Abstract
The spinal nucleus of the bulbocavernosus (SNB) innervates striated muscles, the bulbocavernosus and levator ani (BC/LA), which control penile reflexes. Castration results in shrinkage in the size of SNB somata and dendrites, as well as BC/LA muscle mass. However, there is no information about how quickly these regressive changes occur compared to the rapid effects of castration upon penile reflexes, which are greatly diminished a few days after surgery. Therefore we examined the time course of change in the size of SNB somata after castration of adult male rats. Males were sacrificed 2, 14, or 28 days after either castration or sham surgery and somata were measured in the SNB and in a control population of motoneurons, the retrodorsolateral nucleus (RDLN). BC/LA weight was reduced in castrates compared to intact males 14 and 28 days post surgery, but SNB somata were significantly smaller in castrates only at 28 days after surgery. As has been previously observed, castration did not affect soma size in the RDLN. These data indicate that SNB somata respond more slowly after castration than BC/LA mass or penile reflexes, suggesting that the size of SNB somata cannot account for the loss of penile reflexes. Androgenic effects on SNB somata may contribute to aspects of reproductive behavior that are not apparent in penile reflexes tested ex copula.
Keywords: androgens, neuronal plasticity, sexual dimorphism, retrodorsolateral nucleus
Introduction
The rat lumbosacral spinal cord contains a sexually dimorphic motor nucleus in which males have more and larger motoneurons than do females [1, 2]. This collection of motoneurons, termed the spinal nucleus of the bulbocavernosus (SNB), regulates penile reflexes necessary for successful reproduction by controlling striated muscles [3], the bulbocavernosus (BC) and levator ani (LA) [4], attached to the base of the penis.
Testosterone plays an important role in creating and maintaining the masculine phenotype of the SNB system. Androgens reduce normally occurring cell death in the lumbosacral spinal cord of perinatal males compared to females; this ontogenetic process creates the sex difference in the number of SNB motoneurons [5]. Androgens also maintain the morphology and connectivity of the SNB system in adulthood. This activational role of testosterone affects measures such as soma size [2], the number of synaptic contacts [6], dendritic arborization [7] and BC/LA muscle mass, but does not affect the number of motoneurons [2].
SNB motoneurons are an attractive model to study the effects of androgens on neuronal morphology in adulthood. Virtually all SNB cells express androgen receptors (ARs) [8], are easily identifiable anatomically, and have a well known behavioral function in regulating penile reflexes. Castration eliminates serum concentrations of testosterone within several hours of surgery [9], followed by an almost immediate loss of erectile function and a reduction in penile reflexes [10]. Additionally, restoration of penile reflexes is also quite rapid as long-term castrated males display erections in as little as 6 hours following testosterone treatment [10]. However, there have been no systematic studies examining the temporal dynamics of androgen mediated plasticity in the lumbar spinal cord. In the current report, we examined the time course of castration-induced changes in soma size in the SNB and compared these changes to the size of motoneurons in a control nucleus, the relatively androgen unresponsive motoneurons of the sexually monomorphic retrodorsolateral nucleus (RDLN) that occupy the same lumbar segments as the SNB [11, 12]. RDLN motoneurons innervate the flexor digitorum brevis muscles of the foot [13]. Delineating the time course of testosterone’s action in a simple system may give insight into the morphological changes underlying changes in behavior.
Material and methods
Animals
Male Long Evans rats (N=53; Charles River, Wilmington, MA), housed 2–4 to a cage, were kept on a 12:12 standard light/dark cycle with food and water available ad libitum. Animals were randomly assigned to one of two groups- either castrated or subjected to a sham surgery. Surgeries were performed at 60–70 days of age using isoflourane anesthesia under aseptic conditions. Animals from each group were randomly assigned to be sacrificed at 2 (Sham operated, n=9, Castrated, n=11), 14 (Sham operated, n=6, Castrated, n=9), or 28 (Sham operated, n=9, Castrated, n=11) days post surgery by an overdose of sodium pentobarbital (120mg/kg given intraperitoneally). All procedures were approved by the Michigan State University Institutional Animal Care and Use Committee.
Histology
At the time of tissue collection, blood was taken via a cardiac puncture and plasma testosterone levels were analyzed by radioimmunoassay (details below). Animals were perfused transcardially with normal saline followed by approximately 300 ml 10% neutral buffered formalin. Gonadal status was confirmed and spinal cords, preputial glands, seminal vesicles, and perineal muscles were removed and placed in 10% neutral buffered formalin for at least a month. All tissues but the spinal cord were then trimmed, patted dry on a paper towel and weighed. Spinal cords were placed in phosphate-buffered sucrose (pH 7.4) and stored overnight at 4°C. Using a freezing sliding microtome, alternate 40 μm thick cross sections were collected and mounted onto gelatin-coated glass slides. Samples were allowed to dry at least overnight before staining with thionin for Nissl substance. Slides were dehydrated in a series of graded ethanol solutions, defatted with xylenes, and coverslipped with Permount.
Testosterone Assay
Plasma T concentrations were measured in duplicate using the Coat-a-Count Total Testosterone Kit (Diagnostic Products Corp., Los Angles, CA) radioimmunoassay (RIA) from 50μl of plasma. The lower limit of detection was 0.1 ng/ml and the intra-assay coefficient of variation was 9.0 %.
Analysis
Motoneurons in the SNB and RDLN were selected by an investigator blind to group status. Because the SNB is spread out over lumbar (L) segments L5–L6, care was taken to sample MNs from each of these levels. An average of 20–25 randomly selected motoneurons per animal were sampled in the SNB and RDLN; there are approximately 200 SNB [2] and 300–400 RDLN [11] motoneurons in male rats. Motoneurons from each population were chosen if they met the following criteria: large multipolar neurons that had darkly stained cytoplasm, clear nucleus, intact nuclear envelope, and clearly defined external cellular wall. Using Bioquant software (version 8.00.02), somata were outlined using a computer mouse to generate measures of somata area in μm2. Average somata area was then calculated for each animal and this estimate was used in the statistical analyses.
Statistical Analysis
All statistical analyses were performed using SPSS (v16.0) for Windows. A 3 × 2 factorial design was utilized, with number of days after surgery (2, 14, or 28) and gonadal status (Castrated versus Sham operated) as independent variables to assess the effect of androgens on soma size in the SNB and RDLN. Separate tests were run for each motor pool. When a significant main effect or interaction was observed, a follow up analysis was performed using a one-way ANOVA; where appropriate, post hoc analysis was done using Fisher’s LSD statistic. For all statistical tests, N = number of animals per group and an alpha of 0.05 was used to determine significance.
Results
Spinal Nucleus of the Bulbocavernosus (SNB)
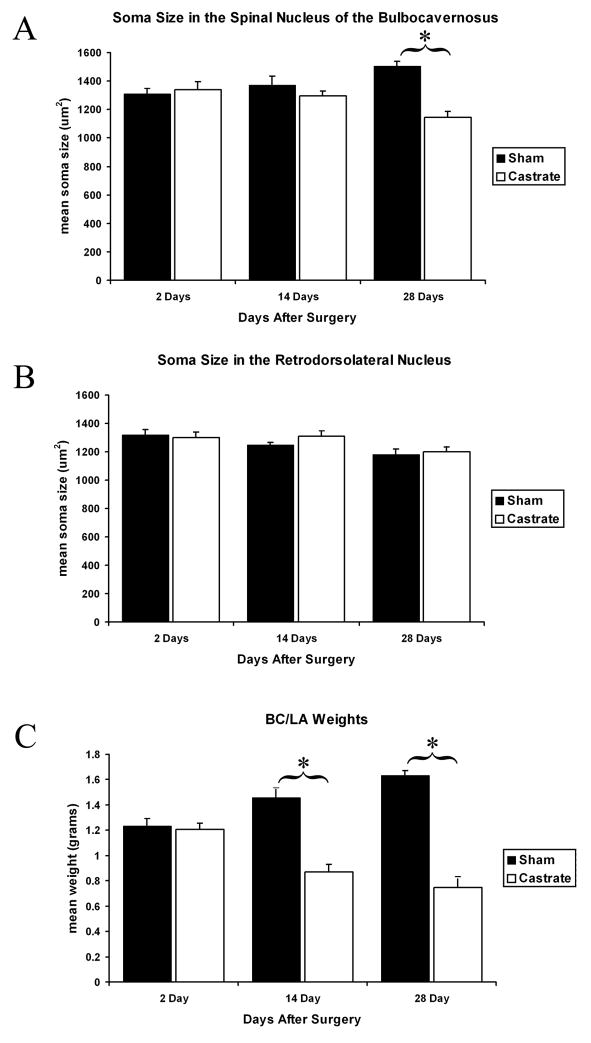
There was a significant main effect of gonadal status on SNB soma size (F1, 47=13.29; p=0.001), in which overall, control (sham surgery) males had larger somata than castrated males. In terms of an effect size, 22% of the variance (partial Eta squared (hp2)=0.22) could be attributed to the presence or absence of the gonads. The number of days after surgery had no main effect on soma size in the SNB (F2, 47=0.021, p=0.98). However, a significant interaction of gonadal status and days after surgery was observed in the SNB (F2, 47=10.48; p=0.0001), where 30.8% of the variance (hp2=0.308) can be attributed to this interaction. Post hoc analysis revealed there was no difference between the sham and castrate groups on days 2 and 14 (p=0.652 and p=0.302, respectively), but somata in the 28 day shams were larger than in the 28 day castrates (p<0.0001, Fig 1A).
Figure 1.
Castration-induced changes in the Spinal Nucleus of the Bulbocavernosus (SNB) system. A) In males subjected to sham surgery at 60–70 days of age, the size of SNB somata increased between 2 and 28 days after surgery. This normal growth was blocked by castration, such that 28 days after surgery the SNB somata of castrates were significantly smaller than those of sham males. There were no significant differences between the groups at either 2 or 14 days post-surgery. B) In the non-androgen responsive motoneurons of the retrodorsolateral nucleus (RDLN), castration had no effect on soma size but age did, with a slight shrinkage in size between 2 and 28 days after surgery in sham males. C) The bulbocavernosus (BC) and levator ani (LA) target muscles innervated by the SNB motoneurons also continued to grow in sham males, an effect presumably dependent on androgens, since castration results in shrinkage of BC/LA weights.
Unexpectedly, comparisons within the sham castrated group across age revealed that somata in the 28 day group (88–98 days old animals) were larger than in the 2 day (62 – 72 days old) group (p=0.004), but were not significantly different from the 14 day group (p=0.066). No differences were detected between the 2 and 14 day groups (p=0.48). These differences cannot be attributed to serum T levels, as no differences were detected between the sham castrated groups sacrificed at different ages (all post hoc comparisons p>0.05, Table 1), and presumably represents continued growth of SNB motoneurons in these young adults. Finally, somata in the 28 Day castrates were smaller compared to somata in both the 14 (p=0.016) and 2 day castrate groups (p=0.0002), but no differences were detected between the 2 and 14 day groups (p=0.408).
Table 1.
Preputial Gland and Seminal Vesicle Weights (in grams) and circulating testosterone levels (in ng/ml) ± SEM.
| 2 Days | 14 Days | 28 Days | ||||
|---|---|---|---|---|---|---|
| Shams | Castrates | Shams | Castrates | Shams | Castrates | |
| Preputial Glands | 0.20 ± 0.02 | 0.23 ± 0.02 | 0.24 ± 0.04 | 0.13 ± 0.02* | 0.21 ± 0.02 | 0.11 ± 0.02* |
|
| ||||||
| Seminal Vesicles | 1.1 ± 0.14 | 0.8 ± 0.03 | 1.58 ± 0.12 | 0.33 ± 0.04* | 2.0 ± 0.08 | 0.29 ± 0.14* |
|
| ||||||
| Testosterone | 3.7±1.11 | ND | 3.26 ±0.52 | ND | 2.88 ±0.42 | ND |
Note: signifies decreased weight compared to sham controls. ND = below the limit of detection of the assay.
Retrodorsolateral Nucleus (RDLN)
In contrast to the SNB, there was no main effect of gonadal status on soma size in the RDLN (F1, 47=0.437, p=0.512). There was however a significant main effect of days after surgery on soma size (F2, 47=5.462, p=0.007), although the change in size was the opposite to that observed for SNB motoneurons (Fig 1B). That is, post hoc analysis revealed that somata in the RDLN of the 28 day group were smaller than in either the 14 (p=0.02) or 2 (p=0.003) day groups while somata in the 2 and 14 day groups did not differ from each other (p=0.574). Additionally, no interaction of days after surgery and gonadal status was observed (F2, 47=0.58, p=0.56).
Bulbocavernosus/Levator Ani (BC/LA) Weights
There was a significant main effect of castration on weight of the BC/LA, the target musculature for SNB motoneurons (F1, 43=88.27, p<0.001). Animals in the castrated group displayed significantly reduced BC/LA weights compared to the shams (Fig 1C). There was no main effect of days post surgery on BC/LA weights (F2, 43=0.324, p=0.726), but there was a significant interaction of gonadal status and days after surgery (F2, 43=22.46, p<0.001). Post hoc analysis of BC/LA weights revealed significant differences between shams and castrates in the 14 (p<0.001) and the 28 (p<0.001) day groups. However, shams and castrates displayed comparable weights in the 2 day group (p=0.8). Comparisons within the sham groups revealed that animals in the 14 and 28 day groups were not significantly different from one another (p=0.08), but both groups displayed heavier muscles than the 2 day group (vs. 14 day, p=0.028; vs. 28 day, p<0.001), again indicating ongoing growth of the SNB system during this developmental period. When BC/LA weights within the castrated groups are compared, we find that most of the decline occurs between two and 14 days, as BC/LA weights of the 2 day group were larger compared to the 14 (p=0.001) and 28 (p<0.001) day groups, but the 14 day group weights were similar compared to the 28 day group weights (p=0.143).
The weights of preputial glands and seminal vesicles confirmed the loss of androgenic stimulation in the castrates (Table 1), but differences between castrates and sham males were significant only at 14 or 28 days after surgery, not at 2 days after surgery. The testosterone assay also confirmed the effectiveness of castration at all three time points (Table 1).
Discussion
We confirmed previous reports that castration reduces the somata of SNB motoneurons. However this effect was only evident 28 days following surgery; we found no significant effects of castration on SNB somata either 2 or 14 days after gonadectomy. The reduction in SNB soma size in the 28 day castrated group was not a function of age—if anything SNB soma size increased over this period in sham males (as has also been reported in 14)—but was presumably attributable to the loss of circulating androgens. Androgenic regulation of soma size in the SNB has often been assessed in chronic castration paradigms [i.e., 28 days or more; for example, see 2, 15, 16]. However, this is the first study to assay the effects of androgen deprivation on SNB somata after shorter delays. Only in the 28 day group do differences become evident, suggesting androgens are having an effect on SNB somata sometime between 15 and 28 days post castration. In contrast to the SNB, androgen withdrawal had a more rapid effect on the target musculature, the BC/LA (as well as seminal vesicles and preputial glands), suggesting that soma size changes happen relatively late in the process. Because androgen appears to act directly on SNB motoneurons to regulate the size of their somata [16], while acting directly on BC/LA muscles to affect their weight [17], these seem to be independent effects of androgen on the SNB system.
The time course for castration-induced changes in soma size in the SNB appears to be similar to that of the sexually dimorphic nucleus of the preoptic area (SDN-POA) [18]. In the SDN-POA, somata shrink 28 days after castration, but are not significantly different 2 or 14 days after surgery. In contrast to this, changes in neuronal somata of the posterodorsal division of the medial amygdala can be seen in as little as 14 days (J. A. Morris, unpublished observations). Together, these data suggest the time course of androgenic effects is tissue dependent.
Tract tracing studies indicate motoneurons of the SNB also innervate a sexually monomorphic muscle, the external anal sphincter [19]. Collins et al. [20] reported that this population of SNB motoneurons does not increase their somata following one month of T treatment, suggesting they are insensitive to androgens. Additionally, it was observed that these motoneurons were smaller compared to SNB motoneurons innervating the BC/LA. Thus, this heterogeneity of SNB motoneurons may have obscured our ability to detect changes in soma size at an earlier time point. However, the observed power [21] for the day by treatment interaction was very high (0.984) suggesting that our sample sizes were sufficient to detect even small changes in soma size.
Penile reflexes mediated by the SNB system are also affected by castration, but the reflexes have been reported absent one day after surgery [22]. Because we see no significant reduction in SNB somata even two weeks post-surgery, the loss of reflexes cannot be attributed to shrinkage of SNB somata. While it was not our intention to address the physiological relevance of soma size changes with this experiment, as this has yet to be determined, it may require a more detailed understanding of BC/LA function than the monitoring of penile reflexes alone. One possibility is that testosterone facilitates the display of penile reflexes by increasing the number of synaptic contacts onto SNB motoneurons; it has previously been reported that 48 hours of testosterone treatment of chronically castrated male rats increased the number of synapses on SNB motoneurons and a concomitant restoration of penile reflexes was noted [23]. However, given that penile reflexes have been observed in as little as 12 hours following androgen treatment [24], the synaptic plasticity may be the result of increased displays of penile reflexes [23]. As such, the functional significance of changes in synaptic inputs also remains to be determined.
Finding that BC/LA muscles shrink within 2 weeks after castration agrees well with previous reports [25]. Interestingly, castration also reduces the expression of AR in the muscles, whereas administration of DHT restored BC/LA weights and AR levels to precastration values after only a week of treatment. Thus, changes in the mass of the BC/LA muscles may actually occur earlier than the 14 day time point we monitored in the current study, and might therefore contribute to the loss of penile reflexes. On the other hand, we saw no difference in BC/LA weight 2 days after castration, when reflexes are severely diminished [22].
Brain derived neurotrophic factor (BDNF), which is expressed in muscle and motoneurons [26, 27], may play a permissive role for androgen-induced plasticity in the SNB, and possibly in the effects on SNB soma size. Severing SNB motor axons has been shown to decrease expression of AR in SNB motoneurons as well as the size of their somata in gonadally intact males [28, 29]. Application of BDNF to the cut ends of SNB axons prevents these morphological changes but only in the presence of androgens [29]. Together, these data suggest that muscle-derived BDNF may be important for maintaining AR expression levels in SNB motoneurons, which in turn allows androgens to maintain the size of SNB somata by acting directly on AR in the motoneurons themselves [16]. Interestingly, 2 weeks of castration results in decreased BDNF expression within the SNB [27], a time course which suggests that loss of muscle derived factors is followed by decreases in motoneuronal AR expression and soma size; however, this time course of changes remains to be empirically determined.
Castration did not affect the size of motoneurons in the RDLN, supporting previous suggestions that androgens play no role in regulating soma size in this population of motoneurons. Indeed, the longer survival time after surgery revealed a decrease in the size of RDLN motoneurons, as the 28 day groups displayed smaller somata than the 2 and 14 day groups. This finding suggests that RDLN motoneuronal somata normally shrink from 60–100 days in rats; the functional significance of this change in size, if any, remains to be determined. Our observations are in agreement with a previous study [13] showing that RDLN motoneurons, on average, do not respond to castration with a decrease in soma size. However, not every cell within the RDLN expresses ARs, whereas virtually all of the motoneurons within the SNB are AR immuno-positive [8, 30]. Because expression of ARs within the motoneuron is required for an increase in SNB soma size in response to testosterone [16], it is possible that a minority of RDLN motoneurons that express AR may have indeed shrunk following castration. If so, then any such effect would have been diluted by the majority of RDLN motoneurons which appear to be AR negative. Thus, a more refined analysis of soma size, separately analyzing AR positive and AR negative RDLN motoneurons, may be insightful.
In summary, androgen deprivation by castration reduces soma size in the rat SNB after 28 days, but this effect is not observed 2 or 14 days post-castration. Comparison of sham-operated control males indicates that SNB somata are normally expanding between 60 to 98 days of age in rats and castration blocks this growth and induces shrinkage as well. Somata in the RDLN were unaffected by castration, but were significantly smaller in the 28 day group, presumably due to normal developmental regression unrelated to androgens.
Acknowledgments
Supported by NS28421. We thank Brittany Dugger for excellent technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- 2.Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- 3.Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- 4.Schrøder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–87. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- 5.Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto A, Micevych PE, Arnold AP. Androgen regulates synaptic input to motoneurons of the adult rat spinal cord. J Neurosci. 1988;8:4168–4176. doi: 10.1523/JNEUROSCI.08-11-04168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- 8.Jordan CL, Padgett B, Hershey J, Prins G, Arnold AP. Ontogeny of androgen receptor immunoreactivity in lumbar motoneurons and in the sexually dimorphic levator ani muscle of male rats. J Comp Neurol. 1997;379:88–98. [PubMed] [Google Scholar]
- 9.Krey LC, McGinnis MY. Time-courses of the appearance/disappearance of nuclear androgen + receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: relationships with gonadotropin secretion. J Steroid Biochem. 1990;35:403–408. doi: 10.1016/0022-4731(90)90247-p. [DOI] [PubMed] [Google Scholar]
- 10.Hart BL, Wallach SJ, Melese-d′Hospital PY. Differences in responsiveness to testosterone of penile reflexes and copulatory behavior of male rats. Horm Behav. 1983;17:274–283. doi: 10.1016/0018-506x(83)90026-0. [DOI] [PubMed] [Google Scholar]
- 11.Jordan CL, Breedlove SM, Arnold AP. Sexual dimorphism and the influence of neonatal androgen in the dorsolateral motor nucleus of the rat lumbar spinal cord. Brain Res. 1982;249:309–314. doi: 10.1016/0006-8993(82)90065-8. [DOI] [PubMed] [Google Scholar]
- 12.Tobin AM, Payne AP. Perinatal androgen administration and the maintenance of sexually dimorphic and nondimorphic lumbosacral motor neuron groups in female Albino Swiss rats. J Anat. 1991;177:47–53. [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie M, Forger NG, Breedlove SM. Sexual dimorphism and androgen effects on spinal motoneurons innervating the rat flexor digitorum brevis. Brain Res. 1991;561:269–273. doi: 10.1016/0006-8993(91)91603-x. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki M, Arnold AP. Androgenic regulation of dendritic trees of motoneurons in the spinal nucleus of the bulbocavernosus: reconstruction after intracellular iontophoresis of horseradish peroxidase. J Comp Neurol. 1991;308:11–27. doi: 10.1002/cne.903080103. [DOI] [PubMed] [Google Scholar]
- 16.Watson NV, Freeman LM, Breedlove SM. Neuronal size in the spinal nucleus of the bulbocavernosus: direct modulation by androgen in rats with mosaic androgen insensitivity. J Neurosci. 2001;21:1062–1066. doi: 10.1523/JNEUROSCI.21-03-01062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rand MN, Breedlove SM. Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol. 1992 Feb;23(1):17–30. doi: 10.1002/neu.480230104. [DOI] [PubMed] [Google Scholar]
- 18.Dugger BN, Morris JA, Jordan CL, Breedlove SM. Gonadal steroids regulate neural plasticity in the sexually dimorphic nucleus of the preoptic area of adult male and female rats. Neuroendocrinology. 2008;88:17–24. doi: 10.1159/000119740. [DOI] [PubMed] [Google Scholar]
- 19.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 20.Collins WF, Seymour AW, Klugewicz SW. Differential effect of castration on the somal size of pudendal motoneurons in the adult male rat. Brain Res. 1992;577:326–330. doi: 10.1016/0006-8993(92)90292-h. [DOI] [PubMed] [Google Scholar]
- 21.O’Keefe DJ. Post hoc power, observed power, a priori power, retrospective power, prospective power, achieved power: sorting out appropriate uses of statistical power analyses. Communication Methods and Measures. 2007;I:291–299. [Google Scholar]
- 22.Gray GD, Smith ER, Davidson JM. Hormonal regulation of penile erection in castrated male rats. Physiol Behav. 1980;24:463–468. doi: 10.1016/0031-9384(80)90237-1. [DOI] [PubMed] [Google Scholar]
- 23.Leedy MG, Beattie MS, Bresnahan JC. Testosterone-induced plasticity of synaptic inputs to adult mammalian motoneurons. Brain Res. 1987;424:386–390. doi: 10.1016/0006-8993(87)91484-3. [DOI] [PubMed] [Google Scholar]
- 24.Gray GD, Smith ER, Davidson JM. Hormonal regulation of penile erection in castrated male rats. Physiol Behav. 1980;24:463–468. doi: 10.1016/0031-9384(80)90237-1. [DOI] [PubMed] [Google Scholar]
- 25.Antonio J, Wilson JD, George FW. Effects of castration and androgen treatment on androgen-receptor levels in rat skeletal muscles. J Appl Physiol. 1999;87:2016–2019. doi: 10.1152/jappl.1999.87.6.2016. [DOI] [PubMed] [Google Scholar]
- 26.Arnold AP, Yang LY. The bulbocavernosus and levator ani (BC/LA) muscle complex expresses BDNF protein. Soc Neurosci Abstr. 1999;25:1269. [Google Scholar]
- 27.Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148:3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- 28.Al-Shamma HA, Arnold AP. Brain-derived neurotrophic factor regulates expression of androgen receptors in perineal motoneurons. Proc Natl Acad Sci U S A. 1997;94:1521–1526. doi: 10.1073/pnas.94.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang LY, Arnold AP. BDNF regulation of androgen receptor expression in axotomized SNB motoneurons of adult male rats. Brain Res. 2000;852:127–139. doi: 10.1016/s0006-8993(99)02225-8. [DOI] [PubMed] [Google Scholar]
- 30.Freeman LM, Padgett BA, Prins GS, Breedlove SM. Distribution of androgen receptor immunoreactivity in the spinal cord of wild-type, androgen-insensitive and gonadectomized male rats. J Neurobiol. 1995;27:51–59. doi: 10.1002/neu.480270106. [DOI] [PubMed] [Google Scholar]