Abstract
Integrins are heterodimeric transmembrane receptors that modulate cell adhesion, migration and signaling. Multiple integrin chains contribute to development and morphogenesis of a given tissue. Here, we analyze the expression of Drosophila integrin alpha chains in the ovarian follicular epithelium, a model for tissue morphogenesis and cell migration. We find expression throughout development of the beta chain, βPS. Alpha chains, however, exhibit both spatial and temporal expression differences. αPS1 and αPS2 integrins are detected during early and mid-oogenesis on apical, lateral, and basal membranes with the βPS chain while αPS3-family integrins (αPS3, αPS4, αPS5) are expressed in anterior cells late in oogenesis. Surprisingly, we find that αPS3-family integrins are dispensable for dorsal appendage morphogenesis but play a role in the final length of the egg, suggesting redundant functions of integrins in a simple tissue. We also demonstrate roles for αPS3βPS integrin in border cell migration and in stretch cells.
Keywords: Drosophila, integrin, ovary, gene expression, protein expression, stretch follicle cell, border cell, RNAi
INTRODUCTION
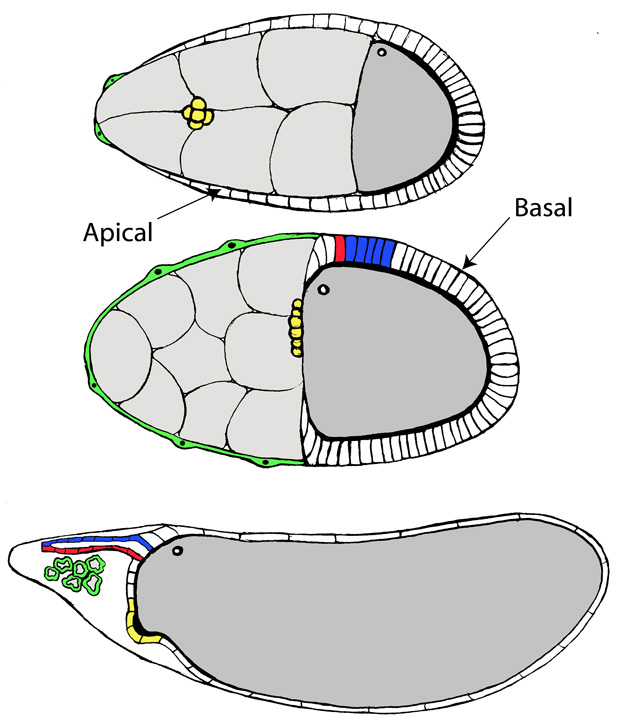
The Drosophila ovary comprises 15–17 smaller ovarioles that produce individual egg chambers in an assembly line-like fashion, and provides an excellent model system in which to study basic mechanisms of cellular and tissue morphogenesis (Spradling, 1993; Horne-Badovinac and Bilder, 2005). Each egg chamber consists of 16 germline cells, including the oocyte and 15 nurse cells that support its development, surrounded by an epithelial layer of somatic follicle cells. The follicle cells have two main roles during the latter half of oogenesis: communication with the oocyte that is essential for patterning the egg chamber and early embryo, and formation of the eggshell (van Eeden and St. Johnston, 1999; Waring, 2000). During eggshell formation, a sequence of migrations and cell shape changes involving subpopulations of the follicle cells (see Fig. 1) results in envelopment of the oocyte and synthesis of specialized eggshell structures such as the dorsal respiratory appendages, the micropyle required for sperm entry, and the door-like operculum that allows hatching of the larva (Berg, 2005; Horne-Badovinac and Bilder, 2005). The cell-cell and cell-matrix adhesive interactions involved in regulating the polarization, movements and functions of the follicle cell epithelium are of interest to understanding how this dynamic cell type contributes to formation of a functional egg.
Figure 1. Illustration of major events during Drosophila egg chamber morphogenesis.
(Top) During Stage 9, most follicle cells migrate posteriorly to cover the oocyte in a columnar epithelium (main body follicle cells) that synthesizes the main part of the eggshell, while a small number of thin stretch cells (green) overlie the germline nurse cells. Border cells (yellow) separate from the anterior follicle cell epithelium and migrate between nurse cells to take up a position at the anterior end of the oocyte by the beginning of Stage 10A. (Middle) In Stage 10B, centripetal cells migrate between the oocyte and nurse cells to complete the covering of the anterior end of the egg, and floor cells (red) and roof cells (blue) are specified that will undergo shape changes to form two tubes that migrate anteriorly (bottom, Stage 12), secreting the dorsal appendage matrix into their lumens. Stretch cells initially coat the outside of the germline cell cluster (middle), but wrap processes completely around each degenerating nurse cell in Stage 10B-12 (bottom). The border cells and centripetal cells contribute to the formation of the micropyle and operculum.
Here we consider the roles of integrins, major adhesion receptors that bind to extracellular matrix (ECM) ligands and that have been extensively characterized in mammalian model systems (Hynes, 2002; Arnaout et al., 2005). Integrins are obligate heterodimers comprising transmembrane alpha and beta subunits, which through various effector proteins, connect to and regulate the actin cytoskeleton, cell migration, proliferation, and survival (Mitra and Schlaepfer, 2006; Delon and Brown, 2007). Integrins play a key role in the development and remodeling of a variety of vertebrate tissues including the brain (Schmid and Anton, 2003), kidney (Chen et al., 2004), and vasculature (Serini et al., 2006) as well as progression of tumors (Guo and Giancotti, 2004). However, expression of multiple integrin chains is a factor complicating the study of basic integrin functions during development. For example, a recent report revealed expression of ten alpha and four beta subunits in the mammalian sclera (Metlapally et al., 2006). Given the difficulty of genetic analysis in mammals, it would be prohibitively complicated to gain insight into how multiple integrin chains are coordinated in a single tissue to control developmental processes.
While in humans there are 18 alpha and 8 beta subunits combining to form at least 24 integrin dimers (Berman et al., 2003), the Drosophila genome encodes five alpha and two beta subunits (Brower, 2003), making genetic manipulation more tractable. The myospheroid (mys) gene encodes the predominantly utilized beta subunit (βPS) in the fly (MacKrell et al., 1988) while the βν subunit is restricted to the gut (Yee and Hynes, 1993). The three characterized fly alpha subunits, αPS1 (encoded by multiple edematous wings, mew; cytological position 11E3–11E8; Brower et al., 1995), αPS2 (inflated, if; 15A5α15A7; Wilcox et al., 1989), and αPS3 (scab, scb; 51E10; Stark et al., 1997; Grotewiel et al., 1998), represent three unique structural families of integrins. Phylogenetic analyses indicate that PS1- and PS2- but not PS3-type integrins are present in vertebrates and have diversified whereas the PS3-type integrins are the only class to show gene duplication in Drosophila (Hughes, 2001). These duplications include genes encoding two unstudied alpha chains, αPS4 (51E11; CG16827) and αPS5 (59E4; CG5372), where the αPS4 gene is adjacent to the 3’ end of scab (Adams et al., 2000). PS1 integrin (αPS1βPS) binds laminin (Gotwals et al., 1994) while PS2 integrin (αPS2βPS) binds RGD-containing ligands such as tiggrin (Fogerty et al., 1994), and the ligand for PS3 integrin (αPS3βPS) has not been well established. Genetic data and immunolabeling suggest laminin as one candidate, which is supported by the spreading of a fraction of PS3 integrin-expressing S2 cells (~20%) on plates coated with a fragment of Drosophila α1,2 laminin (Schock and Perrimon, 2003; Narasimha and Brown, 2004).
Studies in Drosophila have utilized its advanced genetics to answer questions regarding the role of integrins during morphogenetic processes in embryonic development (Schock and Perrimon, 2003; Narasimha and Brown, 2004; Levi et al., 2006; Wada et al., 2006). In the ovary, genetic analyses demonstrate a requirement for both PS1 and PS2 integrins in basal actin polarization in the egg chamber’s follicular epithelium as loss of function mys, mew or if clones resulted in short, rounded eggs (Duffy et al., 1998; Bateman et al., 2001). Other published work has focused on the functional significance of PS1 and PS2 integrins interacting with the ECM in the basement membrane of the follicle cell epithelium (Schotman et al., 2008; Fernández-Miñán et al., 2007; Schneider et al., 2006).
Examination of integrin chains in the well-defined egg chamber model should provide insight into how tissues coordinate expression and localization of multiple integrin chains during a developmental program. In the current study, we have utilized immunohistochemistry, in situ hybridization, clonal analyses, and RNAi to examine the subcellular localization, tissue-level spatial expression, and functional roles of alpha integrin subunits in the latter half of Drosophila oogenesis when the major tissue remodeling occurs via cell migrations and morphology changes. We confirm a recent report that βPS is localized to lateral and apical membranes of follicle cells in addition to their basal membranes (Fernández-Miñán et al., 2007) and extend this result to show that αPS1, αPS2 and αPS3 localize to these membranes in a developmentally dynamic way, suggesting novel roles of integrins during oogenesis. We also provide evidence that multiple alpha subunits cooperate to establish the overall size of the mature egg.
RESULTS AND DISCUSSION
Apical, Lateral, and Basal βPS, αPS1 and αPS2 During Oogenesis
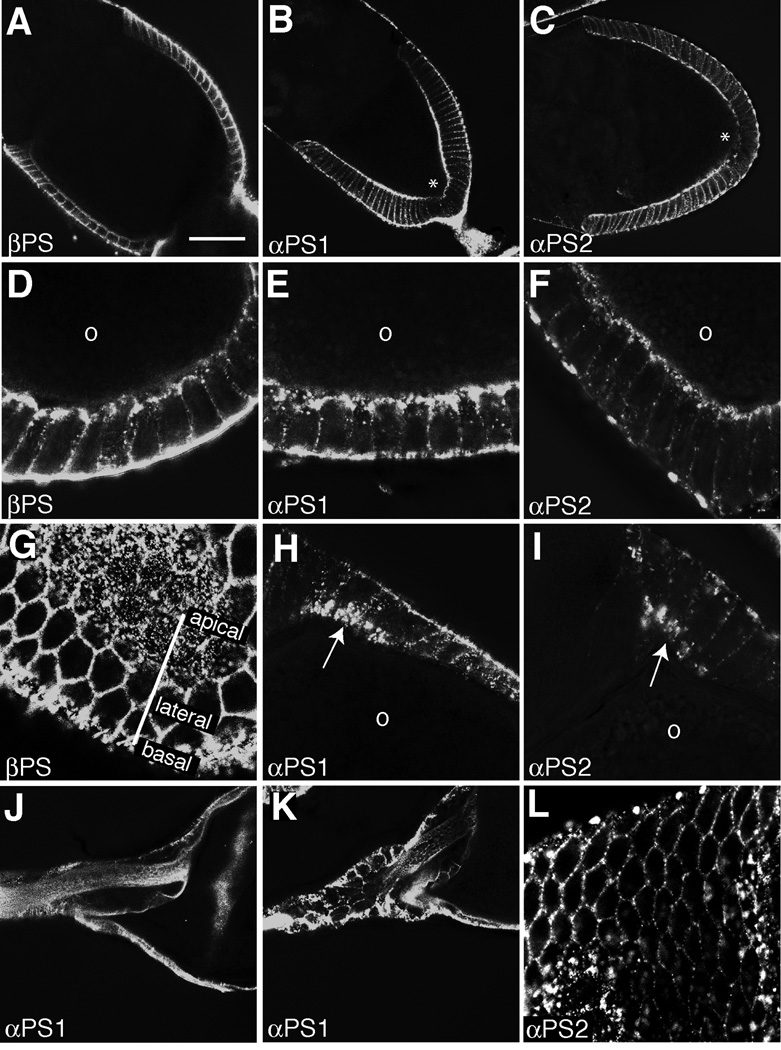
Monoclonal antibodies available through the Developmental Studies Hybridoma Bank allow examination of βPS, αPS1 and αPS2 integrin protein localization (Brower et al., 1984). Using a standard protocol for formaldehyde fixation and immunostaining, we expected to see strong staining of βPS at the basal side of the follicle cells (Bateman et al., 2001), and possibly no staining of αPS1 and αPS2 as these monoclonals were characterized as aldehyde-sensitive (Brower et al., 1984). However, we found significant localization of βPS along lateral and apical follicle cell membranes in addition to basal membranes in egg chambers through Stage 10A (Fig. 2A–D), which was verified by clonal analysis of the mysM2 null allele (Fig. 2E). Migratory border cells also expressed βPS protein at intercellular junctions (Fig. 2C; Llense and Martin-Blanco, 2008). The basolateral staining was prominent and punctate in character, while the apical staining was more dynamic, expressed evenly through early oogenesis and peaking in Stages 9 and 10A columnar follicle cells overlying the oocyte while downregulating in the most posterior follicle cells (Fig. 2C). After Stage 10A, the remaining follicle cell populations began downregulating apically localized βPS, while maintaining lateral and basal localization during dorsal appendage morphogenesis (Fig. 2E–G). Neither βPS nor alpha subunits were detected in the germline. These results confirm observations on the lateral (Pinheiro and Montell, 2004) and apical localization of βPS (Fernández-Miñán et al., 2007; discussion in Denef et al., 2008). Similar staining was observed in mid-oogenesis using the αPS1 antibody (Fig. 2H–K), while the αPS2 antibody showed no reactivity with formaldehyde-fixed tissue (not shown). Later in oogenesis, during the elongation of the oocyte and dorsal appendages, the αPS1 antibody showed little or no staining in the forming dorsal appendages in contrast to continued strong basolateral βPS staining of these follicle cells (Fig. 2F,K).
Figure 2. βPS and αPS1 are dynamically localized to apical, lateral, and basal follicle cell membranes in fixed tissue.
All images are oriented with anterior to the left. (A-G) βPS integrin was visualized with monoclonal antibody CF.6G11 in Stage 6 (A), Stage 9 (C,D), Stage 10A (B), Stage 10B (E,G), and Stage 13 (F) egg chambers. Arrows (A,C,D) indicate apical membrane localization. Arrowheads indicate basal (A,C,D,G) or lateral (F) membrane localization. Asterisks (C) denote downregulation of apical integrin. Presence of GFP (E) marks βPS-null clones. Strong staining between forming dorsal appendages (F) is of stretch cells engulfing nurse cell remnants. (H-K) αPS1 integrin was visualized with monoclonal antibody DK.1A4 in Stage 10B (H-J) and Stage 13 (K) egg chambers. Arrows (H) indicate lateral membrane localization. Scale bars = 20 µm.
To further explore the lateral and apical staining patterns, we turned to antibody staining of unfixed, unpermeabilized ovaries followed by fixation and dissection of egg chambers (based on Brower et al., 1984). Under these conditions, all three antibodies exhibited robust and specific (as verified by clonal analyses; not shown) staining of follicle cell membranes (Fig. 3A–C). Apical staining, especially of βPS and αPS1, was particularly prominent at the apicolateral border, but punctate staining could be seen along the entire apical surface in follicle cell cross-sections (Fig. 3D–F) and in a tangential section showing an apical lawn of punctae (Fig. 3G). Consistent with βPS staining (Fig. 2C), both apical αPS1 and αPS2 were downregulated in the most posterior follicle cells during Stage 9 (Fig. 3B–C, asterisk). Temporally, apical staining peaked at Stage 9 and then was greatly diminished in Stage 10B except in dorsal anterior patches where the dorsal appendage primordia are formed at this stage (Fig. 3H–I). Basolateral staining of αPS1 persisted in dorsal appendage-forming follicle cells at later developmental stages, but was diminished in intensity compared to Stages 9 and 10 and often entirely absent (Fig. 3J,K). The presence of αPS1 and αPS2 is consistent with published data demonstrating that large clones of either mew or if resulted in short, rounded eggs (Bateman et al., 2001). Schotman and colleagues (2001) also examined αPS1 by immunostaining and fluorescent in situ hybridization and found strong basolateral staining during epithelial remodeling but noted that αPS2 was not expressed by follicle cells. This discrepancy with our data may lie in the fact that, although αPS2 was not required for maintainence of the follicular epithelium as a monolayer (Fernández-Miñán et al., 2007), these authors were using aldehyde-fixed tissue when probing for αPS2. Indeed, we were also unable to detect αPS2 under similar conditions, consistent with the αPS2 antibody being strongly aldehyde-sensitive (Brower et al., 1984) while our αPS2 results with unfixed tissue are consistent with both αPS1 and βPS staining.
Figure 3. βPS, αPS1, αPS2 immunolocalization in unfixed egg chambers.
βPS integrin (A,D,G), αPS1 integrin (B,E,H,J,K), and αPS2 integrin (C,F,I,L) were singly labeled with monoclonal antibodies (CF.2C7 for αPS2) in Stage 9 (B-C), Stage 10A (A,D-F), Stage 10B (G-I,L) and Stage 13 (J,K) egg chambers. (G) Tangential section through the Stage 10B follicle cell epithelium demonstrating staining along lateral cell margins and in an apical lawn. (H, I) Cross-sections highlight apical integrin localization in dorsal, anterior dorsal appendage primordia. (J, K) Anterior end of late-stage egg chambers showing autofluorescence of chorion at high detector gain, but reduced (K) to absent (J) lateral, but no apical, staining of αPS1. Asterisks (B,C) denote downregulation of apical integrin. The position of the oocyte is labeled with a lower case ‘o’ in close-up images. Scale bar = 20 µm.
There is precedent for apical localization of integrins in epithelia, generally in cell types where the apical surface can contact other cells. For example, apical integrins are seen in the retinal pigment epithelium (RPE) in frogs (Chen et al., 1997) and mice (particularly αvβ5) where they are involved in circadian phagocytosis of shed outer segments of adjacent photoreceptor cells with which they closely interact (Nandrot et al., 2006; Finnemann et al., 1997). Vascular endothelial cells bear integrins on their luminal surfaces that are involved in functional interactions with plasma proteins and circulating cells (Conforti et al., 1992), and sea urchin blastulae also require apical integrins to organize their cortical actin network (Burke et al., 2000). In the fly Stage 9 and 10A egg chamber, apically localized integrins could be involved in interactions of the follicle cells with the oocyte or in assembly of the vitelline membrane (Spradling, 1993; Waring, 2000), while in Stage 10B and 11, they could be involved in interactions, shape changes, or movements occurring during formation of the epithelial tubes that will synthesize the dorsal respiratory appendages (Berg, 2005). Lateral distribution of βPS integrin has been noted in several developing epithelial tissues in Drosophila such as wing, gut, and salivary glands (Fristrom et al., 1993), although the binding partners for integrins in lateral cell-cell adhesion are not known. In various mammalian cell culture models, integrins were found to localize to sites of epithelial cell-cell contact (Yáñez-Mó et al., 2001; Lampugnani et al., 1991) though a clear role for integrins in epithelial cell-cell adhesion in vivo remains elusive. In two of the Drosophila epithelia, gut and wing, cells lacking integrin function become flattened or cuboidal rather than columnar (Newman and Wright, 1981; Domínguez-Giménez et al., 2007), implicating integrins in the maintenance of columnar morphology. A similar role could be played in the follicle cell epithelium overlying the oocyte, which establishes a columnar morphology in Stage 9 that has greatest height in Stage 10A and then flattens again in Stage 10B (Spradling, 1993). Indeed, it has been recently shown that newly synthesized basolaterally localized integrins are involved in the transition from columnar to cuboidal morphology in the follicular epithelium (Schotman et al., 2008). It would be interesting to see if vitelline membrane assembly or oocyte interactions are also affected in mys, mew, or if clones.
Upregulation of PS3-Family Alpha Integrin Chains During Follicle Cell Movements in Late Oogenesis
We had noted in these studies that the αPS1 and αPS2 chains were poorly expressed after Stage 10B, and data from previous clonal studies suggested that loss of βPS resulted in more severe defects in dorsal appendage formation than did loss of either αPS1 or αPS2 function (Duffy et al., 1998; Bateman et al., 2001). These observations suggested that other alpha chains might be functioning together with βPS in the morphogenesis of dorsal appendages. Thus, we next examined the expression of the remaining Drosophila alpha chains, αPS3, αPS4, and αPS5, using RNA in situ hybridization.
In contrast to αPS1 and αPS2 mRNA expression at low levels in early egg chambers (not shown), we were unable to detect any PS3-family mRNAs until mid-oogenesis when αPS3 mRNA appeared in border cells and in stretch cells at stage 10A (Fig. 4A). The αPS3 mRNA was also strongly expressed at Stages 10B and 11 in two dorsal patches at the anterior end of the oocyte, corresponding to the cells that will form the dorsal appendages (Fig. 4C; Ward and Berg, 2005) and persisted through Stage 13 (Fig. 4E). Thus, the expression of αPS3 is limited to specific sub-populations of follicle cells. The αPS5 mRNA was expressed in all follicle cells overlying the oocyte during Stages 11 and 12, with the most intense staining developing in Stage 12 in cells covering the anterior end of the oocyte and in cells forming the dorsal appendages (Fig. 4B,D). After completion of oocyte enlargement, αPS5 mRNA in main body follicle cells was downregulated at Stage 13 but strongly persisted in the dorsal appendage forming cells (Fig. 4F). The αPS4 mRNA was not strongly expressed, in contrast to the adjacent αPS3 gene, and could only be weakly detected during Stages 12 and 13 in follicle cells at the anterior end of the oocyte that form the operculum and micropyle (Fig. 4G,H).
Figure 4. mRNA expression of the αPS3, αPS4, and αPS5 integrin chains during late oogenesis.
Antisense labeling of αPS3 (A,C,E), αPS4 (G,H), and αPS5 (B,D,F) mRNA in Stage 10A (A), Stage 11 (B,C), Stage 12 (D), and Stage 13 (E-H) egg chambers. Except for a dorsal view in C, all other images are lateral views. Arrows (A,C) denote staining of stretch cells. Asterisk (A) denotes staining of border cells. Scale bar = 20 µm.
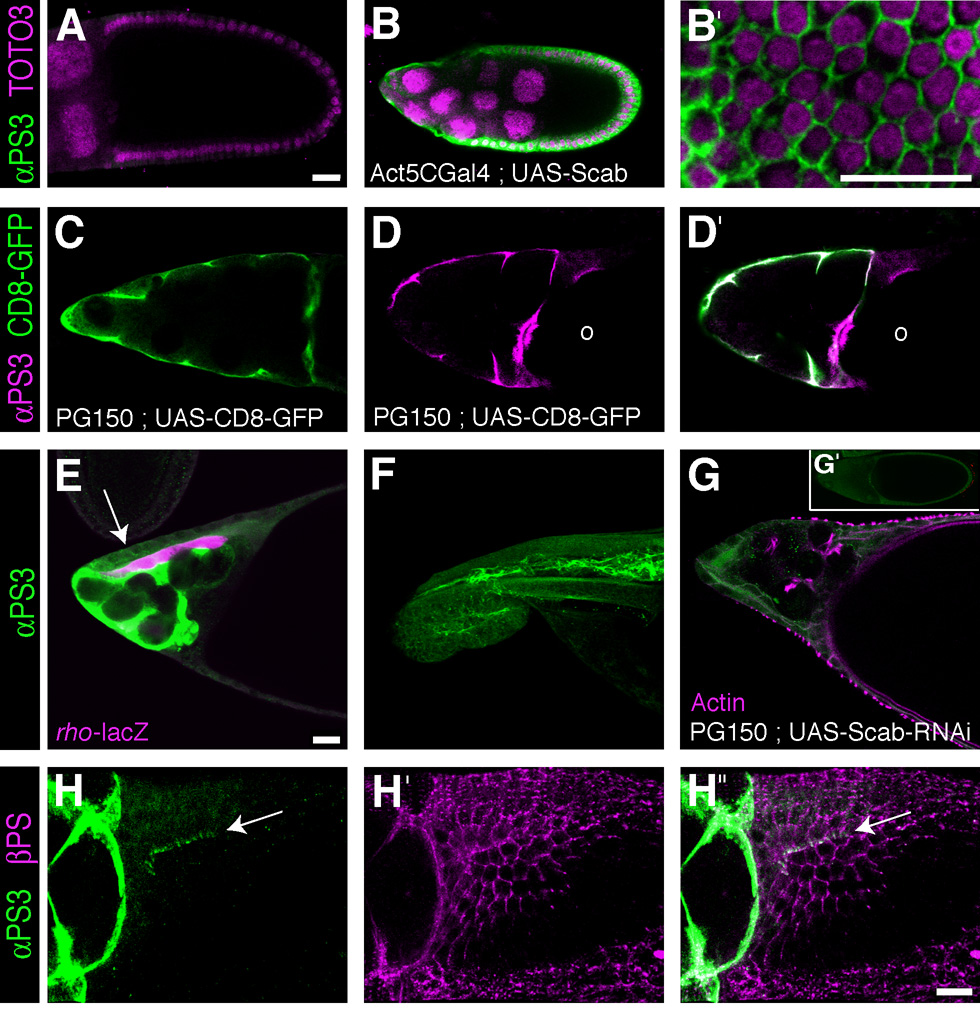
We were able to further examine the expression and localization of the αPS3 chain in the follicle cell epithelium using an antibody raised against a C-terminal peptide common to both αPS3 splice isoforms (Wada et al., 2007). Using this antibody, we could not detect αPS3 protein in early wild-type egg chambers through Stage 10A (Fig. 5A), consistent with mRNA expression slightly preceding protein translation. We confirmed the reactivity of the antibody with αPS3 by staining early egg chambers expressing UAS-scb in all follicle cells. This overexpressed αPS3 was enriched at basolateral but not apical membranes during Stages 9 and 10A egg chambers (Fig. 5B, C), similar to the lateral staining seen for endogenous βPS, αPS1 and αPS2 chains at these stages (Fig. 2,Fig. 3).
Figure 5. αPS3 protein is detected in stretch cells, border cells, and in dorsal appendage primordia.
Colored text corresponds to the respective staining in images as labeled. Genotypes are indicated by white text. (A) A Stage 10A wild-type egg chamber. (B) A Stage 9 egg chamber expressing a scb cDNA. (C,D) A Stage 10A (C) and early Stage 11 (D) egg chamber showing expressing a plasma membrane localized GFP in the stretch cells. In Stage 10A (A,E) there is no staining of stretch cell membranes with anti-αPS3, while this staining is strongly upregulated in Stage 10B (not shown) and 11 (D’). (E) An early Stage 13 egg chamber undergoing dorsal appendage elongation. Anti-lacZ marks rhomboid-expressing floor cells (rho-lacZ), and arrow points to weak staining of lateral membranes of roof cells with anti-αPS3. (F) A late Stage 13 egg chamber in which nurse cells have almost completely degenerated; stretch cells lying between the forming dorsal appendages still stain strongly with anti-αPS3. (G) Stage 13 and Stage 11 (inset) egg chambers expressing scb RNAi in stretch cells show strongly reduced antibody staining in these cells. (H) Confocal projection of the left dorsal appendage primordium in an early Stage 11 egg chamber illustrating apical localization of αPS3βPS integrin. Scale bar = 20 µm.
In later stages, αPS3 immunostaining was detected in three subsets of cells. At Stages 10B and 11, the primordial dorsal-appendage cells exhibited apical localization of αPS3 protein (Fig. 5D), similar to what was seen for aPS1 and aPS2 (Fig. 3H, I), and also had a diffuse punctuate cytoplasmic staining that was not present in follicle cells immediately posterior to the roof cells or on the ventral side of the oocyte. Although the αPS3 mRNA in situ hybridization pattern was most consistent with expression in the entire roof cell population that will form the dorsal portion of the outgrowing tubes (Ward and Berg, 2005), the antibody staining pattern was strongest along the anterior and medial margins of the dorsal appendage primordia (Fig 5H), co-localizing with βPS protein, which was present on all cell membranes (Fig 5H”). It is possible that αPS3 mRNA is selectively translated or its protein membrane-localized in roof cells that are in direct contact with the adjacent floor cells that form an anterior and medial hinge shape at the margin of each appendage primordium and migrate under the roof cells in the center of each dorsoanterior patch to form the base of the outgrowing tubes (Ward and Berg, 2005). Later, in Stages 12 and 13 egg chambers, weak staining was detected at the lateral membranes of cells surrounding the outgrowing dorsal appendages (Fig. 5E).
Other anterior cell types also upregulate expression of αPS3, including border cells, stretch follicle cells and possibly centripetal follicle cells (Horne-Badovinac and Bilder, 2005). Using the stretch cell-specific l(1)3AtPG150 Gal4 driver (Bourbon et al., 2002), we verified that αPS3 protein is absent at Stage 10A (Fig. 5C) and begins to appear during Stage 10B (Fig. 5D), co-localizing with UAS-CD8-GFP. This staining persisted as stretch cells enveloped the individual nurse cells that are dumping their contents into the oocyte (Tran and Berg, 2003) until the end of oogenesis (Fig. 5E,F). Finally, in some but not all Stage 13 egg chambers, staining could also be detected in a posterior patch of follicle cells overlying the oocyte (not shown), consistent with the mRNA expression pattern (Fig. 4E). To verify that the αPS3 immunostaining was specific, we expressed UAS-scb RNAi in stretch cells with the l(1)3AtPG150 driver and saw much reduced labeling of stretch cell membranes (compare Figs. 5D and E with G and G’ inset).
Together these observations show that two of the PS3-family alpha chains, αPS3 and αPS5, are strongly upregulated in late oogenesis and particularly within follicle cells contributing to formation of specialized anterior eggshell structures. Many of these cells are undergoing cell shape changes and migrations, and it is possible that this class of integrins is specialized for signaling required for coordinated cell movements or for interaction with matrix substrates used in the course of these movements. Indeed, embryonic cell migrations during germband extension, dorsal closure, and organization of pericardial cells require the αPS3 chain, although the molecular mechanisms and specificity underlying αPS3 involvement in migration are not understood (Stark et al., 1997; Narasimha and Brown, 2004). Switches from integrins promoting stable attachment to integrins promoting cell migration are well-established for epithelial-to-mesenchymal transitions of mammalian cancer cells (Hood and Cheresh, 2002; Maschler et al., 2005), and can be promoted by growth factors such as TGF-β and IGF-1 (Heino and Massague, 1989; Marelli et al., 2006). In the Drosophila egg chamber, the Notch and Dpp signaling pathways regulate gene expression and cell behavior in anterior follicle cells in late oogenesis (Twombly et al., 1996; Dobens et al., 2005), and could be involved in class switching of integrins through upregulation of PS3-family alpha integrin chains in these cells.
PS3-Family Integrins Make a Small Contribution to Overall Egg Chamber Size and Border Cell Migration but are Dispensable for Major Morphogenetic Programs
To experimentally verify a requirement for αPS3 or αPS5 in dorsal appendage morphogenesis, we generated clones in the follicular epithelium using functional null alleles for scb and αPS5 (see Experimental Procedures). In egg chambers containing large scb clones, the dorsal appendage primordia appeared to be normally patterned, one on each side of the dorsal midline consisting of broad-expressing roof cells (Fig. 6C’) and rhomboid-expressing floor cells (Fig. 6C”) and capable of completing their morphogenetic program described elsewhere in detail (Fig. 6B; Ward and Berg, 2005). Egg chambers containing a majority of scb−null follicle cells underwent normal early development with respect to major cell movements (Fig. 6A,B) but were found to be about 10% shorter along their A-P axis length than egg chambers without large scb clones (Table 1).
Figure 6. scb in stretch cells plays a role in egg axis length but is dispensable for dorsal appendage morphogenesis.
Colored text corresponds to the respective staining in images as labeled. In C, the colored text refers to the white color in each respective images (C’’’, merge) Genotypes are indicated by white text. Genotype in A (stage 10A) applies also to B (Stage 13) and C (Stage 11). (A-C) Egg chambers containing scb loss of function clones marked by absence of GFP. Egg chambers in A and B contained GFP-positive cells in other optical sections (not shown). The small green dots in C, C’, and C’’ outline the GFP-containing clone and is represented in C’’’ by white dots. (D) A Stage 11 egg chamber containing a large clone with loss of scb and αPS5. (E) Bar graph representing the mean ± standard deviation A-P axis egg length from flies expressing stretch cell-specific scb or mys RNAi. Different superscripts (a,b) denote statistical significance (p<0.01; see Experimental Procedures).
Table 1.
A-P axis length of laid eggs with PS3-family integrin loss of function.
| Genotype | Egg length ± std dev, µm (n) | Statistical differences, p<0.01* | |
|---|---|---|---|
| 1 | hsFLP ; RT42B scb / FRT42B hGFP | 478.1 ± 24.1 (70) | 2, 3, 5–10, 12 |
| 2 | hsFLP ; FRT42B scb / CyO | 517.6 ± 18.0 (70) | 1, 3, 4, 9, 11 |
| 3 | Act5C-Gal4 / CyO | 531.5 ± 20.0 (33) | 1, 2, 4–8, 12 |
| 4 | Act5C-Gal4 / + ; UAS-αPS4RNAi | 489.6 ± 28.0 (33) | 2, 3, 6, 9, 10, 12 |
| 5 | dmPG45 / + ; UAS-αPS4RNAi | 503.7 ± 20.6 (33) | 1, 3, 9, 11 |
| 6 | FM7 / + ; UAS-αPS4RNAi | 515.3 ± 15.2 (33) | 1, 3, 4, 11 |
| 7 | Act5C-Gal4 / + ; UAS-αPS5RNAi | 507.1 ± 15.6 (33) | 1, 3, 11 |
| 8 | dmPG45 / + ; UAS-αPS5RNAi | 500.9 ± 16.0 (33) | 1, 3, 9, 11 |
| 9 | FM7 / + ; UAS-αPS5RNAi | 523.9 ± 15.3 (33) | 1, 2, 4, 5, 8, 11 |
| 10 | dmPG45/ FM7 | 516.1 ± 13.7 (33) | 1, 4, 11 |
| 11 | hsFLP ; FRT42B scb αPS5 / FRT42B hGFP | 479.44 ± 18.4 (38) | 2, 3, 5–10, 12 |
| 12 | hsFLP ; FRT42B scb αPS5/ CyO | 509.82 ± 14.4 (38) | 1, 3, 4, 11 |
Denotes genotypes (by row number) producing eggs with statistical differences at p<0.01 for the Bonferroni, Tukey-Kramer, and Kruskal-Wallis multiple comparisons tests. For example, the genotype in row 10 differs statistically from the genotypes in rows 1, 4, and 11.
To further address the functional roles of PS3-family integrin alpha subunits during oogenesis, we expressed RNAi in the follicular epithelium using constitutive drivers (Act5C-Gal4 and dmPG45). Expression of UAS-scbRNAi was lethal under these drivers in addition to hsp70-Gal4 following two consecutive heat shocks, indicating that scb function is essential for survival of adults. Lethality was never observed following heat shock of parent stocks (hsp70-Gal4 and UAS-scbRNAi homozygotes). However, flies expressing UAS-αPS4 and αPS5 RNAi under either constitutive driver were viable, fertile, and lacked any obvious morphogenetic defects (Dinkins and LeMosy, unpublished data). Egg chambers were grossly normal with some minor reduction in the mean length of the egg’s A-P axis, consistent with results from eggs containing scb clones as well as published data for the requirement for PS1 and PS2 integrins in egg elongation (Bateman et al., 2001), though loss of PS4 and PS5 integrins only resulted in a 5–10% reduction in egg length. Table 1 contains the mean value of A-P axis length of laid eggs containing scb and αPS5 clones and expressing αPS4 and αPS5RNAi and genotype controls. For a particular PS3-family alpha chain, Gal4-mediated expression of the respective RNAi construct resulted in females producing shorter eggs (p<0.01, Table 1) than parent stocks for all lines tested except females expressing Act5C-Gal4>UAS-αPS5 RNAi, which is a weaker driver of RNAi in the follicular epithelium than dmPG45 (Fakhouri et al., 2006). Consistent with a minor role for PS5 integrin, late stage egg chambers containing large clones doubly mutant for both scb and aPS5 were not significantly different from egg chambers containing only large scb clones. We conclude that the PS3-family integrins, particularly αPS3, in the follicular epithelium have a minor contribution to the oocyte A-P length.
Given its expression in stretch cells and border cells (Fig. 4A, Fig. 5D–H), we hypothesized that RNAi knockdown of scb would respectively result in egg chambers of reduced length and altered migration of border cells. We expressed UAS-mysRNAi and UAS-scbRNAi in stretch cells under control of l(1)3AtPG150 and observed a modest but significant decrease in A-P axis length of laid eggs in each compared to genotype controls (Fig. 6E; p<0.01). The mean value obtained for loss of function of PS3 integrin in stretch cells was similar to the mean of the population of eggs carrying large scb clones. These results suggest that stretch cell integrins are involved in the process of nurse cell dumping (Wheatley et al., 1995), possibly by contributing to squeezing of the nurse cells as the stretch cells envelop them. Alternatively, it is possible that compromised adhesion of stretch cells to their basement membrane or to the enveloped nurse cells is required for optimum organization of the basal actin network in the main body follicular epithelium (Bateman et al., 2001), though a mechanism whereby this could occur is not clear.
To address the role of PS3 integrin in border cell migration (Fig. 4A, 5D), we expressed UAS-mysRNAi and UAS-scbRNAi in border cells using slbo-Gal4 with and without UAS-puc2A, which encodes active JNK phosphatase and downregulates signaling through the JNK pathway (Martin-Blanco et al., 1998). Loss of mys (Fig. 7D–F) or scb (Fig. 7G–I) resulted in border cells lagging behind the most posterior main body follicle cells migrating toward the oocyte whereas control border cells only expressing GFP were either ahead (Fig. 7B) or parallel with the trailing edge of the posteriorly migrating follicle cells (Fig. 7A). At Stage 10A, border cells should have completed their migrations at the anterior face of the oocyte (Fig. 7C). Occasionally, one or two border cells expressing integrin RNAi would dissociate from the cluster and be left behind (Fig. 7I). We conclude that integrins located at cell-cell contacts in border cells (Pinheiro and Montell, 2004; Llense and Martin-Blanco, 2008) are required for timely migration. Consistent with published data, expression of UAS-puc2A in border cells resulted in loss of cluster integrity (Llense and Martin-Blanco, 2008) where cells covered a larger surface area and often extended long processes during the migratory period (Fig. 7J–L). However, border cells expressing Puc2A with or without integrin RNAi were able to complete their slower migration and contribute to micropyle formation (data not shown). Expression of UAS-mysRNAi or UAS-scbRNAi in border cells also expressing UAS-puc2A produced a range of results. In some instances, border cells with compromised JNK signaling and integrin deficiency were severely lagging in onset of migration (Fig. 7M,P) and characterized by loss of cell-cell adhesion in the cluster (Fig. 7O,R). In other egg chambers, the border cells appeared to migrate in a morphologically normal cluster with proper timing (Fig. 7N,Q). This discrepancy could simply be the result of variation in knockdown of integrins by RNAi and/or timely expression of the puc2A transgene. Our results suggest that PS3 integrin plays a role in timely border cell migration and that its activity is influenced by JNK signaling. Consistent with these observations, it was recently shown that JNK signaling positively regulates expression of scb as well as βPS protein levels during dorsal closure of Drosophila embryonic epidermis (Homsy et al., 2006) and that the zippering of these cells is negatively affected in both JNK and scb loss of function mutants (Wada et al., 2006). In border cells expressing UAS-puc2A, we were unable to detect αPS3 protein using the available antibody (not shown). Other studies in Drosophila support a model where integrin signaling is integrated through JNK signaling during wing development (Kadrmas et al., 2004; Lee et al., 2003), which are consistent with studies of various cell culture models (Cui et al., 2006; Liu-Bryan et al., 2005; Katsumi et al., 2005).
Figure 7. Loss of αPS3βPS integrin in border cells delays migration and disrupts cluster integrity upon downregulation of JNK signaling.
Stage 9 (A,B,D,E,G,J,M,P,Q) and Stage 10A (C,F,H,I,K,L,N,O,R) egg chambers all expressing slbo-Gal4, UAS-GFP (green), which labels border cells, posterior polar cells, and follicle cells at the anterior end of the oocyte. UAS-transgenes and RNAi expressed in border cells for each row are indicated on the left image. Magenta pseudocolored counterstains are shown only to visualize other cell positions: Texas-Red Phalloidin (A,B,C,J,M,P) and Anti-Armadillo (Drosophila β-catenin; D,E,F,G,H,I,K,L,N,O,Q,R). Yellow bars (A,D,F) indicate relative position of border cells to anterior-most migrating follicular epithelium. Note how border cells expressing mys or scb RNAi appear to migrate relatively slowly and can lose cell-cell contacts (I); loss of cell-cell contacts and separation can be more prominent with co-expression of UAS-puc2A (O,R - both z-projections to illustrate absence of long GFP-filopodia).
EXPERIMENTAL PROCEDURES
Fly Stocks and Genetics
We used Oregon R for wild-type integrin staining patterns and mRNA in situ hybridization, and the following Gal4 insertion lines to drive transgenes and RNAi: P{GawB}l(1)3AtPG150, P{GawB}dmPG45, tubP-Gal4, Act5C-Gal4, hsp70-Gal4, and slbo-Gal4. We obtained the following lines from the Vienna Drosophila RNAi Center: UAS-mysRNAi, UAS-scbRNAi, UAS-αPS4 RNAi, UAS-αPS5 RNAi (Dietzl et al., 2007). Mutant and transgenic lines used were mysM2 (null, Januzzi et al., 2002), mewM6 (null, Brower et al., 1995), ifB2 (null, Brabant and Brower, 1993), scb2 (loss of function, Tearle and Nüsslein-Volhard, 1987), P{lacW};l(2)SH1114SH1114 (lethal insertion in the first coding exon of the αPS5 gene from the Szeged Drosophila Stock Centre; Oh et al., 2003), UAS-scab (Wada et al., 2006), rho-lacZ (Ward and Berg, 2005), and UAS-puc2A (active form of JNK phosphatase, Martin-Blanco et al., 1998), UAS-CD8-GFP, and UAS-GFPS65T.
To produce scb and scb, aPS5 follicle cell clones, we recombined chromosomes bearing the integrin lesions with FRT42B and generated yw hsFLP ; FRT42B ‘integrin allele’ / FRT42B GFP.nls. Females were heat shocked twice a day for 60 minutes, for 4 consecutive days at 37°C, and egg chambers were dissected for immunohistochemistry. Mutant integrin clones were noted by the absence of GFP. To produce mys, mew, and if clones, we generated flies of the genotype ‘integrin allele’ FRT19A / UAS-GFPRNAi FRT19A ; UAS-GFPS65T, hsFLP / + ; tubP-Gal4 / + (Levi et al., 2006). Integrin null clones were noted by the presence of GFP.
Immunohistochemistry
Ovaries from Oregon R (OR) females were fixed in 6% formaldehyde in devitellinizing buffer (Verheyen and Cooley, 1994) for 10 minutes under a layer of heptane, blocked with 2.5% normal goat serum in PBS containing 0.5% BSA and 0.3% Triton X-100 (PBT-N), and then incubated with primary antibodies in PBT-N at 4°C (15–18 h). Ovaries were then incubated with secondary antibodies in PBT-N (2 h) at room temperature, washed, and mounted in an antifade solution. For immunostaining of unfixed ovaries, all incubations were at 4°C. Ovaries were incubated 15 min at with 5% normal goat serum in Ringer’s buffer (Verheyen and Cooley, 1994) followed by primary antibody in the same solution overnight. Following 5 washes over 30 minutes in cold buffer, ovaries were incubated with secondary antibodies for 1 h. Following another 5 washes, ovaries were fixed as described above, washed in PBS, and mounted in antifade solution.
The rabbit anti-αPS3 antibody was kindly provided by Dr. Shigeo Hayashi (Wada et al., 2007) and used at a dilution of 1:1000. Monoclonal anti-Broad, anti-Armadillo, anti-βPS (CF.6G11), anti-αPS1 (DK.1A4), and anti-αPS2 (CF.2C7) supernatants (DSHB; Brower et al., 1984) were diluted 1:10 for primary incubation. Anti-LacZ (Rockland Immunochemicals) was used at a dilution of 1:500. Secondary antibodies, including Alexa488- and 546-conjugated goat anti-mouse IgG (Jackson ImmunoResearch), Alexa-488-conjugated anti-rabbit IgG (Molecular Probes), and Cy5-conjugated anti-rabbit IgG (Jackson Immunochemicals) were used at a dilution of 1:200. Texas-Red phalloidin was used to label actin during the secondary incubations.
Whole-mount egg chambers were viewed with a Zeiss LSM510 confocal laser scanning microscope in the MCG Cell Imaging Core Facility. The confocal pinhole was set to 1 µm, and images were acquired with a CCD camera and processed with the Zeiss LSM image examiner and Adobe Photoshop. Measurement of Stage 13 egg chambers containing large clones was performed using the Zeiss LSM image examiner software.
RNA in situ hybridization
A scab cDNA (LP12257) encoding the S isoform of αPS3 was obtained from the Drosophila Genomics Resource Center. This cDNA differs from the L isoform scab cDNA by only 370 bp (of 4294 bp) and in situ probes will hybridize with both isoforms. Exons 1 and 2 of αPS4 and αPS5, respectively, were cloned from OR genomic DNA and inserted into the pBSII-SK- vector. Templates were linearized with appropriate restriction enzymes, and digoxygenin (DIG)-labeled sense and antisense RNA probes were synthesized using the MegaScript T3 and T7 kits, respectively (Ambion). In situ hybridization on paraformaldehyde-fixed OR egg chambers was performed at 56°C according to Lehmann and Tautz (Lehmann and Tautz, 1994). Detection was conducted with alkaline phosphatase-conjugated anti-DIG Fab fragments (Roche) preabsorbed against fixed egg chambers and NBT/BCIP (Roche). Egg chambers were examined with DIC optics at a Zeiss AxioSkop II microscope fitted with a Zeiss MRC5 camera. Images were acquired using AxioVision software and processed using Adobe Photoshop. Sense probes generally gave no signal or very light background in all cells, depending on color reaction development times.
Egg measurements and statistical analyses
Laid eggs from controls or females expressing RNAi constructs were placed on their lateral side and imaged with the Zeiss Axioskop 2 microscope with a 10X objective. Using the objective calibration and AxioVision software, we measured the distance from the micropyle to the posterior-most point of the eggshell. In Table 1, data are presented as mean length of the A-P axis ± standard deviation. Data were analyzed by one-way analysis of variance and differences among the groups were determined by both parametric (Bonferroni, Tukey-Kramer) and non-parametric (Kruskal-Wallis) tests as some of the data were not normally distributed. Differences at p<0.01 were considered to be significant, and statistical results among the three multiple comparison tests were identical with respect to genotypes showing significant differences.
ACKNOWLEDGMENTS
We thank Celeste Berg, Amin Ghabrial, Shigeo Hayashi, Mark Krasnow, Enrique Martin-Blanco, Denise Montell, Laura Nilson, Trudi Schüpbach, and Dave Stein, as well as the Vienna Drosophila RNAi Center and the Szeged and Bloomington Stock Centers, for graciously providing fly stocks and reagents. Anti-Armadillo, Anti-Broad, and the βPS, αPS1, and αPS2 monoclonal antibodies developed by the late Danny Brower were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of NICHD and maintained by the Department of Biological Sciences of The University of Iowa. The anti-αPS3 antibody was kindly provided by Shigeo Hayashi, and the scb cDNA was obtained through the Drosophila Genomics Resource Center. We thank Blaine Prescott for the artwork in Figure 1. V.M.F. received support from the MCG STAR undergraduate research program and M.B.D. received support from the MCG School of Graduate Studies and the American Heart Association.
Grant sponsor: NIH; Grant number: RO1GM067738
REFERENCES
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- Bateman J, Reddy RS, Saito H, Van Vactor D. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr Biol. 2001;11:1317–1327. doi: 10.1016/s0960-9822(01)00420-1. [DOI] [PubMed] [Google Scholar]
- Berg C. The Drosophila shell game: patterning genes and morphological change. Trends Genet. 2005;21:346–355. doi: 10.1016/j.tig.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Berman AE, Kozlova NI, Morozevich GE. Integrins: structure and signaling. Biochemistry (Mosc.) 2003;68:1284–1299. doi: 10.1023/b:biry.0000011649.03634.74. [DOI] [PubMed] [Google Scholar]
- Bourbon HM, Gonzy-Treboul G, Peronnet F, Alin MF, Ardourel C, Benassavag C, Cribbs D, Deutsch J, Ferrer P, Haenlin M, Lepesant JA, Noselli S, Vincent A. A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech Dev. 2002;110:71–83. doi: 10.1016/s0925-4773(01)00566-4. [DOI] [PubMed] [Google Scholar]
- Brabant MC, Brower DL. PS2 integrin requirements in Drosophila embryo and wing morphogenesis. Dev Biol. 157:49–59. doi: 10.1006/dbio.1993.1111. [DOI] [PubMed] [Google Scholar]
- Brower DL. Platelets with wings: the maturation of Drosophila integrin biology. Curr Opin Cell Biol. 2003;15:607–613. doi: 10.1016/s0955-0674(03)00102-9. [DOI] [PubMed] [Google Scholar]
- Brower DL, Bunch TA, Mukai L, Adamson TE, Wehrli M, Lam S, Friedlander E, Roote CE, Zusman S. Nonequivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila: genetic analysis of the alpha PS1 integrin subunit. Development. 1995;121:1311–1320. doi: 10.1242/dev.121.5.1311. [DOI] [PubMed] [Google Scholar]
- Brower DL, Wilcox M, Piovant M, Smith RJ, Reger LA. Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc Natl Acad Sci USA. 1984;81:7485–7489. doi: 10.1073/pnas.81.23.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RD, Murray G, Rise M, Wang D. Integrins on eggs: the betaC subunit is essential for formation of the cortical actin cytoskeleton in sea urchin eggs. Dev Biol. 2000;265:53–60. doi: 10.1016/j.ydbio.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Chen D, Roberts R, Pohl M, Nigam S, Kreidberg J, Wang Z, Heino J, Ivaska J, Coffa S, Harris RC, Pozzi A, Zent R. Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am J Physiol Renal Physiol. 2004;287:F602–F611. doi: 10.1152/ajprenal.00015.2004. [DOI] [PubMed] [Google Scholar]
- Chen W, Joos TO, Defoe DM. Evidence for beta 1-integrins on both apical and basal surfaces of Xenopus retinal pigment epithelium. Exp Eye Res. 1997;64:73–84. doi: 10.1006/exer.1996.0183. [DOI] [PubMed] [Google Scholar]
- Conforti G, Dominguez-Jimenez C, Zanetti A, Gimbrone MA, Jr, Cremona O, Marchisio PC, Dejana E. Human endothelial cells express integrin receptors on the luminal aspect of their membrane. Blood. 1992;80:437–446. [PubMed] [Google Scholar]
- Cui X, Kim HJ, Kuiatse I, Kim H, Brown PH, Lee AV. Epidermal growth factor induces insulin receptor substrate-2 in breast cancer cells via c-Jun NH(2)-terminal kinase/activator protein-1 signaling to regulate cell migration. Cancer Res. 2006;66:5304–5313. doi: 10.1158/0008-5472.CAN-05-2858. [DOI] [PubMed] [Google Scholar]
- Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Denef N, Chen Y, Weeks SD, Barcelo G, Schupbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14:354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dobens L, Jaeger A, Peterson JS, Raftery LA. Bunched sets a boundary for Notch signaling to pattern anterior eggshell structures during Drosophila oogenesis. Dev Biol. 2005;287:425–437. doi: 10.1016/j.ydbio.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Domínguez-Giménez P, Brown NH, Martin-Bermudo MD. Integrin-ECM interactions regulate the changes in cell shape driving morphogenesis of the Drosophila wing epithelium. J Cell Sci. 2007;120:1061–1071. doi: 10.1242/jcs.03404. [DOI] [PubMed] [Google Scholar]
- Duffy JB, Harrison DA, Perrimon N. Identifying loci required for follicular patterning using directed mosaics. Development. 1998;125:2263–2271. doi: 10.1242/dev.125.12.2263. [DOI] [PubMed] [Google Scholar]
- Fakhouri M, Elalayli M, Sherling D, Hall JD, Miller E, Sun X, Wells L, LeMosy EK. Minor proteins and enzymes of the Drosophila eggshell matrix. Dev Biol. 2006;293:127–141. doi: 10.1016/j.ydbio.2006.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Miñán A, Martin-Bermudo MD, Gonzalez-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr Biol. 2007;17:683–688. doi: 10.1016/j.cub.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty FJ, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Fristrom D, Wilcox M, Fristrom J. The distribution of PS integrins, laminin A and F-actin during key stages in Drosophila wing development. Development. 1993;117:509–523. doi: 10.1242/dev.117.2.509. [DOI] [PubMed] [Google Scholar]
- Gotwals PJ, Fessler LI, Wehrli M, Hynes RO. Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc Natl Acad Sci USA. 1994;91:11447–11451. doi: 10.1073/pnas.91.24.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Heino J, Massague J. Transforming growth factor-β switches the pattern of integrins expressed in MG-63 human osteosarcoma cells and causes a selective loss of cell adhesion to laminin. J Biol Chem. 1989;264:21806–21811. [PubMed] [Google Scholar]
- Homsy JG, Jasper H, Peralta XG, Wu H, Kiehart DP, Bohmann D. JNK signaling coordinates integrin and actin functions during Drosophila embryogenesis. Dev Dyn. 2006;235:427–434. doi: 10.1002/dvdy.20649. [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Roles of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Bilder D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232:559–574. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- Hughes AL. Evolution of the integrin alpha and beta protein families. J Mol Evol. 2001;52:63–72. doi: 10.1007/s002390010134. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jannuzi AL, Bunch TA, Brabant MC, Miller SW, Mukai L, Zavortink M, Brower DL. Disruption of C-terminal cytoplasmic domain of betaPS integrin subunit has dominant negative properties in developing Drosophila. Mol Biol Cell. 2002;13:1352–1365. doi: 10.1091/mbc.01-08-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadrmas JL, Smith MA, Clark KA, Pronovost SM, Muster N, Yates JR, III, Beckerle MC. The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J Cell Biol. 2004;167:1019–1024. doi: 10.1083/jcb.200408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280:16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Dejana E, Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112:479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Cho KS, Kim E, Chung J. blistery encodes Drosophila tensin protein and interacts with integrin and the JNK signaling pathway during wing development. Development. 2003;130:4001–4010. doi: 10.1242/dev.00595. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Tautz D. In situ hybridization to RNA. In: Goldstein LSB, Fyrberg EA, editors. Drosophila melanogaster: Practical uses in cell and molecular biology. San Diego: Academic Press; 1994. pp. 576–598. [Google Scholar]
- Levi BP, Ghabrial AS, Krasnow MA. Drosophila talin and integrin genes are required for maintenance of tracheal terminal branches and luminal organization. Development. 2006;133:2383–2393. doi: 10.1242/dev.02404. [DOI] [PubMed] [Google Scholar]
- Liu-Bryan R, Pay S, Schraufstatter IU, Rose DM. The CXCR1 tail mediates beta1 integrin-dependent cell migration via MAP kinase signaling. Biochem Biophys Res Commun. 2005;332:117–125. doi: 10.1016/j.bbrc.2005.04.139. [DOI] [PubMed] [Google Scholar]
- Llense F, Martin-Blanco E. JNK signaling controls border cell cluster integrity and collective cell migration. Curr Biol. 2008;18:538–544. doi: 10.1016/j.cub.2008.03.029. [DOI] [PubMed] [Google Scholar]
- MacKrell AJ, Blumberg B, Haynes SR, Fessler JH. The lethal myospheroid gene of Drosophila encodes a membrane protein homologous to vertebrate integrin beta subunits. Proc Natl Acad Sci USA. 1988;85:2633–2637. doi: 10.1073/pnas.85.8.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manseau L, Baradaran A, Brower D, Budhu A, Elefant F, Phan H, Philip AV, Yang M, Glover D, Kaiser K, Palter K, Selleck S. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev Dyn. 1997;209:310–322. doi: 10.1002/(SICI)1097-0177(199707)209:3<310::AID-AJA6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Marelli MM, Moretti RM, Procacci P, Motta M, Limonta P. Insulin-like growth factor-1 promotes migration in human androgen-independent prostate cancer cells via the alphavbeta3 integrin and PI3-K/Akt signaling. Int J Oncol. 2006;28:723–730. [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martínez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- Menko AS, Philip Beta 1 integrins in epithelial tissues: a unique distribution in the lens. Exp Cell Res. 1995;218:516–521. doi: 10.1006/excr.1995.1186. [DOI] [PubMed] [Google Scholar]
- Metlapally R, Jobling AI, Gentle A, McBrien NA. Characterization of the integrin receptor subunit profile in the mammalian sclera. Mol Vis. 2006;12:725–734. [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Sircar M, Finnemann SC. Novel role for αvβ5-integrin in retinal adhesion and its diurnal peak. Am J Physiol Cell Physiol. 2006;290:C1256–C1262. doi: 10.1152/ajpcell.00480.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimha M, Brown NH. Novel functions for integrins in epithelial morphogenesis. Curr Biol. 2004;14:381–385. doi: 10.1016/j.cub.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Newman SM, Wright TRF. A histological and ultrastructural analysis of developmental defects produced by the mutation lethal(1) myospheroid, in Drosophila melanogaster. Dev Biol. 1981;86:393–402. doi: 10.1016/0012-1606(81)90197-4. [DOI] [PubMed] [Google Scholar]
- Oh SW, Kingsley T, Shin HH, Zheng Z, Chen HW, Chen X, Wang H, Ruan P, Moody M, Hou SX. A P-element insertion screen identified mutations in 455 novel essential genes in Drosophila. Genetics. 2003;163:195–201. doi: 10.1093/genetics/163.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro EM, Montell DJ. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development. 2004;131:5243–5251. doi: 10.1242/dev.01412. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Anton ES. Role of integrins in the development of the cerebral cortex. Cereb Cortex. 2003;13:219–224. doi: 10.1093/cercor/13.3.219. [DOI] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng W-M, Baumgartner S. Perlecan and dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock F, Perrimon N. Retraction of the Drosophila germ band requires cell-matrix interaction. Genes Dev. 2003;17:597–602. doi: 10.1101/gad.1068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotman H, Karhinen L, Rabouille C. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev Cell. 2008;14:171–182. doi: 10.1016/j.devcel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- Stark KA, Yee GH, Roote CE, Williams EL, Zusman S, Hynes RO. A novel alpha integrin subunit associates with betaPS and functions in tissue morphogenesis and movement during Drosophila development. Development. 1997;124:4583–4594. doi: 10.1242/dev.124.22.4583. [DOI] [PubMed] [Google Scholar]
- Tearle R, Nüsslein-Volhard C. Tübingen mutants stock list. DIS. 1987;66:209–226. [Google Scholar]
- Tran D, Berg CA. bullwinkle and shark regulate dorsal-appendage morphogenesis in Drosophila oogenesis. Development. 2003;130:6273–6282. doi: 10.1242/dev.00854. [DOI] [PubMed] [Google Scholar]
- Twombly V, Blackman RK, Jin H, Graff JM, Padgett RW, Gelbart WM. The TGF-β signalling pathway is essential for Drosophila oogenesis. Development. 1996;122:1555–1565. doi: 10.1242/dev.122.5.1555. [DOI] [PubMed] [Google Scholar]
- van Eeden F, St. Johnston D. The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis. Curr Opin Genet Dev. 1999;9:396–404. doi: 10.1016/S0959-437X(99)80060-4. [DOI] [PubMed] [Google Scholar]
- Verheyen E, Cooley L. Looking at Oogenesis. In: Goldstein LSB, Fyrberg EA, editors. Drosophila melanogaster: Practical uses in cell and molecular biology. San Diego: Academic Press; 1994. pp. 545–564. [Google Scholar]
- Wada A, Kato K, Uwo MF, Yonemura S, Hayashi S. Specialized extraembryonic cells connect embryonic and extraembryonic epidermis in response to Dpp during dorsal closure in Drosophila. Dev Biol. 2007;301:340–349. doi: 10.1016/j.ydbio.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Berg CA. Juxtaposition between two cell types is necessary for dorsal appendage tube formation. Mech Dev. 2005;122:241–255. doi: 10.1016/j.mod.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Waring GL. Morphogenesis of the eggshell in Drosophila. Int Rev Cytol. 2000;198:67–108. doi: 10.1016/s0074-7696(00)98003-3. [DOI] [PubMed] [Google Scholar]
- Wheatley S, Kulkarni S, Karess R. Drosophila nonmuscle myosin II is required for rapid cytoplasmic transport during oogenesis and for axial nuclear migration in early embryos. Development. 1995;121:1937–1946. doi: 10.1242/dev.121.6.1937. [DOI] [PubMed] [Google Scholar]
- Wilcox M, DiAntonio A, Leptin M. The function of PS integrins in Drosophila wing morphogenesis. Development. 1989;107:891–897. doi: 10.1242/dev.107.4.891. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M, Tejedor R, Rousselle P, Sánchez-Madrid F. Tetraspanins in intercellular adhesion of polarized epithelial cells: spatial and functional relationship to integrins and cadherins. J Cell Sci. 2001;114:577–587. doi: 10.1242/jcs.114.3.577. [DOI] [PubMed] [Google Scholar]
- Yee GH, Hynes RO. A novel, tissue-specific integrin subunit, beta nu, expressed in the midgut of Drosophila melanogaster. Development. 1993;118:845–858. doi: 10.1242/dev.118.3.845. [DOI] [PubMed] [Google Scholar]