Abstract
In neonatal mice ultrasonic vocalizations have been studied both as an early communicative behavior of the pup-mother dyad and as a sign of an aversive affective state. Adult mice of both sexes produce complex ultrasonic vocalization patterns in different experimental/social contexts. All these vocalizations are becoming an increasingly valuable assay for behavioral phenotyping throughout the mouse life-span and alterations of the ultrasound patterns have been reported in several mouse models of neurodevelopmental disorders. Here we also show that the modulation of vocalizations by maternal cues (maternal potentiation paradigm) – originally identified and investigated in rats - can be measured in C57Bl/6 mouse pups with appropriate modifications of the rat protocol and can likely be applied to mouse behavioral phenotyping. In addition we suggest that a detailed qualitative evaluation of neonatal calls together with analysis of adult mouse vocalization patterns in both sexes in social settings, may lead to a greater understanding of the communication value of vocalizations in mice. Importantly, both neonatal and adult USV altered patterns can be determined during the behavioural phenotyping of mouse models of human neurodevelopmental and neuropsychiatric disorders, starting from those in which deficits in communication are a primary symptom.
Keywords: ultrasonic vocalizations, maternal potentiation, animal models of neurodevelopmental disorders
1. Introduction
In the neonatal house mouse Mus musculus, isolation-induced ultrasonic vocalizations (USVs) are whistle-like sounds with a single component at frequencies between 30 kHz and 90 kHz (Branchi et al., 2001. The rate of calling follows a clear ontogenetic profile, peaking around the eighth day after birth and decreasing to zero when outbred pups are two weeks old {Noirot, 1969#30; Elwood and Keeling, 1982). Considerable differences have been found among strains (Roubertoux et al., 1996).with C57Bl/6 showing lower calling rate and earlier profile peak [around postnatal day (PND) 3] than other inbred mice (BALB/c, DBA, A/J (Hennessy et al., 1980; Roubertoux et al., 1996; Sewell, 1970; Thornton et al., 2005).
Since their first description, neonatal USVs were interpreted as a communicative behavior (Zippelius and Schleidt, 1956). Functional significance of such vocalizations have then been extensively debated. It has been postulated that these sounds are the incidental by-product of a physiological response to a thermal challenge, e.g. the reflexive abdominal compression reaction that helps return venous blood to the heart (Blumberg and Alberts, 1990; Blumberg and Sokoloff, 2001).
However, it is a sound ethological evidence that pup vocalizations elicit maternal orientation/approach and retrieval (Cohen-Salmon et al., 1985; Ehret and Bernecker, 1992; Noirot, 1972; Smotherman et al., 1974) and reduce attacks or rough manipulation by the dam (Ihnat et al., 1995; Noirot, 1966). Focussing on the USV receiver, the dam, Farrell and Alberts showed that rat mothers approach and maintain orientation to a vocalizing pup far more than virgin females, such orientation appears immediately after delivery, increase during the first week of life of the offspring and decline by the time of weaning, being regulated, at least partially by maternal hormones (Farrell and Alberts, 2002a; Farrell and Alberts, 2002b). A dynamic relationship between maternal responsiveness and pup calling rate has been shown in mice by comparing maternal responsiveness to USVs in two different strains. Using a three-compartment cage test where the mother, to reach the pups, had to cross the central part of the cage containing olfactory cues from a potentially infanticidal male, authors showed that C57Bl/6 mothers scored higher in maternal responsiveness than BALB/c females, and their pups emitted fewer calls than BALB/c pups and suggested that maternal responsiveness, (i.e. mother promptness to respond to pups’ needs) might be a key factor tuning the rate of ultrasonic emission of the offspring (D’Amato et al., 2005).
USVs can be quantitatively analysed, can be elicited by measurable stimuli, and can be analysed with limited handling of the pup. In addition, neonatal USVs could also map onto later development of adult anxiety profiles (Dichter et al., 1996). The present review underscores the reliability of the evaluation of the mouse USVs as a behavioural endpoint targeting the communicative competencies of this species. This issue appears extremely relevant for the construction of validated experimental models of developmental and psychiatric disorders characterised by communication deficits among the pathological signs, e.g autism spectrum disorders. Indeed, whereas USVs have been studied extensively in a rodent ethological perspective in the last decades, only very recently they are systematically becoming a core feature (and an effective tool) in behavioural phenotyping of both adult and neonatal mutant mice modelling several neuropsychiatric and neurodevelopmental disorders, starting from those associated with communicative/social deficits (Moy and Nadler, 2008).
2. Measuring USV in mouse pups: the role of neurotransmitters
Several pharmacological studies have been conducted to evaluate the role of different neurotransmitter systems on the regulation of USV signalling in rodents and this information has been previously reviewed (Branchi et al., 2001; Hofer, 1996). Here we reported USV data from mouse lines with genetic manipulations of neurotransmitter receptor subtypes, and reduced or elevated synaptic levels of a targeted neurotransmitter; only those pharmacological studies that have formed the background information for a subsequent analysis of USVs in transgenic mice are also mentioned.
Serotonin (5HT)
Pharmacological studies have shown that serotoninergic drugs affect USV of mouse pups, often through a mechanism associated with sedative or thermoregulatory actions (Nastiti et al., 1991). Ultrasonic calling has therefore been included in behavioural phenotyping studies focused on lines of mice with targeted mutations in the 5HT1a and 5HT1b receptor genes. Knockout (KO) mice for the 5HT1b receptor emitted fewer vocalizations after neonatal social isolation than control wildtype (WT) littermates (Brunner et al., 1999). Interestingly the same authors showed that mutant 5HT1b dams spent significantly more time outside the nest as compared to WT mothers, despite no differences in latency to retrieve their pups (Brunner et al., 1999). When the role played by the maternal environment on the USV profile emitted by infants was analysed in greater detail, interesting result were found (Weller et al., 2003). In the neonatal isolation paradigm, both 5HT1a and 5HT1b KO mice vocalized less than WT littermates, thus confirming previous results. Genotype of dams, however, had a dramatic effect on heterozygous 5HT1a pups. Heterozygous offspring of 5HT1a KO dams unexpectedly emitted more USVs than genetically identical pups reared by WT dams (Weller et al., 2003).
From a methodological viewpoint these results also highlight the importance of maternal factors that appear to shape the neonatal and adult behavioural phenotype of the offspring. Brunner, Weller, and coworkers recommend a breeding procedure of heterozygous crossings, to minimize variation of the maternal environment and provide within-litter controls.
Cannabinoids (CBs)
Pharmacological studies had indicated that CBs modulate USV production by mechanisms entirely independent from hypothermia in rats (McGregor et al., 1996). More recently, prenatal exposure to the cannabinoid CB1 receptor agonist WIN 55,212-2 was shown to decrease the rate of separation-induced USV in 10-day-old rats (Antonelli et al., 2005). Behavioural analysis of CB-1 KO mice thus included USV among the endpoints, reporting a total lack of the characteristic developmental peak in separation induced USV (Fride et al., 2005).
GABA
GABA B (1a or 1b) KO mice have been behaviourally characterised (Jacobson et al., 2006), but despite clear evidence from pharmacological studies in which baclofen or benzodiazepines had anxiolytic effects decreasing isolation induced neonatal USVs (Cirulli et al., 1994; Nastiti et al., 1991), but so far no data are available about USVs in GABA B KO pups.
Opioid
Opioid modulation of isolation induced USV in neonatal rats has been reported by several authors (Carden et al., 1991; Kehoe and Blass, 1986; Nelson and Panksepp, 1998). Pharmacological studies showed a reduction in vocalization after treatment with μ opioid receptor agonists (Carden et al., 1991). Early behavioural phenotyping of mice lacking μ opioid receptors found decreased USV, interpreted as a sign of decreased responsiveness to maternal separation (Moles et al., 2004). Interestingly these mouse pups failed to show higher USV production on the second consecutive isolation from the mother (the maternal potentiation paradigm, described below): these results were interpreted as a decreased social bonding in neonatal mice lacking μ opioid receptor (Moles et al., 2004).
Oxytocin
In 9–10-day-old rats, exogenous administration of oxytocin reduced the rate of USV emitted by neonatal rats that had been socially isolated (Insel and Winslow, 1991). Treatment with an oxytocin antagonist, however, did not have the opposite effect, leaving USV unchanged (Insel and Winslow, 1991). Null mutant mouse pups lacking oxytocin displayed fewer USVs than their wildtype controls (Winslow et al., 2000). This counterintuitive finding (given that exogenous oxytocin also decreased calling) has been interpreted as evidence that in the absence of oxytocin social separation is not perceived as a distress and does not induce USV (Winslow et al., 2000; Winslow and Insel, 2002). In addition, data from male KO mouse pups lacking the oxytocin receptor showed fewer USVs in 7-day-old knockout mice (Takayanagi et al., 2005), consistent with the hypothesis that oxytocin neurotransmission is necessary for the perception of social separation and the subsequent USV response.
Vasopressin
The first experiments to evaluate the possible role of the peptide arginine vasopressin (AVP) on the modulation of ultrasonic vocalizations found that in rats exogenous administration of vasopressin decreased ultrasonic vocalizations (Winslow and Insel, 1993). Using the antagonists available at that time, i.e. AVP1 vs AVP2 antagonists, the effects of exogenously administered vasopressin appeared to be regulated primarily by AVP1 receptors (Winslow and Insel, 1993). Recent pharmacological studies provided evidence for the AVP1b receptor subtype in the regulation of USV in neonatal rats. The recently developed selective AVP1b antagonist (SSR 149-451) significantly decreased neonatal USV in rats (Hodgson et al., 2007).
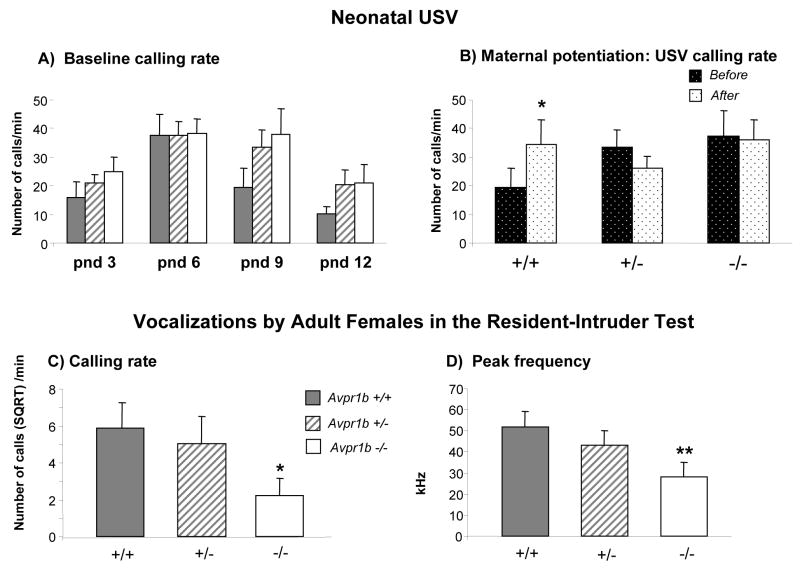
Neonatal USVs of AVP1b receptor KO mouse pups have been recently measured (Scattoni et al., 2008). Despite the large pharmacological evidence for a role of AVP1b receptors in the modulation of neonatal USV during social isolation, no evidence of genotype differences were detected in baseline levels of emission. AVP1b null mutant pups did show a deficit in USVs in the neonatal maternal potentiation USV paradigm, and also in USVs emitted by adult females during social interaction with an unfamiliar female partner (see Figure 1) (Scattoni et al., 2008). Differences between pharmacological evidence and the behavioural phenotype of mutant mice are likely due to: i) developmental effects of genetic modification, both prenatally and postnatally; ii) intrinsic species-specific regional patterns of expression for neuropeptide receptors. The regional distribution of AVP1a receptors in praire and montane voles differs from the neuroanatomical distributions in rats and mice, providing a paradigmatic example of the limitations in predicting mouse behavioural phenotypes that are primarily based on pharmacological data gathered in another rodent species, e.g. rat (Young et al., 1997).
Figure 1.
Ultrasonic vocalizations (USVs) in Avpr1b mice. A) Number of USV on postnatal day (pnd) 3, 6, 9 and 12 in response to social separation during a five minute session. No consistent genotype differences were detected across the four ages tested. B) Number of USVs emitted on pnd 9 during the maternal potentiation test by pups during the second five min separation session, following a five min reunion. Before: first period of five min isolation from the mother and siblings. After: second period of isolation, following five min of reunion with the mother and entire litter. Avpr1b +/+ mice emitted more calls (*p< .05) during the second separation after reunion, displaying the expected maternal potentiation. Avpr1b +/− and Avpr1b −/− mice failed to show an effect of the reunion with their mother and siblings on number of calls emitted during the second separation. C) Number of USVs emitted by resident female mice (four months of age) when exposed to a C57Bl/6J adult female intruder during the Resident-Intruder test. Avpr1b −/− emitted significantly fewer USVs in comparison to Avpr1b +/+ (*p<.05). D) Avpr1b −/− emitted calls with lower peak frequencies than Avpr1b +/+ (**p <.01) during the Resident-Intruder test. Data for number of USVs (panel C) are expressed as square root mean ± SEM. In the graphs A, C and D, data are expressed as mean ± SEM. Panel A and B Avpr1b +/+ (n = 9); Avpr1b +/− (n = 31); Avpr1b −/− (n = 17). Panel C and D Avpr1b +/+ (n = 10); Avpr1b +/− (n = 11); Avpr1b −/− (n = 11). Modified from (Scattoni et al., 2008).
Other Neuropeptides
In agreement with the proposed physiological roles for the substance P-neurokinin 1(NK1) receptor system in regulating stress responses in humans and rodents (Holmes et al., 2003), genetic deletion of the substance P receptor NK1 significantly reduced USVs on PND 8 and 9 (Rupniak et al., 2000). No information is available on adult USV patterns in these mice which are characterised at adulthood by reduced aggression and by decreased emotionality in the plus-maze (Santarelli et al., 2001).
3. Investigating USVs in mouse models of neurodevelopmental disorders
Recently, USV analysis has been applied to studies of mouse models of neurodevelopmental disorders (Branchi and Ricceri, 2002; Ricceri et al., 2007b). USV pattern was analysed in a mouse model of Down syndrome (Ts65Dn) carrying a partial trisomy of the chromosome 16 that includes the region homologous to the human chromosome 21. In these mice, the ontogenetic profile of USV emissions is delayed by 4 days, with Ts65Dn mice showing a peak of emission on pnd 9 whereas the peak was evident on pnd 5 in the WT controls (Holtzman et al., 1996).
Jimpy is a shortened life-span (pnd 30) murine mutant line showing recessive sex-linked inheritance. The genetic defect consists of a point mutation in the proteolipid protein (PLP) gene and produces a severe CNS myelin deficiency that is associated with a variety of complex abnormalities affecting all glial populations (Vela et al., 1998). Jimpy males produced fewer USV than their normal male littermates, beginning at postnatal day 2 and persisting throughout the first postnatal week (Bolivar and Brown, 1994). As observed in Ts65Dn mice, the USV deficit is accompanied by a delay in acquiring developmental milestones and by a reduced body weight gain and reduced locomotor activity. The reduced vocalization rate had no consequences on maternal retrieval behaviour (Bolivar and Brown, 1995).
Reeler mice, with a mutation in the gene for reelin, an extracellular-matrix protein involved in plasticity of dendritic spines and synaptic transmission, showed alterations in USV patterns with a clear gene-dose dependency (Laviola et al., 2006). Null mutant reeler mice emitted fewer calls than WT controls, and heterozygotes emitted USV at an intermediate level (Laviola et al., 2006). These reductions in USV calls were, however, associated with body weight decreases, and were reversed by epigenetic factors including prenatal exposure to an organophosphate, suggesting that increased availability of acetylcholine early in development could have played a sort of compensatory role (Laviola et al., 2006). Furthermore, the deficient profile of null mutant reeler pups was counteracted by repeated maternal separations, which are known to stimulate hypothalamic-pitituary axis activation and release of corticosteroid hormone (Ognibene et al., 2007).
One of the most intriguing USV patterns is seen in neonatal Foxp2 mutant mice (Shu et al., 2005). The FOXP2 gene codes for a transcription factor (forkhead box transcription factor) which has been identified in humans as the gene responsible for verbal dyspraxia, dysphasia, and other severe language and speech disorders affecting the KE family (Vargha-Khadem et al., 2005). Heterozygous and homozygous FoxP2 KO pups showed a selective decrease in USV vocalization emitted after neonatal isolation on postnatal days 6 and 10 (Shu et al., 2005). These results were interpreted as supporting the role of Foxp2 in regulating neural development and social communication.
Somatic growth, somatosensory reflexes and USVs have been examined in Mecp21lox mutant mice, a mouse model of Rett Syndrome (Picker et al., 2006). Somatic development increases steadily and appears similar to WT controls in both Mecp2 null male and Mecp2 heterozygous female mice. However, beginning at postnatal day 5, both Mecp2 null males and heterozygous females exhibited dramatic increases in USV in response to social isolation (Picker et al., 2006) This is the earliest and most prominent sign detected in MECP2 mutant mice. Elevated USV levels might indicate either an altered response to social isolation or alteration of respiratory function, an issue that is receiving increasing attention in mouse models of Rett syndrome in the last years (Ogier et al., 2007; Viemari et al., 2005). Further testing should clarify the contributions of each of these factors in the altered behavioural response. Interestingly, USV are increased in infancy after cholinergic blockade (Kehoe et al., 2001) and cholinergic deficits have been reported for Rett individuals (Wenk and Hauss-Wegrzyniak, 1999). These data have translational significance, because they suggest that the early deficits noted in Rett individuals may be mimicked in the mouse models, and candidate USV as a neonatal behavioral response that can be used as an assay at early ages to evaluate therapeutic interventions.
Finally, analysis of the behavioural phenotype of Fibroblast growth Factor 17 (Fgf17) KO mice - a putative mouse model of selected aspects of schizophrenia - revealed selective alterations in the social domain, including a general deficit in USVs and decreased reactivity to social novelty in a social recognition task associated with a reduced frontal cortex activation (Scearce-Levie et al., 2007). Fgf17 is a trophic factor involved in neural embryonic development and regional organization of cortical layers in cerebellum, inferior colliculus and frontal cortex in rodents (Cholfin and Rubenstein, 2007). Fgf17 gene lies within the 8p22-21 chromosomal linkage region for schizophrenia and two different genes coding for Fgf ligands are also within schizophrenia linkage sites.
These latter data thus suggests that USVs can be analysed fruitfully also in behavioural phenotyping of mouse models of complex human neuropsychiatric diseases.
4. Maternal potentiation procedure as a model for USV modulation: mouse pups are not little rat pups
In socially isolated rat pups, the rate of USV emission is dramatically increased during the second consecutive separation, i.e. a 5 minute separation, followed by 5 minutes of contact with the mother, followed immediately by a second 5 minute separation (Shair et al., 2005). This phenomenon, called “maternal potentiation,” has been extensively characterised during the second postnatal week in the rat species (for a detailed review see (Shair, 2007)). Maternal potentiation of USV has been reported in rat pups after a 5 minute reunion with a passive anesthetised dam, as well as with an active anaesthetised, behaviourally active dam (Hofer et al., 1996; Kraebel et al., 2002). USV potentiation during the second separation can also occur after a brief contact with the sire, provided that pups are reared with their sire in the home cage (Brunelli et al., 1998). A more detailed analysis conducted in 10-day-old neonatal rats showed that maternal reunion not only increases the subsequent calling rate but also induces qualitative changes in ultrasonic emission, namely increased average amplitude and average bout size (i.e. number of USV/bout) (Myers et al., 2004).
In rat pups, the maternal potentiation of USV is a robust phenomenon occurring in different experimental settings, independent of thermal cues, and resilient to adverse early experiences such as hypoxia at birth (Venerosi et al., 2006) and selective cholinergic lesions of the cholinergic basal forebrain (Ricceri et al., 2007a). Maternal potentiation is not species-specific for rats, since it also occurs in 15-day-old guinea pigs (Hennessy et al., 2006). In this species, however, potentiation of USV response after a brief reunion with the dam is evident not as an increase of USV response, rather number of USV did not differ between first and second isolations whereas control pups not exposed to the brief maternal reunion showed a significant reduction of calls from the first to second isolation.
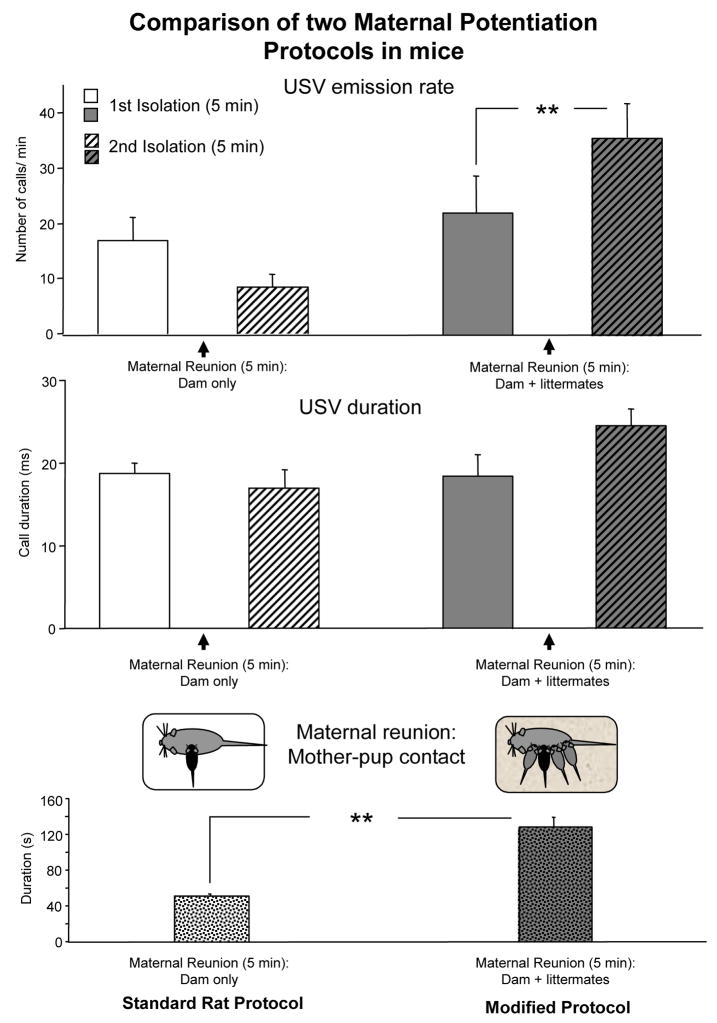
Early findings in mice indicate that maternal potentiation of USV is not as robust, and may depend on the mouse strain. Total lack of maternal potentiation has been reported in the CD-1 Swiss derived albino outbred strain (Branchi et al., 2004). By contrast, some recent maternal potentiation data collected in C57Bl/6J 8-day-old pups indicate that maternal potentiation of USV can be detected using an experimental protocol modified from the rat one, with reunion occurring in the home cage with both mother and littermates (see figure 2 for details). Indeed, in such a condition, time spent by the dam in contact with the experimental pups is significantly increased. This enhances the contrast experienced by the pups between maternal reunion and second isolation and results in potentiation of USV response.
Figure 2.
Effects of two maternal potentiation protocols in 8-day-old C57Bl/6J mice: in the first method, shown in the left column, maternal reunion between first and second isolation consisted in returning the pup to dam only, following the standard protocol for rats. In the second protocol, shown in the right column, maternal reunion consisted of returning the pup to the home cage with the dam and the littermates. Data are mean + sem, n = 10 litters per protocol [methods for the USV recording and statistical analysis are as described in Scattoni et al. 2007]. Upper panel: number of USV per min emitted by the experimental pup (black pup in the diagram) during the first and second isolation periods, ** p< 0.01 after Tukey HSD posthoc comparison performed on the interaction protocol x maternal potentiation factor F (1, 18) =23.6, p<0.01. Middle panel: mean duration of USV emitted by the experimental pup (black pup in the diagram) during the first and second isolation periods; protocol x maternal potentiation factor F (1, 18) =4.5, p<0.05, Tukey HSD posthoc comparison performed on this interaction just missed statistical significance. Lower panel: time spent by the dam in active contact with the experimental pup (black pup in the diagram) during maternal reunion, ** main effect of protocol F (1, 18) =48.9, p<0.01.
In addition to such evidence of maternal potentiation in pups of the inbred strain C57Bl/6J, maternal potentiation was also evident at postnatal day 12 in WT controls, but significantly reduced in pups with a null mutation in the mu opioid receptor, (Moles et al., 2004). Similarly, maternal potentiation was detectable in a line of AVP1b receptor knockout mice with a mixed C57Bl/6J and 129/SvJ genetic background, in which USV patterns have been analysed in greater detail (Scattoni et al., 2008). In 9-day-old WT control pups, maternal potentiation was found both in terms of number and duration of calls. Duration appeared to be a more sensitive parameter than number of calls, and call amplitude was not affected by the dam reunion (Scattoni et al., 2008).
It must be also noted; however, that maternal potentiation of the USV response has not been found in another mutant mouse line with C57Bl/6 background, the Fgf17 knockout line (Cholfin and Rubenstein, 2007). Such a discrepancy might be due to procedural differences such as age of testing or dam reunion modality (dam in the experimental cage vs pup back in the home cage with mother and littermates). Detailed analysis of differential reunion procedures and age-related differences upon maternal potentiation phenomenon in C57Bl/6 inbred mouse pups merits further investigation.
AV1b receptor knockout data illustrate the additional informative value of the maternal potentiation USV response. This mouse line represents the only targeted gene mutation so far in which baseline USV did not differ in the three genotypes analysed (+/+, +/− and −/−), while genotype emerged when the maternal potentiation paradigm was applied. In contrast, in the Mecp2, Oxytocin and Foxp2 mutants, only baseline USV differed between genotypes (Picker et al., 2006; Shu et al., 2005; Winslow et al., 2000), while in the mu opiate receptor mutants, both baseline and maternal potentiation deficits were detected (Moles et al., 2004). These findings thus suggest that the maternal potentiation response could be a useful additional endpoint in early behavioural phenotyping of mouse lines in which the neonatal responsiveness to maternal cues represents the target interest.
5. Recent advances in USV analysis: from quantity to quality
The development of sound spectrographic analysis allows additional insights into both environmental and genetic factors shaping USV response. This advance in instrument technology provided user-friendly hardware and software to characterize and measure acoustic signals, adding a qualitative analysis to the more common quantitative measures as shown by (Branchi et al., 1998). These authors were the first to demonstrate that USVs emitted by neonatal laboratory mice were differently shaped when recorded under different ecologically relevant conditions (odor from the nest, social isolation, low temperature-isolation, tactile stimulation, or odor from a conspecific adult unfamiliar male).
Analysis of sonograph patterns also revealed significant effects of prenatal malnutrition on USV response that were not evident in terms of call rates (Tonkiss et al., 2003). Detailed sonographic analysis showed that the reduction of the number of USV following neonatal ethanol exposure is not a general reduction of all wave-types, but rather a selective reduction of certain waveforms (rising frequency, u-shaped and 3 sweeps calls) (Barron and Gilbertson, 2005). Further analyses using playback approaches could clarify whether these selective changes have a specific impact on dam responses. Detailed analysis of acoustic parameters of the neonatal USV has also offered the opportunity to evaluate the auditory cortical response of the dam to such USVs (Liu et al., 2006). This methodological approach has indicated that auditory cortical encoding of the same playback USV may differ between mothers and naïve females (Liu and Schreiner, 2007). Cortical responses for USV detection and discrimination were seen in mothers responding the natural pup vocalizations, but not observed when non-communicative artificial sound ensembles were presented (Liu and Schreiner, 2007).
6. Adult mouse vocalizations: a still unexploited tool in mouse behavioural phenotyping?
Males
The USV emission of adult mice has been primarily reported in reproductive contexts (Nyby, 2001), with males being responsible of most of the calls (Maggio et al., 1983; Whitney and Nyby, 1979). At variance with rats, adult mice USV are not detectable during agonistic encounters in laboratory settings (Nyby, 2001). Only recently, however, mouse USV have been detected in adolescent C57Bl/6J and Balb/cJ mice of both sexes during social interaction undergoing since weaning a repeated schedule (four days) of social housing followed by one-day social isolation (Panksepp et al., 2007). Interestingly, a detailed qualitative analysis showed significant strain differences in frequency distribution of waveforms with prevalence of downwards and complex sonograms in C57Bl/6J mice and prevalence of upwards and inverted U-shaped sonograms in Balb/cJ mice (Panksepp et al., 2007).
The exposure to a female partner or to the urine induces a clear USV response in adult male mice with previous reproductive experience (Holy and Guo, 2005; Maggio et al., 1983; Nyby, 2001; Whitney and Nyby, 1979). The quantitative analysis performed by Holy and Guo illustrated for the first time that the male vocalizations are characterised by temporal sequences that are specific for each individual (Holy and Guo, 2005).
Very recently the female-induced male vocalization response has been used in the behavioural phenotyping of cholinergic (muscarinic) and dopamine receptor knockout mice focused on the reward mechanisms underlying male/female recognition and sexual reward. These data, although still preliminary, point to a decreased USV response in M2 or M5 receptor knockout male mice, whereas the same response is unchanged in M4 and D2 knockout mice (Wang et al., 2008).
Females
The production of USV during female-female mouse encounters is a sound phenomenon discovered by Maggio and Whitney in 1985 (Maggio and Whitney, 1985), even though sporadic reports of ultrasonic emissions by female mice appeared also previously (D’Udine et al., 1982). Maggio and Whitney systematically investigated the USV emitted during female-female interaction in mice showing that female mice during encounters with other females emit a large number of USV, at absolute rates comparable to those of the male-female interaction. When the female-female encounter occurs within a resident/intruder experimental paradigm, the resident female emits a great number of ultrasonic calls (Moles and D’Amato F, 2000) as demonstrated by anesthetizing alternatively the members of the pair.
Such female resident-intruder paradigm has been recently used to analyse social behaviour of mice lacking the AVP1b receptor gene (Scattoni et al., 2008). Null mutation of the Avpr1b gene resulted in reduced UVs emission by adult females during the resident-intruder test, while social sniffing levels were unaltered. While some authors have questioned the role of the female mouse vocalization in naturalistic conditions (Nyby, 2001), USV during resident-intruder interactions in laboratory conditions has been interpreted as a main factor contributing to the establishment of female social dominance hierarchies (Maggio and Whitney, 1985). These calls may serve as communication signals, enhancing physical proximity and enabling social information gathering (Maggio and Whitney, 1985; Moles et al., 2007). Since the Avpr1b −/− showed social sniffing responses (i.e. physical proximity with the intruder) that were not significantly different from WT controls, these data lend support to the role of USVs emitted by the resident female in other forms of communication, e.g. the establishment of hierarchical ranks (Scattoni et al., 2008).
The growing literature on USV in mice suggests that this behavioural response is likely to be useful to i) characterize basal profiles and selected qualitative aspects of mother-pup interaction and ii) investigate additional adult social responsiveness in different inbred strains. Importantly, both neonatal and adult USV deviant patterns can be determined in investigations of behavioural phenotypes in several mouse models of human neurodevelopmental and neuropsychiatric disorders, starting from those in which deficits in communication are a primary symptom.
Table 1.
Representative vocalization studies in mutant mouse models
| Mouse model | Ultrasonic vocalizations | Age range | Description | References |
|---|---|---|---|---|
| FOXP2 knock out mice | Decreased emission rate | PND 6 | Decrease emission rate in (−/+) mice, no emission in(−/−) mice. Both whistles and clicks are reduced in (−/+) mice | Shu et al, 2005 |
| Ts65Dn (Down syndrome mouse model) | Delayed ontogenetic profile | PNDs 3 - 13 | Ultrasonic vocalization profile was delayed by 4 days. Peak expression of USVs was observed on PND 9 in Ts65Dn mice, on PND 5 in 2n control mice | Holtzman et al. 1996 |
| MeCP2 (Rett syndrome mouse model) | Increased emission rate | PNDs 3 -12 | Increased emission rate since PND 5 in hemizygous null male mice, since PND 7 in heterozygous females | Pickers et al. 2006 |
| Reelin | Decreased emission rate | PNDs 3 - 9 | Decreased emission rate throughout the first postnatal week. | Laviola et al. 2006; Ognibene et al. 2007 |
| FGF17 | Decreased emission rate | PND 8 | Decreased emission rate during two repeated isolation periods | Scearce-Levie et al. 2007 |
| AVP1b receptor Knock out mice | Lack of maternal potentiation effect in knockout and heterozygous | PND 9 | Increased emission rate (maternal potentiation) after a brief reunion with the mother in wt controls, not in heterozygous and knockout; increased call duration in all groups (wt, heterozygous and knockout). | Scattoni et al. 2008 |
Acknowledgments
This research was supported by ISS-NIH 0F14 “Neurobehavioral phenotyping of genetically modified mouse models of mental retardation” (LR), by the National Institute of Mental Health Intramural Research program (JNC), and in part by the NIMH Intramural Research Program (Z01-MH-02179).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonelli T, Tomasini MC, Tattoli M, Cassano T, Tanganelli S, Finetti S, Mazzoni E, Trabace L, Steardo L, Cuomo V, Ferraro L. Prenatal exposure to the CB1 receptor agonist WIN 55,212–2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb Cortex. 2005;15:2013–2020. doi: 10.1093/cercor/bhi076. [DOI] [PubMed] [Google Scholar]
- Barron S, Gilbertson R. Neonatal ethanol exposure but not neonatal cocaine selectively reduces specific isolation-induced vocalization waveforms in rats. Behav Genet. 2005;35:93–102. doi: 10.1007/s10519-004-0859-2. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Alberts JR. Ultrasonic vocalizations by rat pups in the cold: an acoustic by-product of laryngeal braking? Behav Neurosci. 1990;104:808–817. doi: 10.1037//0735-7044.104.5.808. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Sokoloff G. Do infant rats cry? Psychol Rev. 2001;108:83–95. doi: 10.1037/0033-295x.108.1.83. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Brown RE. The ontogeny of ultrasonic vocalizations and other behaviors in male jimpy (jp/Y) mice and their normal male littermates. Dev Psychobiol. 1994;27:101–110. doi: 10.1002/dev.420270204. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Brown RE. Selective retrieval of jimpy mutant pups over normal male littermates by lactating female B6CBACa-Aw-J/A-Ta jp mice. Behav Genet. 1995;25:75–80. doi: 10.1007/BF02197244. [DOI] [PubMed] [Google Scholar]
- Branchi I, Campolongo P, Alleva E. Scopolamine effects on ultrasonic vocalization emission and behavior in the neonatal mouse. Behav Brain Res. 2004;151:9–16. doi: 10.1016/S0166-4328(03)00277-8. [DOI] [PubMed] [Google Scholar]
- Branchi I, Ricceri L. Transgenic and knock-out mouse pups: the growing need for behavioral analysis. Genes Brain Behav. 2002;1:135–141. doi: 10.1034/j.1601-183x.2002.10301.x. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic vocalizations by infant laboratory mice: a preliminary spectrographic characterization under different conditions. Dev Psychobiol. 1998;33:249–256. doi: 10.1002/(sici)1098-2302(199811)33:3<249::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Brunelli SA, Masmela JR, Shair HN, Hofer MA. Effects of biparental rearing on ultrasonic vocalization (USV) responses of rat pups (Rattus norvegicus) J Comp Psychol. 1998;112:331–343. doi: 10.1037/0735-7036.112.4.331. [DOI] [PubMed] [Google Scholar]
- Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci U S A. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Santucci D, Laviola G, Alleva E, Levine S. Behavioral and hormonal responses to stress in the newborn mouse: effects of maternal deprivation and chlordiazepoxide. Dev Psychobiol. 1994;27:301–316. doi: 10.1002/dev.420270505. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon C, Carlier M, Roubertoux P, Jouhaneau J, Semal C, Paillette M. Differences in patterns of pup care in mice. V--Pup ultrasonic emissions and pup care behavior. Physiol Behav. 1985;35:167–174. doi: 10.1016/0031-9384(85)90331-2. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet. 2005;35:103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Brunelli SA, Hofer MA. Elevated plus-maze behavior in adult offspring of selectively bred rats. Physiol Behav. 1996;60:299–304. doi: 10.1016/0031-9384(95)02222-8. [DOI] [PubMed] [Google Scholar]
- D’Udine B, Robinson DJ, Oliverio A. An analysis of single-gene effects on audible and ultrasonic vocalizations in the mouse. Behav Neural Biol. 1982;36:197–203. doi: 10.1016/s0163-1047(82)90189-3. [DOI] [PubMed] [Google Scholar]
- Ehret G, Bernecker C. Categorical perception of mouse-pup ultrasounds in the temporal domain. Anim Behav. 1992;43:409–416. [Google Scholar]
- Elwood RW, Keeling F. Temporal organization of ultrasonic vocalizations in infant mice. Dev Psychobiol. 1982;15:221–227. doi: 10.1002/dev.420150306. [DOI] [PubMed] [Google Scholar]
- Farrell WJ, Alberts JR. Maternal responsiveness to infant Norway rat (Rattus norvegicus) ultrasonic vocalizations during the maternal behavior cycle and after steroid and experiential induction regimens. J Comp Psychol. 2002a;116:286–296. doi: 10.1037/0735-7036.116.3.286. [DOI] [PubMed] [Google Scholar]
- Farrell WJ, Alberts JR. Stimulus control of maternal responsiveness to Norway rat (Rattus norvegicus) pup ultrasonic vocalizations. J Comp Psychol. 2002b;116:297–307. doi: 10.1037/0735-7036.116.3.297. [DOI] [PubMed] [Google Scholar]
- Fride E, Suris R, Weidenfeld J, Mechoulam R. Differential response to acute and repeated stress in cannabinoid CB1 receptor knockout newborn and adult mice. Behav Pharmacol. 2005;16:431–440. doi: 10.1097/00008877-200509000-00016. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Li J, Lowe EL, Levine S. Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev Psychobiol. 1980;13:573–584. doi: 10.1002/dev.420130603. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Miller EE, Shair HN. Brief exposure to the biological mother “potentiates” the isolation behavior of precocial Guinea pig pups. Dev Psychobiol. 2006;48:653–659. doi: 10.1002/dev.20180. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Higgins GA, Guthrie DH, Lu SX, Pond AJ, Mullins DE, Guzzi MF, Parker EM, Varty GB. Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacol Biochem Behav. 2007;86:431–440. doi: 10.1016/j.pbb.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology. 1996;21:203–217. doi: 10.1016/0306-4530(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Brunelli SA, Masmela J, Shair HN. Maternal interactions prior to separation potentiate isolation-induced calling in rat pups. Behav Neurosci. 1996;110:1158–1167. doi: 10.1037//0735-7044.110.5.1158. [DOI] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, Johnson RM, Chen K, Sun Y, Carlson E, Alleva E, Epstein CJ, Mobley WC. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci U S A. 1996;93:13333–13338. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihnat R, White NR, Barfield RJ. Pup’s broadband vocalizations and maternal behavior in the rat. Behav Processes. 1995;33:257–272. doi: 10.1016/0376-6357(94)00028-f. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT. Central administration of oxytocin modulates the infant rat’s response to social isolation. Eur J Pharmacol. 1991;203:149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Bettler B, Kaupmann K, Cryan JF. GABAB1 receptor subunit isoforms exert a differential influence on baseline but not GABAB receptor agonist-induced changes in mice. J Pharmacol Exp Ther. 2006;319:1317–1326. doi: 10.1124/jpet.106.111971. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Blass EM. Opioid-mediation of separation distress in 10-day-old rats: reversal of stress with maternal stimuli. Dev Psychobiol. 1986;19:385–398. doi: 10.1002/dev.420190410. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Callahan M, Daigle A, Mallinson K, Brudzynski S. The effect of cholinergic stimulation on rat pup ultrasonic vocalizations. Dev Psychobiol. 2001;38:92–100. doi: 10.1002/1098-2302(200103)38:2<92::aid-dev1001>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kraebel KS, Brasser SM, Campbell JO, Spear LP, Spear NE. Developmental differences in temporal patterns and potentiation of isolation-induced ultrasonic vocalizations: influence of temperature variables. Dev Psychobiol. 2002;40:147–159. doi: 10.1002/dev.10022. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Gaudino C, Marino R, Keller F. Paradoxical effects of prenatal acetylcholinesterase blockade on neuro-behavioral development and drug-induced stereotypies in reeler mutant mice. Psychopharmacology (Berl) 2006;187:331–344. doi: 10.1007/s00213-006-0426-z. [DOI] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory Cortical Detection and Discrimination Correlates with Communicative Significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio JC, Maggio JH, Whitney G. Experience-based vocalization of male mice to female chemosignals. Physiol Behav. 1983;31:269–272. doi: 10.1016/0031-9384(83)90186-5. [DOI] [PubMed] [Google Scholar]
- Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- McGregor IS, Dastur FN, McLellan RA, Brown RE. Cannabinoid modulation of rat pup ultrasonic vocalizations. Eur J Pharmacol. 1996;313:43–49. doi: 10.1016/0014-2999(96)00511-0. [DOI] [PubMed] [Google Scholar]
- Moles A, Costantini F, Garbugino L, Zanettini C, D’Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav Brain Res. 2007;182:223–230. doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Moles A, D’Amato F R. Ultrasonic vocalization by female mice in the presence of a conspecific carrying food cues. Anim Behav. 2000;60:689–694. doi: 10.1006/anbe.2000.1504. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- Myers MM, Ali N, Weller A, Brunelli SA, Tu AY, Hofer MA, Shair HN. Brief maternal interaction increases number, amplitude, and bout size of isolation-induced ultrasonic vocalizations in infant rats (Rattus norvegicus) J Comp Psychol. 2004;118:95–102. doi: 10.1037/0735-7036.118.1.95. [DOI] [PubMed] [Google Scholar]
- Nastiti K, Benton D, Brain PF. The effects of compounds acting at the benzodiazepine receptor complex on the ultrasonic calling of mouse pups. Behav Pharmacol. 1991;2:121–128. [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Noirot E. Ultra-sounds in young rodents. I. Changes with age in albino mice. Anim Behav. 1966;14:459–462. doi: 10.1016/s0003-3472(66)80045-3. [DOI] [PubMed] [Google Scholar]
- Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5:371–387. doi: 10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- Nyby JG. Auditory communication among adults. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. New York: CRC; 2001. pp. 3–18. [Google Scholar]
- Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Macri S, Laviola G. Neurobehavioural disorders in the infant reeler mouse model: interaction of genetic vulnerability and consequences of maternal separation. Behav Brain Res. 2007;177:142–149. doi: 10.1016/j.bbr.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker JD, Yang R, Ricceri L, Berger-Sweeney J. An altered neonatal behavioral phenotype in Mecp2 mutant mice. Neuroreport. 2006;17:541–544. doi: 10.1097/01.wnr.0000208995.38695.2f. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Cutuli D, Venerosi A, Scattoni ML, Calamandrei G. Neonatal basal forebrain cholinergic hypofunction affects ultrasonic vocalizations and fear conditioning responses in preweaning rats. Behav Brain Res. 2007a;183:111–117. doi: 10.1016/j.bbr.2007.05.035. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res. 2007b;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, Martin B, Le Roy I, Beau J, Marchaland C, Perez-Diaz F, Cohen-Salmon C, Carlier M. Vocalizations in newborn mice: genetic analysis. Behav Genet. 1996;26:427–437. doi: 10.1007/BF02359487. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Carlson EC, Harrison T, Oates B, Seward E, Owen S, de Felipe C, Hunt S, Wheeldon A. Pharmacological blockade or genetic deletion of substance P (NK(1)) receptors attenuates neonatal vocalisation in guinea-pigs and mice. Neuropharmacology. 2000;39:1413–1421. doi: 10.1016/s0028-3908(00)00052-6. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille ET, Blier P, Hen R, Heath MJ. Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc Natl Acad Sci U S A. 2001;98:1912–1917. doi: 10.1073/pnas.041596398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, Rubenstein JL, Mucke L. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2007 doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- Shair HN. Acquisition and expression of a socially mediated separation response. Behav Brain Res. 2007;182:180–192. doi: 10.1016/j.bbr.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shair HN, Brunelli SA, Hofer MA. Lack of evidence for mu-opioid regulation of a socially mediated separation response. Physiol Behav. 2005;83:767–777. doi: 10.1016/j.physbeh.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci U S A. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Hahn ME, Schanz N. Genetic and developmental influences on infant mouse ultrasonic calling. III. Patterns of inheritance in the calls of mice 3–9 days of age. Behav Genet. 2005;35:73–83. doi: 10.1007/s10519-004-0857-4. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Bonnie KE, Hudson JL, Shultz PL, Duran P, Galler JR. Ultrasonic call characteristics of rat pups are altered following prenatal malnutrition. Dev Psychobiol. 2003;43:90–101. doi: 10.1002/dev.10124. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6:131–138. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- Vela JM, Gonzalez B, Castellano B. Understanding glial abnormalities associated with myelin deficiency in the jimpy mutant mouse. Brain Res Brain Res Rev. 1998;26:29–42. doi: 10.1016/s0165-0173(97)00055-6. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Cutuli D, Chiarotti F, Calamandrei G. C-section birth per se or followed by acute global asphyxia altered emotional behaviour in neonate and adult rats. Behav Brain Res. 2006;168:56–63. doi: 10.1016/j.bbr.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS ONE. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A, Leguisamo AC, Towns L, Ramboz S, Bagiella E, Hofer M, Hen R, Brunner D. Maternal effects in infant and adult phenotypes of 5HT1A and 5HT1B receptor knockout mice. Dev Psychobiol. 2003;42:194–205. doi: 10.1002/dev.10079. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Hauss-Wegrzyniak B. Altered cholinergic function in the basal forebrain of girls with Rett syndrome. Neuropediatrics. 1999;30:125–129. doi: 10.1055/s-2007-973476. [DOI] [PubMed] [Google Scholar]
- Whitney G, Nyby J. Cues that elicit ultrasounds from adult male mice. Amer Zool. 1979;19:457–463. [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Effects of central vasopressin administration to infant rats. Eur J Pharmacol. 1993;233:101–107. doi: 10.1016/0014-2999(93)90354-k. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Zippelius HM, Schleidt WM. Ultraschall-laute bej jungen mausen (Ultrasonic vocalization in infant mice) Naturwissenschaften. 1956;43:502–503. [Google Scholar]