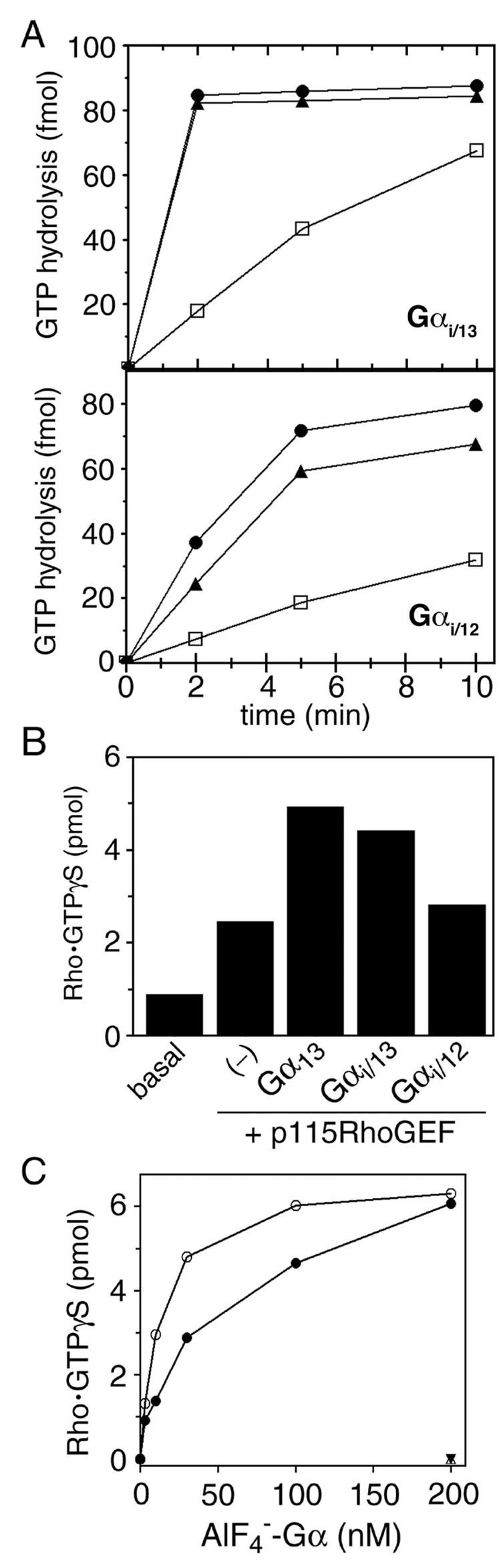
Gα12 and Gα13 interact with the RH domain of p115RhoGEF or LARG in an AlF4−-dependent manner (15, 16). As shown in Figure 2, Gαi/12 and Gαi/13 also interacted with RH domain-containing fragments of p115RhoGEF or LARG in an AlF4−-dependent manner, as assessed by GST pull-down assays. We also could isolate high affinity complexes of each chimeric G protein with the RH domain of LARG by size exclusion chromatography (data not shown). We next examined whether these same p115RhoGEF or LARG fragments exhibit GAP activity for the chimera. Both fragments accelerated the GTPase activity of Gαi/12 and Gαi/13 in single-turnover GTPase assays (Fig. 3A), with a faster response in Gαi/13 than in Gαi/12, as previously observed for their wild-type counterparts (15, 16).
Figure 2.
Gαi/13 or Gαi/12 chimera interact with RH domains in an activation-dependent manner. GST-p115-RH or GST-LARG-RH were incubated with purified His6-Gαi/13 (upper panels) or His6-Gαi/12 (lower panels), either in the absence or presence of AlF4−, and then GST-RH was pulled down using glutathione Sepharose 4B resin. Protein eluted from washed beads was resolved by SDS-PAGE and either immunoblotted (IB) with anti-Gα13 (B860) or anti-Gα12 (J168) antibody, or stained by Coomassie Brilliant Blue (CBB).
Figure 3.
Activity of purified Gαi/13 and Gαi/12 in reconstitution assays. (A) Single-turnover hydrolysis of GTP bound to Gαi/13 (upper panel) or Gαi/12 (lower panel) was measured at 15°C in the absence (□) or presence of 100 nM GST-p115-RH (●) or 100 nM GST-LARG-RH (▲). Data are from one experiment, which is representative of three experiments. (B) Stimulation of p115RhoGEF activity by Gαi/13 but not Gαi/12. GTPγS binding to His6-RhoA was measured after incubation for 5 min at 30°C in the presence of p115RhoGEF (5 nM) and indicated AlF4−-activated Gα subunit (100 nM). Data are the mean of duplicate determinations from one experiment, which is representative of three experiments. (C) Dose-dependent stimulation of p115RhoGEF activity by Gαi/13. GTPγS binding to His6-RhoA by p115RhoGEF (5 nM) was evaluated after 5 min at 30°C, and included the indicated concentration of either AlF4−-activated wild-type Gα13 (○) or Gαi/13 (●). Samples lacking RhoA but containing p115RhoGEF and either AlF4−-activated wild-type Gα13 (△) or Gαi/13 (▼) were assayed in parallel.
Regulation of RhoGEF activity by Gαi/13 or Gαi/12
Gα13, but not Gα12, directly stimulates p115RhoGEF-mediated nucleotide exchange on RhoA (13). To assess whether our chimera could similarly regulate RhoGEF activity, we measured GTPγS binding to RhoA in the presence of p115RhoGEF and AlF4−-activated Gαi/12 or Gαi/13. As shown in Figure 3B, Gαi/13, but not Gαi/12, could stimulate p115RhoGEF. The dose-response curve of Gαi/13 for p115RhoGEF activation is similar to that of wild-type Gα13 (Fig. 3C). Thus, Gαi/12 and Gαi/13 regulate effector molecules with specificity similar to their wild-type counterparts.
In summary, Gαi/12 and Gαi/13 chimeras retain the specific biochemical properties of their wild-type counterpart subunits. Although the N-terminal helices of Gα subunits are involved in binding Gβγ subunits, regulation of effector molecules, and specific interaction with receptors (33, 37, 38), substitution of the N-terminal helix of either Gα12 or Gα13 with that of Gαi1 did not affect its interaction with Gβγ or RhoGEFs insofar as our assays could detect. Thus, the native N-terminal helix of Gα12/13, while important for receptor selectivity (38), is not essential for the regulation of RhoGEFs.
Structure determination of Gαi/12 and Gαi/13
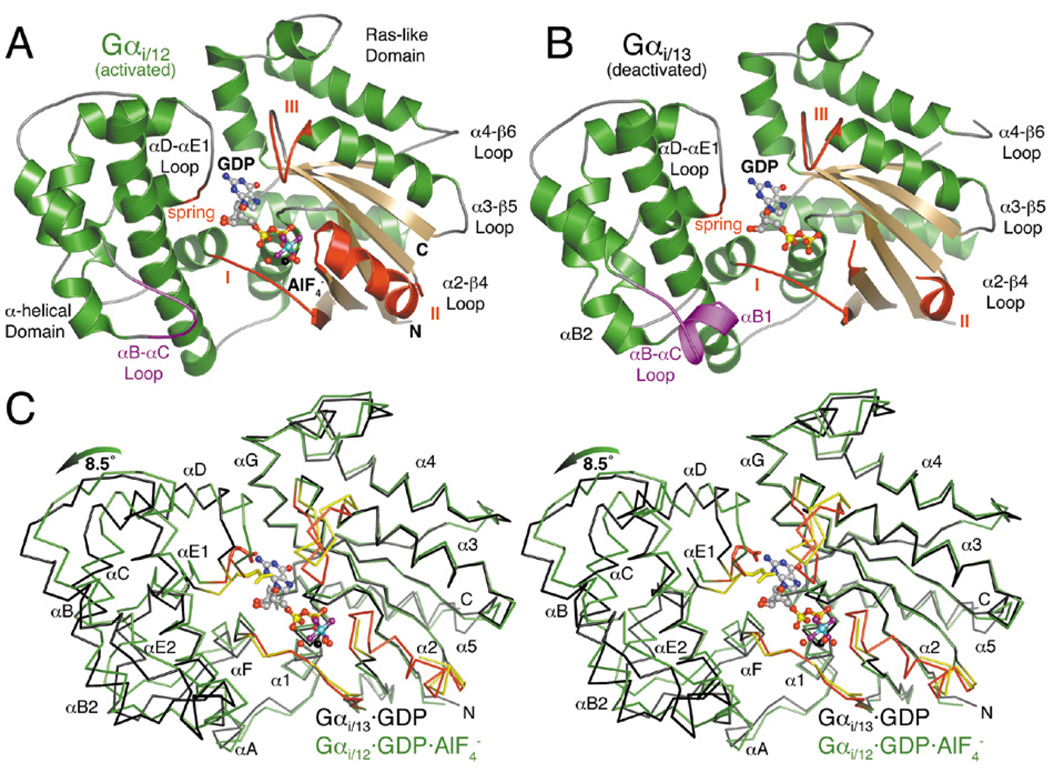
Both Gαi/12 and Gαi/13 were activated with AlF4− prior to crystallization, and their structures were determined by molecular replacement (Table I). As is typical for structures of monomeric Gα subunits, the N-terminal helices and extreme C-termini are disordered. The structure of Gαi/12·GDP·AlF4− spans residues 54 to 371, and therefore contains only wild-type residues (Fig. 4A). Gαi/13 was resolved in a GDP-bound state that most closely resembles the deactivated structures of Gαi and Gαt (Fig. 4B, Fig. 5B). In its structure, only one residue derived from Gαi at the chimeric N terminus is observed, and a portion of switch II (residues 226 to 232) and two residues in the α4-β6 loop (residues 339 to 340) are additionally disordered (Fig. 4B). It is somewhat surprising that the Gαi/13 protein crystallized in a deactivated state because both Gαi/12 and Gαi/13 were activated using the same protocol, formed stable complexes with RH domains (Fig. 2) and were resistant to trypsin digestion (Fig. S2). However, AlF4− binding is reversible, and different crystallization conditions likely lead to different proportions of proteins in activated and deactivated states. The relatively low pH used for the crystallization of Gαi/13 (pH 4.8), for example, could destabilize the activated, AlF4−-bound state and allow the deactivated protein to crystallize (39). Previously reported Gα·AlF4− complexes were crystallized at pH values 5.5 or higher.
Figure 4.
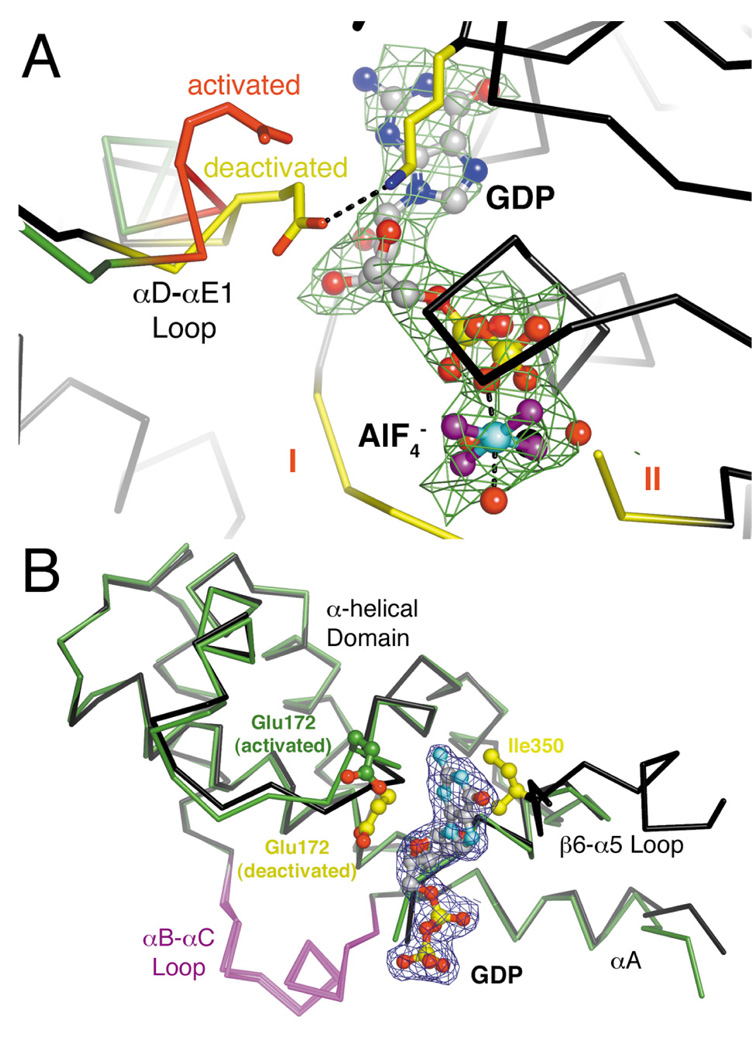
Structures of Gα12/13 subunits in activated and deactivated states. (A) The activated Gαi/12·GDP·AlF4− complex. The region of the αB-αC loop distinct from that of Gαi/13 is colored purple. The three conformationally flexible “switch regions” of the Gα subunit are red and labeled with Roman numerals (I–III). A fourth, apparently Gα12/13-specific element (the “spring”) is likewise colored red. The Mg2+·GDP·AlF4− ligand complex is shown as a ball-and-stick model, with carbons colored gray, oxygens red, nitrogens blue, phosphates yellow, fluorines purple, aluminum cyan and magnesium black. Waters are shown as red spheres. (B) The deactivated Gαi/13·GDP complex adopts an unusually open conformation in which the α-helical domain has rotated ~8.5° away from the Ras-like domain. Switch II is almost completely disordered, and switch III has rotated away (up in the figure) from the nucleotide binding site. (C) Stereo view of the Cα traces of Gαi/12·GDP·AlF4− (green with red switch regions) and Gαi/13·GDP (black with yellow switch regions), superimposed using their Ras-like domains. The glutamate side chain in the “spring” of each α-helical domain (E175 in Gα12, E172 in Gα13) is shown as a stick model.
Figure 5.
The active sites and α-helical domains of activated and deactivated Gα12/13 subunits. (A) The activation-dependent “spring” of the α-helical domain. In the deactivated (GDP-bound) state, Gαi/13-E172 (yellow) is extended to form a salt bridge with Lys292 and packs against the nucleotide. In the activated (AlF4−-bound) state, the spring (red) adopts a curled conformation and Gαi/12-Glu175 does not contact the bound nucleotide. Only the Ras-like domain of Gαi/13 is shown for clarity. Electron density from a 2.5 σ |Fo|-|Fc| omit map from the Gαi/12·GDP·AlF4− crystal structure is shown as green wire cage. (B) Superposition of the activated and deactivated conformations of the Gα13 α-helical domain. The Cα-trace of the α-helical domain (residues 74 to 201) and the β6-α5 loop (residues 345 to 357) of the Gαi/13·GDP structure are colored black, and the α-helical domain from the Gα13/i-5·p115RhoGEF complex is colored green. The αD-αE1 loop in the α-helical domain and the linker between the Ras-like and α-helical domains (N-terminal to αA) exhibit the most profound conformational changes upon nucleotide hydrolysis. Electron density from a 1.0 σ 2|Fo|-|Fc| map derived from the Gαi/13·GDP crystal structure is shown as green wire cage.
Comparison of the Gαi/12 and Gαi/13 structures
Like other Gα subunits, Gαi/12 and Gαi/13 are composed of a nucleotide-binding domain homologous to that of Ras, into which an α-helical domain is inserted (Fig. 4). The most dramatic difference between Gαi/12·GDP·AlF4− and Gαi/13·GDP is an ~8.5° rotation of the α-helical domain away from the Ras-like domain in Gαi/13·GDP (Fig. 4C). Among previous Gαi and Gαt crystal structures, the Ras-like and α-helical domains differ in relative orientation only by ~2.5° between their activated and deactivated states. Thus, the Gαi/13 structure is unusually “open” in conformation. This is most likely due to its activation state and not to differences in primary sequence between Gα12 and Gα13 (see below discussion).
In addition to the three switch regions, which are well known to exhibit activation-dependent conformational changes, the Ras-like domains of Gαi/12 and Gαi/13 exhibit differences in the α4-β6 loop, which is disordered and one residue longer in Gαi/13. In other Gα subunits, this loop, along with the C-terminus, is thought to contribute to receptor specificity (reviewed in (40)). More subtle differences are also evident in the backbone of the α3-β5 loop, a region known to interact with effector proteins in Gαs and Gαt (4, 41), although this could be affected by an effector-like crystal contact in the Gαi/12 crystal lattice (Fig. S3). Excluding these regions, the Ras-like domains of Gαi/12 and Gαi/13 are quite similar, as might be expected by their 70% sequence identity. They superimpose with a root mean squared deviation (RMSD) of 0.75 Å for 175 equivalent Cα atoms (Fig. 4C).
The α-helical domains of Gαi/12 and Gαi/13 exhibit more profound differences. In Gαi/13, the N-terminus of αA is kinked due to the presence of Pro86 (Asp93 in Gα12), and the αB-αC loop is four residues longer and harbors an additional helix (αB1; Fig. 4B). Omitting these regions, the domains superimpose with an RMSD of 0.7 Å for 101 equivalent Cα atoms. Intriguingly, the αD-αE1 loop of Gαi/13 (residues 171 to 173, immediately adjacent to the nucleotide binding site) adopts a strikingly different conformation from the analogous loops in the activated Gαi/12 and Gα13/i-5 structures, even though these loops have identical primary structure (Fig. 5A, 5B). Therefore, the αD-αE1 loop also appears to exhibit a novel activation-dependent conformational change.
Comparison of Gα12/13 subunits with other Gα subfamilies
In line with their sequence identities (43% and 39%, respectively), the Ras-like domains of Gα12/13 are more similar to Gαi than Gαs, with RMSDs of superposition of 1.1 Å and 2.2 Å for 184 and 170 equivalent Cα atoms, respectively. Regions exhibiting obvious structural differences among the Ras-like domains of Gα12/13, Gαi, and Gαs are the α4-β6 loop, which projects further away from the Ras-like domain in Gα12/13 subunits, and the β5-α4 loop (Fig. 4C). The cleft formed between the α2 (switch II) and α3 helices and their C-terminal loops is increasingly recognized as the effector binding site of Gα (17, 41, 42). In Gα12/13 subunits, the α3-β5 loop is most similar to that of Gαt. Differences between the α-helical domains of Gα12/13 and Gα subunits from other subfamilies have been described previously (17).
Activation-Dependent Conformational changes in Gα12/13 subunits
Comparison of the structures of Gαi/12·GDP·AlF4−, Gαi/13·GDP and Gα13/i-5·GDP·AlF4− reveals conformational changes that appear to be linked to GTP hydrolysis in Gα12/13 subunits (Fig. 4 and Movie S1). This important deactivation mechanism has previously only been described for the Gαi subfamily of G proteins. In the Gαi/12·GDP·AlF4− structure, the three switch regions and the α-helical domain adopt a conformation similar to those of the activated structures of Gαi, Gαt and Gαs. In Gαi/13·GDP, switch I retains this activated conformation except for residues 206 to 210 at the C terminus of the switch, which shift by as much as 2 Å towards the α-helical domain (Fig. 4C), a conformation previously observed in several GDP-bound structures of Gαi (34, 43). Less of switch II is disordered in the Gαi/13·GDP structure than in Gαi1·GDP, and switch III rotates 13° away from the active site to a position nearly identical to that observed in the Gαt·GDP complex (44).
As described above, the α-helical domain of Gαi/13 also appears to undergo activation-dependent conformational changes (Fig. 4C, 5B) by rotating away from the Ras-like domain and by alteration of the αD-αE1 loop (residues 171 to 173 in Gαi/13). In other subfamilies, Gα13-Glu172 is substituted by aspartate, which interacts with an invariant lysine in the Ras-like domain (Gα13-Lys292) and packs against the purine ring and ribose sugar of GDP. The unusually open conformation of Gαi/13·GDP allows the longer Gα13-Glu172 side chain to form an analogous salt bridge (Fig. 5A), although in electron density maps this side chain appears less ordered than its neighboring residues. Contrarily, in activated structures of Gα12 and Gα13/i-5, the αD-αE1 loop is puckered, apparently to avoid steric collisions, and Gα13-Glu172 makes relatively few contacts with the bound nucleotide (Fig. 5). Thus, the αD-αE1 loop thus acts like an activation-dependent “spring” that appears to push the α-helical domain away from the Ras-like domain in the Gαi/13·GDP structure. This transition may be facilitated by the loss of contacts between the α-helical domain and switch III upon GTP hydrolysis.
To better understand whether these conformational differences are coupled to GTP hydrolysis and not to differences in primary structure, we need atomic structures of the activated and deactivated forms of both Gαi/12 and Gαi/13. Currently, Gαi/12·GDP has not been crystallized, and we only have the Gα13/i-5 chimera to represent the activated structure of Gα13. Despite this, the 70% sequence identity of Gα12 and Gα13 and the 100% identity of their αD-αE1 loops suggest that they undergo similar conformational changes upon deactivation. Indeed, the activated structures of Gαi/12 and the Gα13/i-5 chimera are remarkably similar despite their even higher sequence disparity. Furthermore, in the Gαi/13·GDP structure, the αD-αE1 loop adopts the same conformation as those of activated and deactivated structures of Gαi, Gαt and Gαs, suggesting that this is the energetically preferred state, which can only be attained in Gα12/13 subunits after GTP hydrolysis and outward rotation of the helical domain. However, as in any crystal structure, we cannot totally exclude the possibility that the unusually open conformation of the Gαi/13·GDP structure was also influenced by lattice contacts or crystallization conditions.
If the open conformation of the Gαi/13·GDP structure and the structural transition of the αD-αE1 loop indeed represent activation-dependent conformational changes in Gα12/13 subunits, what might their physiological significance be? Because the Ras-like and α-helical domains of Gα13 are both known to interact with the N-terminal fragment p115RhoGEF (17), the open conformation of deactivated Gαi/13·GDP could help discourage interactions with p115RhoGEF or LARG upon GTP hydrolysis. Another consequence of the open conformation of Gαi/13·GDP is that the nucleotide appears less solvent accessible in deactivated Gαi/13 than in activated Gα12/13 subunits (~14.5 Å2 of accessible surface area in the Gαi/12·GDP·AlF4− and Gα13/i-5·GDP·AlF4− complexes, and 6.9 Å2 in the Gαi/13·GDP complex). The opposite is true in Gαi subunits (e.g. 18.8 Å2 in the structure of Gαi·GDP, and 3.5 Å2 in Gαi·GDP·AlF4−). These differences in the substrate binding site could influence rates of nucleotide exchange and/or hydrolysis, and thus the duration of signals in response to the activation of Gα12/13-coupled receptors.
Structural insights into the interactions of Gα12 and Gα13 with effectors
The best-characterized Gα12/13 effector is the N-terminal fragment of p115RhoGEF, which includes its RH domain (17). Because the Gα13/i-5-p115RhoGEF structure determination employed a Gα subunit with a chimeric effector-binding site, we modeled the p115RhoGEF RH domain complex with our essentially wild-type Gαi/13 and Gαi/12 structures. The resulting model reveals that the chimeric residues in the Gα13/i-5-p115RhoGEF interface either do not make significant contributions or are conservatively substituted in Gαi/13 and Gαi/12. In line with our observation that the RH domains of p115RhoGEF and LARG bind Gαi/12 and Gαi/13 equally well (Fig. 2), the structures of Gαi/12 and Gαi/13 have no significant amino acid differences among the residues that contribute to their respective RH domain binding sites. However, Gα12 and Gα13 subunits do exhibit differences in their GAP response to p115RhoGEF and LARG. The region immediately N-terminal to the RH domain of p115RhoGEF is responsible for its GAP activity and binds in the cleft formed between the Ras-like and α-helical domains (17, 19), where it contacts αA and the αB-αC loop. While the interacting residues within αA are conserved in Gα12 and Gα13, their αB-αC loops have dramatically different structure (Fig. 4). Diminished or detrimental interactions between the shorter αB-αC loop of Gαi/12 and p115RhoGEF could therefore account for the higher GAP response of Gαi/13 with respect to Gαi/12.
Another relatively-well characterized effector target of activated Gα12/13 subunits is cadherin (10). Recently, it was shown that mutation of residues 244 to 249 within β4 of Gα12 lead to uncoupling of Gα12 from p115RhoGEF and LARG, but not E-cadherin (45). Based on the crystal structure of Gαi/12, this substitution is expected to disrupt the effector-binding site of the G protein, in accordance with the loss of p115RhoGEF and LARG binding. Cadherin either binds elsewhere on Gαi/12, or is less reliant on a properly structured effector-binding pocket.
Conclusions
Elucidation of the molecular mechanisms by which Gα subunits regulate their own activity as well as the activity of their effector target is required not only to understand the regulation of many cellular processes, but also to develop drugs designed to modulate such processes. Gα12 and Gα13 have similar biochemical properties (20, 21), but distinct functional roles in cell signaling, such that Gα13 knockout mice die around embryonic day E10 due to a vascular system formation defect (46), whereas Gα12 knockout mice are viable and without any obvious phenotype (47). In this study, we demonstrate a simple and efficient method to produce fully functional, soluble forms of Gα12 and Gα13 that enabled us to determine their crystal structures. These structures reveal molecular differences not only between these two subunits, but also with those of other Gα subunits. By determining the activated structure of Gα12 and the deactivated structure of Gα13, we fortuitously observed novel conformational changes that are likely coupled to GTP hydrolysis. If true, then it can no longer be assumed that the transition upon deactivation will necessarily be the same in all Gα subfamilies. In the future, these chimeric subunits can be used in biochemical and crystallographic analyses to decipher their differential ability to regulate the growing number of effectors that interact with Gα12/13 subunits (12). Remarkably, the chimeric approach we used to generate soluble Gα12/13 subunits in this paper can be applied successfully to other, formerly intractable Gα subunits, even from other subfamilies, as evidenced by our successful structural studies of the analogous Gαi/q chimera in complex with GRK2 and Gβγ (48).
Supplementary Material
Figure S1. Alignment of the amino-terminal regions of Gαi1, Gα12, and Gα13.
Figure S2. Gαi/13 is activated by aluminum tetrafluoride.
Figure S3. Pseudo-effector crystal contact between the effector-binding region of Gαi/12 and a symmetry-related α-helical domain.
Movie S1. Conformational changes proposed to occur upon deactivation of Gα12/13 subunits.
This material is available free of charge via the Internet at http://pubs.acs.org
Acknowledgments
We would like to thank Yi Li and Beth Dulin for technical assistance. Diffraction data were collected at beam line 17-ID of the Industrial Macromolecular Crystallography Association Collaborative Access Team (IMCA-CAT) at the Advanced Photon Source (Argonne, IL). These facilities are supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Illinois Institute of Technology (IIT), executed through the IIT Center for Synchrotron Radiation Research and Instrumentation. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract No. W-31-109-Eng-38.
Abbreviations
- βME
β-mercaptoethanol
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- G protein
guanine nucleotide binding protein
- Gα
heterotrimeric G protein α subunit
- Gβγ
heterotrimeric G protein β and γ subunits
- GPCR
G protein-coupled receptor
- GDP
guanosine-5’-diphosphate
- GST
glutathione-S-transferase
- GTP
guanosine-5’-triphosphate
- HEPES
4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid
- LARG
leukemia-associated RhoGEF
- p115RhoGEF
p115 Rho guanine nucleotide exchange factor
- RGS
regulator of G protein signaling
- RH
RGS homology
- SDS
sodium dodecyl sulfate
Footnotes
Support was provided by National Institute of Health Grant HL071818, American Heart Association Scientist Development Grant 0235273N and an American Cancer Society Research Scholar grant (to JJGT), National Institutes of Health Grants GM61454 and NS41441 and an American Heart Established Investigator Award 0040006N (to TK) and a Predoctoral fellowship from the American Heart Association Midwest Affiliate 0315253Z (to BK).
We refer to the core domain of RGS4 as an RGS homology (RH) domain in order to distinguish between the fold of this domain and its function. For example, while RGS proteins serve as Gα GAPs, domains in G protein-coupled receptor kinases and axin that are homologous to those found in RGS proteins (RH domains) have distinct functions.
References
- 1.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 2.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 3.Berman DM, Kozasa T, Gilman AG. The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J Biol Chem. 1996;271:27209–27212. doi: 10.1074/jbc.271.44.27209. [DOI] [PubMed] [Google Scholar]
- 4.Tesmer JJG, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4−-activated Giα1: stabilization of the transition state for GTP hydrolysis. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 5.Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992;17:383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- 6.Dhanasekaran N, Dermott JM. Signaling by the G12 class of G proteins. Cell Signal. 1996;8:235–245. doi: 10.1016/0898-6568(96)00048-4. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead IP, Zohn IE, Der CJ. Rho GTPase-dependent transformation by G protein-coupled receptors. Oncogene. 2001;20:1547–1555. doi: 10.1038/sj.onc.1204188. [DOI] [PubMed] [Google Scholar]
- 8.Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol. 2000;40:459–489. doi: 10.1146/annurev.pharmtox.40.1.459. [DOI] [PubMed] [Google Scholar]
- 9.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 10.Meigs TE, Fields TA, McKee DD, Casey PJ. Interaction of Gα12 and Gα13 with the cytoplasmic domain of cadherin provides a mechanism for β-catenin release. Proc Natl Acad Sci U S A. 2001;98:519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi Y, Katoh H, Mori K, Negishi M. Gα12 and Gα13 interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr Biol. 2002;12:1353–1358. doi: 10.1016/s0960-9822(02)01034-5. [DOI] [PubMed] [Google Scholar]
- 12.Kurose H. Gα12 and Gα13 as key regulatory mediator in signal transduction. Life Sci. 2003;74:155–161. doi: 10.1016/j.lfs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274:5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki N, Nakamura S, Mano H, Kozasa T. Gα12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci U S A. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Singer WD, Sternweis PC, Sprang SR. Structure of the p115RhoGEF rgRGS domain-Gα13/i1 chimera complex suggests convergent evolution of a GTPase activator. Nat Struct Mol Biol. 2005;12:191–197. doi: 10.1038/nsmb888. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Singer WD, Wells CD, Sprang SR, Sternweis PC. Mapping the Gα13 binding interface of the rgRGS domain of p115RhoGEF. J Biol Chem. 2003;278:9912–9919. doi: 10.1074/jbc.M212695200. [DOI] [PubMed] [Google Scholar]
- 19.Wells CD, Liu MY, Jackson M, Gutowski S, Sternweis PM, Rothstein JD, Kozasa T, Sternweis PC. Mechanisms for reversible regulation between G13 and Rho exchange factors. J Biol Chem. 2002;277:1174–1181. doi: 10.1074/jbc.M105274200. [DOI] [PubMed] [Google Scholar]
- 20.Kozasa T, Gilman A. Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of α12 and inhibition of adenylyl cyclase by αz. J Biol Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 21.Singer WD, Miller RT, Sternweis PC. Purification and characterization of the α subunit of G13. J Biol Chem. 1994;269:19796–19802. [PubMed] [Google Scholar]
- 22.Yasukawa T, Kanei-Ishii C, Maekawa T, Fujimoto J, Yamamoto T, Ishii S. Increase of solubility of foreign proteins in Escherichia coli by coproduction of the bacterial thioredoxin. J Biol Chem. 1995;270:25328–25331. doi: 10.1074/jbc.270.43.25328. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura S, Kreutz B, Tanabe S, Suzuki N, Kozasa T. Critical role of lysine 204 in switch I region of Gα13 for regulation of p115RhoGEF and leukemia-associated RhoGEF. Mol Pharmacol. 2004;66:1029–1034. doi: 10.1124/mol.104.002287. [DOI] [PubMed] [Google Scholar]
- 24.Tanabe S, Kreutz B, Suzuki N, Kozasa T. Regulation of RGS-RhoGEFs by Gα12 and Gα13 proteins. Methods Enzymol. 2004;390:285–294. doi: 10.1016/S0076-6879(04)90018-3. [DOI] [PubMed] [Google Scholar]
- 25.Otwinoski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 26.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr D Biol Crystallogr. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 27.Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 28.Winn MD. An overview of the CCP4 project in protein crystallography: an example of a collaborative project. J Synchrotron Radiat. 2003;10:23–25. doi: 10.1107/s0909049502017235. [DOI] [PubMed] [Google Scholar]
- 29.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for the building of protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 30.DeLano W. The PyMOL Molecular Graphics System. San Carlos, CA, USA: DeLano Scientific; 2002. [Google Scholar]
- 31.Kozasa T. Purification of G protein subunits from Sf9 insect cells using hexahistidine-tagged α and βγ subunits. Methods Mol Biol. 2004;237:21–38. doi: 10.1385/1-59259-430-1:21. [DOI] [PubMed] [Google Scholar]
- 32.Linder M, Ewald D, Miller R, Gilman A. Purification and characterization of Goα and three types of Giα after expression in Escherichia coli. J Biol Chem. 1990;265:8243–8251. [PubMed] [Google Scholar]
- 33.Neer EJ, Pulsifer L, Wolf LG. The amino terminus of G protein α subunits is required for interaction with βγ. J Biol Chem. 1988;263:8996-70. [PubMed] [Google Scholar]
- 34.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 35.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 36.Brandt DR, Ross EM. GTPase activity of the stimulatory GTP-binding regulatory protein of adenylate cyclase, Gs. Accumulation and turnover of enzyme-nucleotide intermediates. J Biol Chem. 1985;260:266–272. [PubMed] [Google Scholar]
- 37.Hepler JR, Biddlecome GH, Kleuss C, Camp LA, Hofmann SL, Ross EM, Gilman AG. Functional importance of the amino terminus of Gqα. J Biol Chem. 1996;271:496–504. doi: 10.1074/jbc.271.1.496. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi Y, Katoh H, Negishi M. N-terminal short sequences of α subunits of the G12 family determine selective coupling to receptors. J Biol Chem. 2003;278:14936–14939. doi: 10.1074/jbc.M301409200. [DOI] [PubMed] [Google Scholar]
- 39.Schlichting I, Reinstein J. pH influences fluoride coordination number of the AlFx phosphoryl transfer transition state analog. Nat Struct Biol. 1999;6:721–723. doi: 10.1038/11485. [DOI] [PubMed] [Google Scholar]
- 40.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 41.Slep KC, Kercher MA, He W, Cowan CW, Wensel TG, Sigler PB. Structural determinants for regulation of phosphodiesterase by a G protein at 2.0 Å. Nature. 2001;409:1071–1077. doi: 10.1038/35059138. [DOI] [PubMed] [Google Scholar]
- 42.Tesmer J, Sunahara R, Gilman A, Sprang S. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα·GTPγS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 43.Kimple RJ, Kimple ME, Betts L, Sondek J, Siderovski DP. Structural determinants for GoLoco-induced inhibition of nucleotide release by Gα subunits. Nature. 2002;416:878–881. doi: 10.1038/416878a. [DOI] [PubMed] [Google Scholar]
- 44.Lambright DG, Noel JP, Hamm HE, Sigler PB. Structural determinants for activation of the α-subunit of a heterotrimeric G protein. Nature. 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 45.Meigs TE, Juneja J, Demarco CT, Stemmle LN, Kaplan DD, Casey PJ. Selective uncoupling of Gα12 from Rho-mediated signaling. J Biol Chem. 2005;280:18049–18055. doi: 10.1074/jbc.M500445200. [DOI] [PubMed] [Google Scholar]
- 46.Offermanns S, Mancino V, Revel JP, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science. 1997;275:533–536. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- 47.Gu JL, Muller S, Mancino V, Offermanns S, Simon MI. Interaction of Gα12 with Gα13 and Gαq signaling pathways. Proc Natl Acad Sci U S A. 2002;99:9352–9357. doi: 10.1073/pnas.102291599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesmer V, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJG. Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gβγ complex. Science. 2005 doi: 10.1126/science.1118890. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Alignment of the amino-terminal regions of Gαi1, Gα12, and Gα13.
Figure S2. Gαi/13 is activated by aluminum tetrafluoride.
Figure S3. Pseudo-effector crystal contact between the effector-binding region of Gαi/12 and a symmetry-related α-helical domain.
Movie S1. Conformational changes proposed to occur upon deactivation of Gα12/13 subunits.
This material is available free of charge via the Internet at http://pubs.acs.org