Abstract
The iboga alkaloid congener, 18-methoxycoronaridine (18-MC), decreases self-administration of multiple drugs of abuse. Here, in a biased procedure, we investigated whether 18-MC would have a similar effect on the acquisition, expression and reinstatement of a cocaine conditioned place preference (CPP) in male, Sprague-Dawley rats. While 18-MC attenuated acquisition of a cocaine CPP, it had no effect on CPP expression, and enhanced the reinstatement of cocaine CPP following extinction. Our results are consistent with those obtained using ibogaine, but reinforce the notion that acquisition, expression and reinstatement of a CPP likely involve separate mechanisms.
Keywords: Conditioned place preference, 18-methoxycoronaridine, cocaine, reinstatement
Introduction
A congener of the indole alkaloid ibogaine, 18-methoxycoronaridine (18-MC), decreases self-administration of a range of abused substances, including cocaine [3]. It functions as an antagonist at α3β4 nicotinic acetylcholine receptors (nAChRs), a subtype which is highly expressed in the medial habenula and interpeduncular nucleus. Local administration of α3β4 nAChR antagonists (including 18-MC) into the habenulo-interpeduncular pathway reduces drug self-administration [5, 6]. This pathway both directly and indirectly modulates the mesolimbic dopamine circuit, traditionally the locus of reward in the brain [13]. Consequently, 18-MC likely acts in a novel fashion to reduce the rewarding properties of drugs of abuse. One possibility is that 18-MC disrupts the associations between the drug (unconditioned stimulus) and conditioned cues supporting drug-seeking.
Conditioned place preference (CPP) is a form of Pavlovian conditioning that measures the ability of a drug to elicit a preference to a previously unpleasant or neutral stimulus. Unlike self-administration, the response to a previously neutral stimulus can be assessed in a drug-free state. Furthermore, three elements of place preference learning, i.e., acquisition, expression, and reinstatement following extinction can be assessed. As these three elements likely involve different neurobiological mechanisms [2, 8, 9], their manipulation by 18-MC may provide further insight into the mechanism(s) by which 18-MC affects reinforcement. Here, we measured the effects of 18-MC on acquisition, expression and reinstatement of a cocaine CPP.
Materials and Methods
Animals and drugs
A total of 90 male, Sprague-Dawley rats (250–275g; Taconic, Germantown, NY) were group-housed in a colony room maintained on a 12h light-dark cycle (lights on: 0700 h) and were provided with ad libitum food and water. All procedures followed the “Guide for the Care and Use of Laboratory Animals” (National Academy of Sciences, 1996). 18-Methoxycoronaridine HCl (Albany Molecular Research, Albany, NY) was dissolved in phosphate buffer (VEH) and injected intraperitoneally (i.p.) at a volume of 2 ml/kg. Cocaine HCl (Sigma, St. Louis, MO) was dissolved in sterile saline (SAL) and injected i.p. at a volume of 1 ml/kg.
Apparatus
Place preference chambers (71 × 25 × 46 cm) were constructed of three Plexiglas™ compartments with different wall colors and floors, separated by guillotine doors. The middle compartment (11 × 25 × 46 cm) was grey with a smooth grey floor. The outer compartments (30 × 25 × 46 cm) consisted of a white-walled compartment with a wire mesh floor and a black-walled compartment with a stainless steel rod floor. A ceiling-mounted digital video camera (Panasonic), and Any-Maze™ video tracking software (Stoelting Inc., Wood Dale, IL) were used to record and analyze activity in the chamber, including time spent in each compartment, and the number of crosses between compartments.
Procedure
Animals were habituated by handling them for 3 consecutive days prior to testing. The test procedure was somewhat similar to that used by Mueller and Stewart [12], and consisted of 3 consecutive phases: preconditioning, conditioning and test. Preconditioning took place over two consecutive days, during which animals were placed in the CPP chamber and allowed to explore all three compartments for 15 min. The time spent in each compartment was recorded only on the second day, as the first day served as an acclimation to the chambers. For all experiments (i.e., acquisition, expression and reinstatement), cocaine was paired with the white compartment, which was the non-preferred side for most animals. A different group of animals was used for each experiment.
Experiment 1. CPP acquisition
After preconditioning, animals were divided into four groups (n = 8–10): 1) VEH ; 2) 18-MC ; 3) VEH + cocaine ; and 4) 18-MC + cocaine. Conditioning began 48 h after the preconditioning day and lasted for 6 consecutive days. On Days 1, 3, and 5, rats were injected with drug and confined to the white compartment for 30 min. On these days, 40 mg/kg 18-MC (or VEH) was given 15 min prior to 15 mg/kg cocaine (or SAL) - that is, all rats received 2 injections. On Days 2, 4, and 6, all rats were injected with SAL and confined to the black compartment for 30 min. The CPP test took place 48 h after the last conditioning day, during which time rats were placed in the center compartment in a drug-free state and allowed to explore the entire chamber for 15 min.
Experiment 2: CPP expression
Two groups of rats were used to test the effects of 18-MC on expression of a cocaine place preference. In this experiment, rats were injected with cocaine (15 mg/kg) on Days 1, 3, and 5 and confined to the white compartment for 30 min. On Days 2, 4, and 6, rats were given a SAL injection and confined to the black compartment for 30 min. The expression test took place 48 h after the last conditioning session, during which time the animals were injected with either VEH or 40 mg/kg 18-MC, 45 min prior to being placed in the center compartment and were allowed to explore the chamber for 15 min.
Experiment 3: Extinction and Reinstatement of CPP
A different group of rats underwent an identical conditioning procedure as in Experiment 2, but no animals received 18-MC during the CPP expression test. One day after the expression test, rats underwent extinction by placing them in the center compartment in a drug-free state and allowing them to explore all three compartments for 15 min. During these tests, the time spent in each compartment was recorded. Extinction trials continued for each rat until the time in the drug-paired compartment was within 5% of the time spent during preconditioning, for 2 out of 3 consecutive days. Testing for cocaine induced reinstatement of CPP took place the day after each rat met the extinction criterion. Rats were divided into two groups and injected with 40 mg/kg 18-MC, or VEH, 45 min prior to the test session. Immediately before the test session, all rats were given a priming injection of cocaine (10 mg/kg), placed in the center of the CPP chamber and allowed to explore all three compartments for 15 min.
Data analysis
Preference scores were expressed as the difference between the time spent in the drug-paired compartment during the CPP test and the preconditioning test. Groups were compared using one-way analysis of variance (ANOVA) followed by post hoc Newman-Keuls test, or unpaired t-tests.
Results
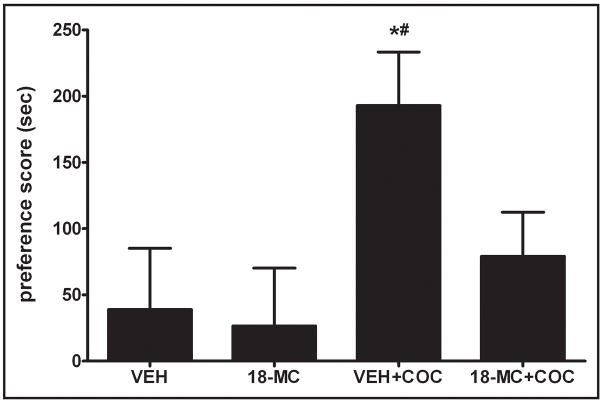
As mentioned, most animals exhibited a spontaneous preference for the white compartment during preconditioning. Across all experiments, time spent in the white compartment during preconditioning was 243.8 ± 11.3 sec compared with 367.0 ± 14.8 sec in the black compartment (t=5.54; p < 0.01; n=90). In Experiment 1, 18-MC prevented the acquisition of a cocaine CPP (Fig. 1). Animals previously co-injected with 18-MC and cocaine (18-MC+COC group) during conditioning had a mean preference score of 79.0 ± 33.0 sec which was significantly lower than those in the VEH+COC group (192.9 ± 40.3 sec; p < 0.05). In order to assure that 18-MC alone did not produce a place preference or place aversion, rats were injected with 18-MC and VEH on alternate days for a period of 6 days, and then were tested for any resulting place preference or aversion. Fig 1 shows that 18-MC, when delivered alone, did not itself elicit a place preference or an aversion. The preference score for animals given only 18-MC was 26.7 ± 43.5 sec which was not different from those injected with only VEH (38.9 ± 46.1 sec; p > 0.05).
Figure 1.
18-MC attenuates acquisition of a cocaine CPP. Preference scores indicate the difference between the time spent in the drug-paired compartment during the CPP test and the preconditioning test. Difference scores were derived by An * denotes significant difference from VEH group; # indicates significant difference from 18-MC+COC group; p < 0.05; one-way ANOVA followed by Newman-Keuls test.
While 18-MC attenuated the acquisition of a cocaine place-preference, it did not have an effect on the expression of cocaine CPP when administered 45 min prior to the expression test (Experiment 2). When 18-MC was administered 45 min prior to the expression test, it had no effect on animals’ preference for the drug-paired side. Mean preference scores (± SEM) were 114.0 ± 30.1 for the VEH group (n = 16) and 158.3 ± 87.8 for the 18-MC group (n = 8; p > 0.05).
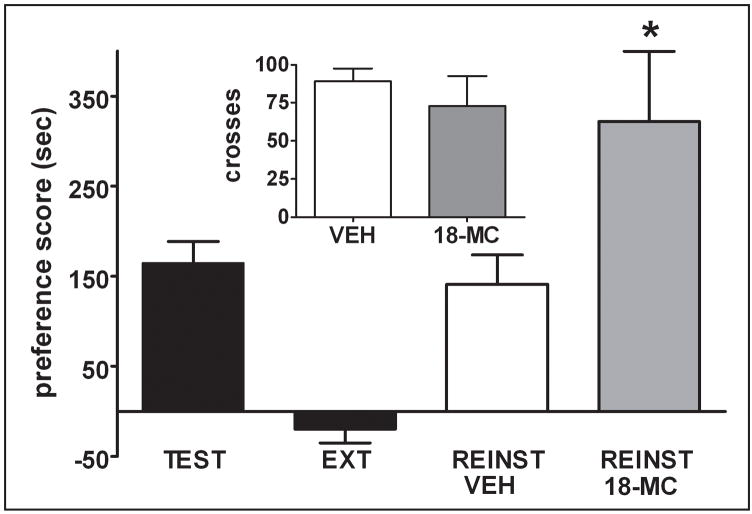
In order to examine the effect of 18-MC on cocaine-primed reinstatement of an established CPP after extinction (Experiment 3), a different group of rats was tested for cocaine CPP, and then underwent an extinction procedure consisting of repeated daily access to all three compartments of the CPP chamber while in a drug-free state. The mean number of days required to meet the criterion for extinction was 5.4 ± 0.51 (n = 31). The mean time spent on the drug-paired side after extinction was 210.7 ± 11.0 sec which was significantly less than the time spent on this side during the expression test (395.4 ± 16.1; t = 11.25; p < 0.01). For the reinstatement test, rats were given a VEH or 18-MC injection 45 min prior to a priming injection of COC (10 mg/kg). Unexpectedly, rats pretreated with 18-MC spent more time on the drug-paired side than rats given a VEH injection (Fig. 2). The effect of 18-MC on cocaine-induced reinstatement cannot be attributed to a change in locomotor activity induced by 18-MC, which was administered 45 minutes before the test. Activity, as measured by the number of crosses within the place preference apparatus was not significantly different between the two treatment groups (VEH: 89.1 ± 8.4 vs 18-MC: 72.9 ± 19.6; p > 0.05).
Figure 2.
Pretreatment with 18-MC enhances cocaine-induced reinstatement of CPP. Rats given 18-MC (40 mg/kg) prior to reinstatement showed an enhanced degree of reinstatement. Preference scores reflect the difference between the time spent in the drug-paired compartment during the CPP test and during preconditioning. * indicates significant difference from VEH during reinstatement (p < 0.05; t-test). Inset: no significant difference in activity between VEH and 18-MC groups was found during reinstatement test. Shown are the number of crosses between the compartments during the 15 min test.
Discussion
In these experiments, the iboga alkaloid congener, 18-MC, differentially affected the acquisition, expression and reinstatement of a cocaine CPP. It was not surprising that 18-MC blocked the acquisition of a cocaine CPP, as this component has been associated with the direct rewarding properties of a drug, and 18-MC also decreases cocaine self-administration [3]. However, 18-MC had no effect on CPP expression, and enhanced cocaine-induced reinstatement of CPP following extinction. Contrasting effects on CPP acquisition and expression have been previously reported (e.g.,[2, 7–9, 19, 20]), suggesting different neurobiological mechanisms underlie these processes. Still, there is little consensus regarding the precise neurotransmitter systems or brain regions that regulate CPP acquisition, expression and reinstatement [1].
These results are consistent with those obtained using ibogaine, the parent compound of 18-MC. Like 18-MC, ibogaine disrupted acquisition of morphine[15] and amphetamine CPP [11], without affecting CPP expression [10, 11]. Furthermore, the two compounds did not elicit an aversion or a preference when administered alone[15]. The action of both 18-MC and ibogaine may be due to their role as α3β4 nicotinic receptor antagonists [14]. This nAChR subtype is densely populated along the habenulo-interpeduncular pathway, which has been referred to as an “alternate reward pathway” due to the existence of reciprocal connections between it and the mesolimbic dopamine pathway [13, 18]. Local injections of 18-MC into the medial habenula or interpeduncular nucleus reduce drug self-administration[5, 6]. Moreover, the habenulo-interpeduncular pathway has been recently implicated in mediating drug withdrawal [16].
An enhanced cocaine-induced reinstatement of CPP by 18-MC probably does not reflect a summation of rewarding properties of 18-MC and cocaine, as 18-MC did not induce a place preference when given alone. One possible interpretation of our findings is that 18-MC behaved as a stressor, classically known to enhance reinstatement of self-administration[17]. However, it might then be assumed that 18-MC would elicit a conditioned place aversion when delivered alone, which was not the case. Similarly, since 18-MC had no rewarding or aversive effects when tested alone there appear to be no anxiolytic or anxiogenic effects of 18-MC that could influence the results with cocaine. Rather, it is possible that 18-MC diminishes the perceived effect of cocaine and more time was spent on the drug-paired side during reinstatement in order to compensate for this diminished perception. The effect of 18-MC on reinstatement of drug self-administration has not been investigated, nor has ibogaine been tested in this manner. Further research is necessary to determine how 18-MC is able to enhance reinstatement in this model.
The dose of 18-MC used in these experiments, 40 mg/kg, was appropriate as it blocks self-administration of cocaine and morphine and acutely decreases dopamine release from the nucleus accumbens [3]. It should be noted that the acquisition component of CPP differs from expression and reinstatement of the task in that acquisition is tested in a drug-free state. The terminal half-life of 18-MC in vivo is approximately 100 minutes [4]. Since 18-MC was injected 45 minutes prior to the expression and reinstatement phases, it is not possible to rule out state-dependent effects during these phases, except that 18-MC exhibited contrasting effects during these procedures, with no effect on expression but enhancement of cocaine-induced reinstatement. Furthermore, 18-MC administered prior to the reinstatement test did not change locomotor activity, as indicated by the number of crosses among the compartments.
In conclusion, we have found that the iboga alkaloid congener, 18-MC, reduces the acquisition of a cocaine CPP, but has no effect on CPP expression, and actually enhances the cocaine-primed reinstatement of CPP. Although the acquisition, expression and reinstatement of CPP appear to involve separate and different mechanisms, the precise details responsible for their differences remain to be determined.
Acknowledgments
SOURCE OF FUNDING
This research was supported by NIDA Grant DA 016283 (SDG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguilar MA, Rodriguez-Arias M, Minarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2008 doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–50. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- 3.Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- 4.Glick SD, Maisonneuve IM, Hough LH, Kuehne ME, Bandarage UK. (+/−)-18-Methoxycoronaridine: A novel iboga alkaloid congener having potential anti-addictive efficacy. CNS Drug Rev. 1999;5:27–42. [Google Scholar]
- 5.Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur J Pharmacol. 2006;537:94–8. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Glick SD, Sell EM, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration. Eur J Pharmacol. 2008 doi: 10.1016/j.ejphar.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo N, Garcia MM, Taylor BK, Zadina JE, Harlan RE. Blockade of micro-opioid receptors in the medial thalamus inhibits acquisition, but not expression, of morphine-induced conditioned place preference. Neuroscience. 2008;151:948–54. doi: 10.1016/j.neuroscience.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 8.Hand TH, Stinus L, Le Moal M. Differential mechanisms in the acquisition and expression of heroin-induced place preference. Psychopharmacology (Berl) 1989;98:61–7. doi: 10.1007/BF00442007. [DOI] [PubMed] [Google Scholar]
- 9.Hiroi N, White NM. The ventral pallidum area is involved in the acquisition but not expression of the amphetamine conditioned place preference. Neurosci Lett. 1993;156:9–12. doi: 10.1016/0304-3940(93)90426-l. [DOI] [PubMed] [Google Scholar]
- 10.Luxton T, Parker LA, Siegel S. Ibogaine fails to interrupt the expression of a previously established one-trial morphine place preference. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:857–72. doi: 10.1016/0278-5846(96)00064-4. [DOI] [PubMed] [Google Scholar]
- 11.Moroz I, Parker LA, Siegel S. Ibogaine interferes with the establishment of amphetamine place preference learning. Exp Clin Psychopharmacol. 1997;5:119–22. doi: 10.1037//1064-1297.5.2.119. [DOI] [PubMed] [Google Scholar]
- 12.Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–36. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- 14.Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW. Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration. Eur J Pharmacol. 2004;492:159–67. doi: 10.1016/j.ejphar.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Parker LA, Siegel S, Luxton T. Ibogaine attenuates morphine-induced conditioned place preference. Exp Clin Psychopharmacol. 1995;3:344–348. [Google Scholar]
- 16.Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–8. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J Comp Physiol Psychol. 1981;95:781–91. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- 19.Tolliver BK, Sganga MW, Sharp FR. Suppression of c-fos induction in the nucleus accumbens prevents acquisition but not expression of morphine-conditioned place preference. Eur J Neurosci. 2000;12:3399–406. doi: 10.1046/j.1460-9568.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 20.Zarrindast MR, Lashgari R, Rezayof A, Motamedi F, Nazari-Serenjeh F. NMDA receptors of dorsal hippocampus are involved in the acquisition, but not in the expression of morphine-induced place preference. Eur J Pharmacol. 2007;568:192–8. doi: 10.1016/j.ejphar.2007.04.015. [DOI] [PubMed] [Google Scholar]