Abstract
Purpose
To evaluate the impact of rotational setup errors on dose distribution in spinal stereotactic body radiotherapy (SBRT).
Methods and Materials
39 Cone Beam CT (CBCT) scans from 16 SBRT treatment courses were analyzed. Alignment (including rotation) to the treatment planning CT was performed, followed by translational alignment that reproduced the actual positioning. The planned fluence was then applied to determine the delivered dose to the targets and organs at risk.
Results
The mean PTV volume was 71.01 mL (SD ± 60.05, range 22.62 – 250.65 mL). Prescribed dose (to the 62 – 82% isodose) was 14 – 30 Gy in one to six fractions. The average rotational displacements were 0.38 ± 1.21, 1.12 ± 1.82 and −0.51 ± 2.0 degrees with maximal rotations of −4.29, 5.76 and −6.64 degrees along the x (pitch), y (yaw), and z (roll) axes, respectively.
PTV coverage changed by an average of −0.07 Gy (SD ± 0.20 Gy) between the rotated and the original plan, representing 0.92% of prescription dose (SD ± 2.65%).
For the spinal cord, planned with 2 mm expansion to create a planning organ at risk volume (PRV), the difference in minimum dose to the upper 10% of the PRV volume was 0.03 ± 0.3 Gy (maximum 0.9 Gy). Other organs at risk saw insignificant changes in dose.
Conclusions
PRV expansion generally assures safe treatment delivery in the face of typically encountered rotations. Given the variability of delivered dose within this expansion for certain cases, caution should be taken to properly interpret doses to the cord when considering clinical dose limits.
Keywords: SBRT, CBCT, IGRT, Rotation
Introduction
Stereotactic body radiation therapy (SBRT) is emerging as an effective and safe treatment option for patients with both metastatic and primary malignancies involving the spine. In SBRT, a high dose of radiation is delivered to the target, utilizing either a single fraction or a small number of fractions, with a high degree of precision. Clinical data suggest that for a selected group of patients spinal SBRT may result in more rapid and long-lasting pain alleviation as well as better local control (1 – 6), compared to conventional radiotherapy (7 – 11). This has not been investigated in prospectively randomized studies though. Additional potential advantages include reduced patient discomfort and the ability to treat lesions in previously irradiated sites.
Given the ablative doses, the tight margins that are used in SBRT, and the close proximity of the target to the spinal cord, accurate targeting is essential to ensure adequate tumor coverage and sparing of adjacent normal tissues. Any rotational deviation of the patient, introduced by change in gross position and/or articulation of the spine, may potentially induce significant position and dose uncertainty, especially at the target edges. With the introduction of 3-dimensional (3D) volumetric imaging for treatment guidance [e.g. cone beam CT (CBCT)], rotational setup errors can be more easily detected and more accurately measured. To date, however, few stereotactic treatment systems are equipped to conveniently measure and correct for all axes of rotation. Tomotherapy units can account for body roll via simple angular offset of the beamlet pattern. Conventional linear accelerators can theoretically account for rotation about the anterior-posterior axis by changing the offset pedestal angle. Combining all three rotations (pitch, yaw, and roll) in a single correction involves a couch that can rotate, as well as tilt and roll. Both research and commercial examples of such modifications have been described and used, although the potential to correct the entire range of rotational variations seen, as well as the dosimetric impact of not doing so, have not been fully explored.
In our clinic, spinal SBRT positioning is based on alignment of a treatment planning CT scan with a cone-beam CT acquired at the start of the treatment fraction. Although rotational variations may be present for a given treatment positioning, translation-only setup adjustments are made. In order to minimize the impact of potential rotational variations on the delivered dose, the translational alignment is biased to most accurately align the interface of tumor and spinal cord.
Recently, Murphy provided valuable general guidance on rotational offsets management (12). He has shown that if translation-only corrections are to be made then the rigid registration should be based on selected landmarks that are close to the treatment site and should include only the translational degrees of freedom. Registration using all six degrees of freedom but applying only the translational shifts will almost always result in a worse setup compared to translation-only registration and position correction. This is due to the separation of the nominal pivot point from the focus of anatomy that is most critical to accurately aligning the patient. Our clinical positioning philosophy follows a manual alignment paradigm guided by the same philosophical principal. By applying a translated position of the patient which places the pivot point at the most proximal interface between the target and spinal cord, we expect to minimize variation in cord position and as a result limit the impact of rotations on dose deviations to this proximal structure.
Using patients’ CBCT data, a retrospective study was performed to evaluate the effect of the uncorrected rotational setup errors, using our clinical paradigm for positioning, on delivered spinal SBRT dose distributions.
Methods and Materials
Patient data
Under IRB approval, 39 CBCT scans from 16 SBRT treatment courses of 14 consecutive patients were analyzed. All patients had a histologic diagnosis of malignant neoplasm and had spinal or paraspinal involvement. 11 patients received SBRT as the initial treatment for their spinal disease, while 5 others had received radiation therapy to the same region prior to SBRT. The treatment sites were thoracic in 8 patients, lumbar in 7, and sacral in 1 patient. The mean PTV volume was 71.01 mL (range 22.62 – 250.65 mL) and the mean PTV cranio-caudal extent was 5.29 cm (3.0 – 8.5 cm).
Doses of 14–30 Gy were prescribed to the 62–82% isodose line (IDL), and delivered in one to six fractions. Patient characteristics and treatment parameters are summarized in Table 1.
Table 1.
Target and treatment characteristics
| Total 16 lesions in 14 patients | |
|---|---|
| Site of Spinal Involvement | |
| Thoracic spine | 8 |
| Lumbar spine | 7 |
| Sacrum | 1 |
| Vertebral body tumors | 10 |
| Paraspinal tumors | 6 |
| Previous RT to same site | |
| Yes | 5 |
| No | 11 |
| PTV volume (mL) | |
| Average (±SD) | 71.01 ± 60.05 |
| Range | 22.62 – 250.65 |
| PTV length (cm) | |
| Average (±SD) | 5.29 ± 1.95 |
| Range | 3.0 – 8.5 |
| PRV volume (mL) | |
| Average (±SD) | 11.76 ± 6.64 |
| Range | 4.18 – 26.77 |
| SBRT dose [total dose (Gy)/no. of fractions)] | |
| 14/1 | 5 |
| 15/1 | 1 |
| 16/1 | 1 |
| 15/3 | 3 |
| 18/3 | 3 |
| 24/3 | 1 |
| 25/5 | 1 |
| 30/6 | 1 |
SBRT procedure
Immobilization during simulation and subsequent treatment was achieved by using a customized body vacuum bag and evacuating the space between the patient and a covering mylar sheet (Bodyfix, Medical Intelligence, Schwabmunchen, Germany). CT scans defined the reference patient model for planning, with 3 mm axial slice thickness and spacing. For spinal cord visualization, intravenous contrast was given in the majority of cases. Image fusion with MR images was performed whenever feasible. The target volume and spinal cord, as well as other organs at risk (OAR), were delineated. The PTV consisted of the GTV (tumor in the vertebral body, para-spinal soft tissue, or combination of the two) expanded by 2–5 mm, plus the adjacent vertebral body for true bone metastases. The spinal cord was expanded by 2 mm in the axial plane to create a planning organ at risk volume (PRV). In certain circumstances, including especially cases involving lesions below the spinal cord, the entire spinal canal was delineated as PRV. When we started our SBRT program, the PRV volume was expanded longitudinally to the length of one vertebral body above and below the PTV. Later on, our definition changed to only 9 mm above and below the PTV. Close proximity or even overlap of the PTV and PRV was allowed. Ten cases had PTV-PRV separations 0.1 cm or less extending along 0.3 – 3.6 cm of the PRV length. Five of these cases contained PTV-PRV overlap.
For single fraction treatments, the dose to the PRV was constrained such that no more than 10% of the PRV would receive 10 Gy or more (13). For treatments prescribed over three fractions, this dose constraint was 6 Gy per fraction. For five fraction treatments, the PRV limit was 5 Gy per fraction. SBRT was planned and delivered using multiple (8–17) static beams. An effort was made to use co-planar beams only whenever possible. Fields were primarily shaped to block out the PRV and other organs at risk. Any fields with a maximum aperture of less than 1 cm width were excluded during the planning process. Dose was prescribed to the lowest isodose line covering 95% of the PTV, typically the 60 – 80% IDL. The prescription dose was individualized according to normal tissue dose constraints and history of prior irradiation to the same site, and ranged between 14 – 30 Gy in one to six fractions. Dose was calculated with tissue heterogeneity correction.
For each treatment fraction, patients were immobilized and set up using laser alignment of skin marks. A CBCT was then acquired and aligned with the treatment planning CT using a manual translation only alignment tool (OBI/CBCT workstation, Varian Medical Systems, Palo Alto, CA). As noted above, the translation was optimized so that the primary interface of the spinal canal and GTV matched as closely as possible between the treatment planning and cone beam CT images. The alignment was approved by a physician before table adjustments were applied and treatment proceeded.
Dosimetry study
Complete (6 degrees of freedom) regional rigid body alignment of the CBCT to the treatment planning CT was performed using an in-house automated alignment tool that used mutual information as a metric for goodness-of-fit. The region of interest for the automated alignment was the PTV and its vicinity, accomplished by cropping anatomy that extended more than 1 cm from the involved tumor and vertebral bodies in all directions. Subsequently, a manual alignment was performed using the treatment planning system (UMPLAN, University of Michigan, Ann Arbor, MI) that reproduced the actual clinical translation-only setup adjustment. The treatment planning CT in the orientation of the CBCT was then used for dose calculation with a resolution of 2 mm. The planned fluence pattern was applied to the rotated and translation-corrected CT to determine the dose actually delivered to the targets and organs at risk. Differences between planned and delivered doses were evaluated using criteria related to initial plan acceptance, including target coverage, normal tissue doses, and dose-volume constraints.
Results
The average and standard deviation of the rotational setup errors were 0.38 ± 1.21, 1.12 ± 1.82 and −0.51 ± 2.0 degrees along the x (pitch), y (yaw), and z (roll) directions, respectively. The maximal rotations observed were −4.29, 5.76 and −6.64 degrees, around the x, y and z axes.
The change in target coverage was minimal. The average difference between the rotated and the original plan in the dose covering 95% of the PTV was −0.07 Gy (SD ± 0.20 Gy) per fraction, representing 0.92% (SD ± 2.65%) of the prescribed dose. The maximal difference in 95% PTV coverage was −0.7 Gy. The mean dose per fraction to the target changed only slightly, by an average of −0.02 Gy (SD ± 0.07 Gy).
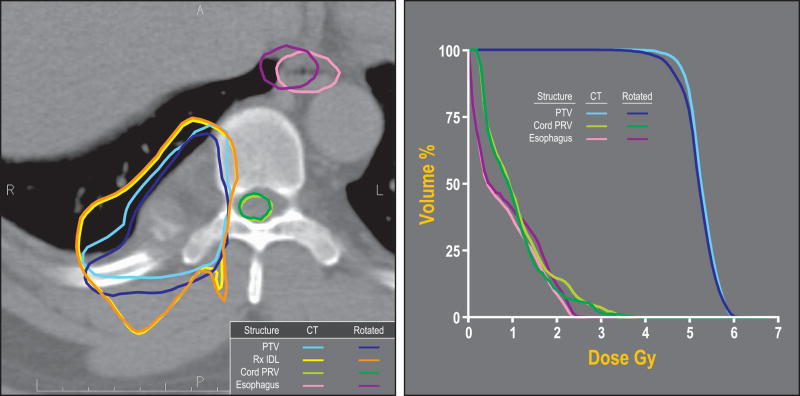
For most cases, the estimated delivered dose to the PRV changed only minimally compared to the planned dose, and was well within the PRV dose limit. The average difference in D10, the minimum dose to the upper 10 percent of the PRV volume, between the rotated and the original plan was 0.03 Gy (SD ± 0.3 Gy). For single fraction cases, the estimated delivered dose to 0.01 ml of the PRV was 13 ± 2.58 Gy compared to 13.02 ± 2.76 Gy in the initial plan. For three fraction cases, the delivered dose to 0.01 ml of the PRV was 4.4 ± 1.02 Gy compared to 4.38 ± 1.11 Gy in the original plan. An example of the resulting position of target and normal structures relative to the prescription dose surface as well as the planned and delivered DVH’s are shown in Figure 1.
Figure 1.
(a) Planned and actual position of target and normal structures relative to the prescription dose surface projected on the treatment planning CT. This case was treated with six fractions of 5 Gy prescribed to the 78% isodose line. The fraction shown here had one of the largest rotations (−0.38, 3.66 and −6.64 degrees around the x, y, and z axes respectively). Shown are the planned PTV (light blue), planned PRV (light green) and esophagus (pink). Rotation-corrected structures are: PTV (in dark blue), PRV (dark green) and esophagus (purple). The planned and rotated 5 Gy isodose lines are shown in yellow and orange, respectively.
(b) Dose-volume histogram of the original plan and the actual delivered treatment of the same fraction.
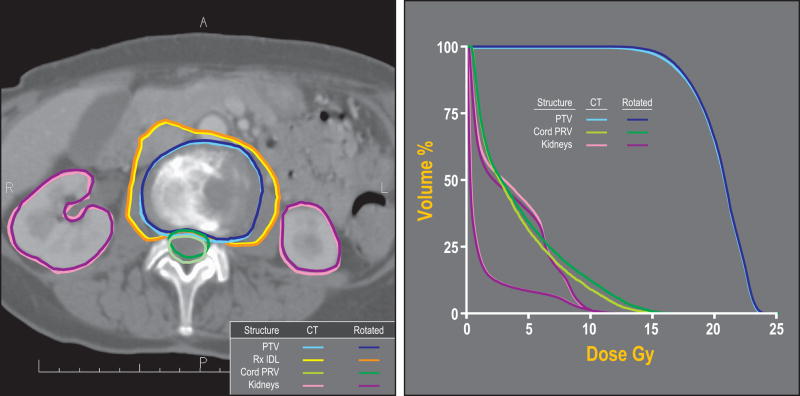
In five patients, the absolute difference in D10 to the PRV was ≥0.5 Gy, with a maximum difference of 0.9 Gy in one case. For three of these cases, the estimated delivered D10 to the PRV was greater than the calculated D10 in the treatment plan, while in two others it was smaller than the planned D10. The magnitude of rotation in three of these cases was high: −4.29 degrees around the x axis in one case and greater than 5 degrees around the y axis in two others. The PTV and PRV volumes and lengths were considerably higher than the average in only one of these cases. In four of the five patients with the highest deviation in delivered versus planned PRV dose, the shortest distance between PTV and PRV was 0.1 cm or less or the two volumes even overlapped. For these cases, the length of cord in which this overlap or near approach existed extended from 1.5 – 3.6 cm. The remaining cases with differences <0.5 Gy to D10 of the PRV consisted of six with PTV-PRV proximity 0.1 cm or less, which extended along 0.3 – 3.3 cm of the PRV. Two of the latter cases involved overlap between the PTV and PRV. Figure 2 shows an example case with such proximity, and relatively larger dose increase under a typically observed rotation magnitude. The characteristics of the five cases are summarized in Table 3. It should be noted however, that while the maximal difference in D10 to the PRV was 0.9 Gy, D10 to the spinal cord differed by a maximum of 0.5 Gy.
Figure 2.
(a) Planned and actual position of target and normal structures relative to the prescription dose surface in a single fraction treatment (14 Gy prescribed to the 63% isodose line). This case, which had minor rotational setup errors (0.92, 1.57, and −0.77 degrees) but close proximity of the PTV and PRV, showed the largest difference between the planned and estimated delivered D10 to the PRV (0.9 Gy).
Shown are the planned PTV (light blue), planned PRV (light green), and the kidneys (pink). Rotation-corrected PTV, PRV and kidneys are shown in dark blue, dark green and purple, respectively. The planned and rotated 14 Gy isodose lines are shown in yellow and orange, respectively (b) Dose-volume histogram of the original plan and the actual delivered treatment.
Table 3.
Characteristics of cases with largest observed changes in delivered versus planned PRV doses
| Δ D10 to the PRV (Gy) | PTV length (cm) | PTV volume (mL) | X rotation (degrees) | Y rotation (degrees) | Z rotation (degrees) | |
|---|---|---|---|---|---|---|
| 1 | 0.55 | 3.6 | 37.1 | −4.29 | 1.9 | 1.92 |
| 2 | 0.9 | 4.2 | 78.3 | 0.92 | 1.57 | −0.77 |
| 3 | −0.55 | 7.2 | 119.2 | −1.0 | 5.76 | −3.15 |
| 4 | −0.55 | 3 | 40.6 | 1.04 | 1 | 0.37 |
| 5 | 0.5 | 5.7 | 46.45 | 0.95 | 5.4 | 0.2 |
For the seven patients who received single fraction SBRT, D10 for the PRV was 9.95 ± 1.83 Gy, compared to 9.78 ± 1.63 Gy calculated in the initial plan. To increase the validity of a percent volume criterion, the cord and PRV lengths were retrospectively reduced in cases that were planned with the longer PRV definition, and the 9 mm longitudinal extensions past the PTV were applied. This reduction in critical structure length caused a violation of the D10 cord dose volume constraint in the original treatment plans in four cases. Three of these cases had delivered to planned D10 differences of 0.5 Gy or higher. All four lesions were at the level of the cauda equina where the whole spinal canal was delineated as the PRV. None of these patients developed neurologic toxicity, with a mean follow-up of 10 months (range 6 – 20 months).
Other organs at risk, such as the esophagus, trachea and kidneys saw insignificant changes in dose. The difference between the estimated actual maximal dose and the planned maximal dose to the esophagus (defined as the maximum dose to 0.01 mL) ranged between −0.9 − 0.3 Gy with an average of −0.04 Gy. Similarly, that average difference for the trachea was −0.28 Gy with a range of −1.5 - 0.01 Gy. The mean difference in kidney volume receiving ≥8Gy for single fraction treatment, ≥5Gy per fraction for three fraction treatment and ≥4Gy per fraction for 5–6 fraction treatment was 0.21% (range 0 – 1.0%), 0.24% (−0.24 – 2.3%) and 0%, respectively.
Discussion
Accurate targeting is crucial for spinal SBRT, because of the narrow margins, the steep dose gradient around the target, and close proximity of target tissue to the spinal cord. In this study, we evaluated the rotational deviations observed during treatment positioning and their impact on dose distribution in spinal SBRT. Analyzing data from 39 SBRT fractions, we found rotational deviations of 0.38 ± 1.21, 1.12 ± 1.82 and −0.51 ± 2.0 degrees along the x (pitch), y (yaw), and z (roll) directions, respectively. These data are within the range of rotational setup deviations reported in the literature (14).
The clinical impact of these rotational setup deviations on the target dose was minimal. The estimated delivered dose to 95% of the PTV changed by only −0.07 Gy (SD ± 0.20 Gy) compared to the original treatment plan. The difference between the delivered and planned mean PTV dose was −0.02 Gy (SD ± 0.07 Gy).
This minimal effect of rotational setup errors on the target dose is in good agreement with other publications on this subject. Chuang et al. studied the effects of residual target motion during spinal SBRT delivery in six patients with spinal metastases treated with CyberKnife (15). They found that most residual rotational errors fell within ± 1°, with sporadic errors of 2°. For all cases, the prescription dose to the target (the dose prescribed to 95% of the target volume) varied by a maximum of 2.5%.
Astreinidou et al. simulated normally distributed patient displacements and recalculated the dose for each setup deviation on 8 head and neck IMRT plans (16). Their data indicate that random translational errors of σ=2 mm and rotational deviations of σ=1° in all three directions did not affect the CTV primary volume receiving 95% of the prescribed dose regardless of the PTV margin used. The rotational setup deviations alone, however, had no effect on the coverage of the CTV primary and had only a negligible effect on the CTV elective coverage. It should be noted that the origin of rotations in this study was the target center. In clinical practice, they noted that the rotation is more likely to be around the axis through the spine (i.e. distal to the target center), and thus might affect the dose distribution in a different way from what was observed in their study.
The effect of rotational setup errors on the dose to organs at risk other than the cord was negligible. The rotational influence on the dose to the cord was more complex. In our study, the estimated delivered D10 to the PRV changed only minimally compared to the dose calculated in the initial plan. Nevertheless, in five patients a difference of 0.5 Gy or greater was found. The maximal dose uncertainty to the PRV due to rotation was 0.9 Gy, which may be clinically significant under certain circumstances. However, the maximal effect of rotation on the dose to the spinal cord itself was only 0.5 Gy.
Similar findings are reported in the literature. Guckenberger et al. simulated translational and rotational patient positioning errors in IMRT plans of nine patients with spinal malignancies undergoing conventionally fractionated or hypofractionated RT (17). Their endpoint was the minimum dose delivered to five volume percent (D5) of the spinal canal which was used as the PRV. Applying rotations of 0.5 – 7.5 degrees increased the dose to the PRV by 3 ± 2% on average. Simulation of both translational and rotational setup errors increased D5 of the PRV by 24 ± 14%, which was not significantly different compared to simulation of translational errors alone. However, in one case that had rotations of 2.8 and 3 degrees around the x and y axes, respectively, the D5 of the PRV was 14% higher than calculated in the initial treatment plan.
Onimaru et al. investigated the translational and rotational setup errors in five patients with spinal schwannoma using a fluoroscopic real-time tracking radiation therapy system employing three fiducial markers (18). The rotations after manual setup were 2.3 ± 5.9, 0.1 ± 4.6, and −1.6 ± 3.1 degrees around the x, y, and z axes, respectively. The difference between the planned dose and estimated actual dose to the spinal cord was generally minimal. Only in one patient was the actual dose to the cord considerably higher than the planned dose.
In the above described study by Chuang et al. (15), for most cases, the spinal cord dose varied by 4% or less due to residual target motion during SBRT delivery. In four out of the six cases analyzed, the effect of the residual target motion (translation and rotation) did not exceed spinal cord tolerance. In one case a significant dose uncertainty to the cord was found (>1.5 Gy).
Kim et al simulated the residual head roll after translational correction for 6 head and neck IMRT cases (19). Comparison between the recalculated plans and the original ones demonstrated an average increase of maximal dose to the spinal cord of 3.1% and 6.4% for a 3° and 5° head roll, respectively.
For most spinal SBRT cases, it appears that rotational setup errors have insignificant effect on the delivered dose to either the target or the cord if the concept of a PRV is introduced. With appropriate immobilization, translation-only corrections appear to be sufficiently safe to not warrant the extra complexity of rotational adjustments. Yet, in selected cases the dose uncertainty to the cord introduced by rotational errors may be clinically significant. This should be kept in mind when establishing dose constraints for the cord or PRV. Caution is warranted, especially if the dose over the cord is escalated. Our data, although small, suggest that close proximity (e.g. 1 mm) of the PTV to the PRV and higher magnitude of rotation [e.g. greater than 2 degrees as reported recently by Wang et al (20)] may serve as predictors for patients who may benefit from correction of both translational and rotational setup errors.
Methods of correcting rotations should certainly not be ignored. Commercial (21) and in-house (22) systems represent a technology that may have many practical uses. The issue explored here is to what extent practice standards should require such rotational corrections, with their related complexity in accurate measurement and safe implementation. Other investigations have included more analytic approaches to changing linear accelerator configuration to implement rotation adjustments (23) and optimizing the related translation to minimize the impact of rotation as done in this study. Increased awareness of the impact of residual uncertainties as part of an overall position management plan will help with continuing propagation of safe, highly precise treatments to targets near critical structures such as the spinal cord.
Table 2.
Results
| Mean | SD | Range | |
|---|---|---|---|
| Rotational setup errors | |||
| x rotation (degrees) | 0.38 | ±1.21 | −4.29 – 2.18 |
| y rotation (degrees) | 1.12 | ±1.82 | −1.54 – 5.76 |
| z rotation (degrees) | −0.51 | ±2.00 | −6.64 – 3.39 |
|
| |||
| Δ D covering 95% PTV (Gy) | −0.07 | ±0.20 | −0.70 – 0.30 |
|
| |||
| Δ Mean PTV dose (Gy) | −0.02 | ±0.07 | −0.23 – 0.10 |
|
| |||
| Δ D10 to the PRV (Gy) | 0.03 | ±0.30 | −0.55 – 0.90 |
|
| |||
| Δ D10 to the cord (Gy) | 0.15 | ±0.19 | −0.15 – 0.50 |
|
| |||
| Δ Dmax to esophagus (Gy) | −0.04 | ±0.30 | −0.90 – 0.30 |
|
| |||
| Δ Dmax to trachea (Gy) | −0.28 | ±0.54 | −1.5 – 0.01 |
|
| |||
| Δ Vol of each kidney(%) receiving | |||
| ≥ 8 Gy | 0.21 | 0.39 | 0 – 1 |
| ≥ 5 Gy | 0.24 | 0.57 | −0.24 – 2.3 |
| ≥ 4 Gy | 0 | 0 | 0 |
Acknowledgments
Supported in part by NIH P01CA59827
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7(2):151–60. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 2.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193–9. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 3.Gerszten PC, Burton SA, Quinn AE, Agarwala SS, Kirkwood JM. Radiosurgery for the treatment of spinal melanoma metastases. Stereotact Funct Neurosurg. 2005;83(5–6):213–21. doi: 10.1159/000091952. [DOI] [PubMed] [Google Scholar]
- 4.Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3(4):288–95. doi: 10.3171/spi.2005.3.4.0288. [DOI] [PubMed] [Google Scholar]
- 5.Ryu S, Rock J, Rosenblum M, Kim JH. Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg. 2004;101 (Suppl 3):402–5. [PubMed] [Google Scholar]
- 6.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71(2):484–90. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 8.Huguenin PU, Kieser S, Glanzmann C, Capaul R, Lutolf UM. Radiotherapy for metastatic carcinomas of the kidney or melanomas: An analysis using palliative end points. Int J Radiat Oncol Biol Phys. 1998;41(2):401–405. doi: 10.1016/s0360-3016(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Hodgson D, Chow E, et al. A phase II trial of palliative radiotherapy for metastatic renal cell carcinoma. Cancer. 2005;104(9):1894–1900. doi: 10.1002/cncr.21410. [DOI] [PubMed] [Google Scholar]
- 10.Roos DE, Turner SL, O’Brien PC, et al. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05) Radiother Oncol. 2005;75(1):54–63. doi: 10.1016/j.radonc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch bone metastasis study. Radiother Oncol. 1999;52(2):101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 12.Murphy MJ. Image-guided patient positioning: if one cannot correct for rotational offsets in external-beam radiotherapy setup, how should rotational offsets be managed? Med Phys. 2007;34(6):1880–3. doi: 10.1118/1.2731485. [DOI] [PubMed] [Google Scholar]
- 13.Ryu S, Jin JY, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109(3):628–636. doi: 10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- 14.Jin JY, Ryu S, Rock J, et al. Evaluation of residual patient position variation for spinal radiosurgery using the novalis image guided system. Med Phys. 2008;35(3):1087–1093. doi: 10.1118/1.2839097. [DOI] [PubMed] [Google Scholar]
- 15.Chuang C, Sahgal A, Lee L, et al. Effects of residual target motion for image-tracked spine radiosurgery. Med Phys. 2007;34(11):4484–4490. doi: 10.1118/1.2790587. [DOI] [PubMed] [Google Scholar]
- 16.Astreinidou E, Bel A, Raaijmakers CP, Terhaard CH, Lagendijk JJ. Adequate margins for random setup uncertainties in head-and-neck IMRT. Int J Radiat Oncol Biol Phys. 2005;61(3):938–944. doi: 10.1016/j.ijrobp.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Guckenberger M, Meyer J, Wilbert J, et al. Precision required for dose-escalated treatment of spinal metastases and implications for image-guided radiation therapy (IGRT) Radiother Oncol. 2007;84(1):56–63. doi: 10.1016/j.radonc.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Onimaru R, Shirato H, Aoyama H, et al. Calculation of rotational setup error using the real-time tracking radiation therapy (RTRT) system and its application to the treatment of spinal schwannoma. Int J Radiat Oncol Biol Phys. 2002;54(3):939–947. doi: 10.1016/s0360-3016(02)03014-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim GY, Pawlicki T, Le QT, Luxton G. Linac-based on-board imaging feasibility and the dosimetric consequences of head roll in head-and-neck IMRT plans. Med Dosim. 2008;33(1):93–99. doi: 10.1016/j.meddos.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Shiu A, Wang C, et al. Dosimetric effect of translational and rotational errors for patients undergoing image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2008;71(4):1261–1271. doi: 10.1016/j.ijrobp.2008.02.074. [DOI] [PubMed] [Google Scholar]
- 21.Jin JY, Yin FF, Tenn SE, Medin PM, Solberg TD. Use of the BrainLAB ExacTrac X-Ray 6D system in image-guided radiotherapy. Med Dosim. 2008;33(2):124–34. doi: 10.1016/j.meddos.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Hornick DC, Litzenberg DW, Lam KL, et al. A tilt and roll device for automated correction of rotational setup errors. Med Phys. 1998;25(9):1739–40. doi: 10.1118/1.598355. [DOI] [PubMed] [Google Scholar]
- 23.Rijkhorst EJ, van Herk M, Lebesque JV, Sonke JJ. Strategy for online correction of rotational organ motion for intensity-modulated radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;69(5):1608–17. doi: 10.1016/j.ijrobp.2007.08.042. [DOI] [PubMed] [Google Scholar]