Abstract
CD28 is required for the development of regulatory T cells (Tregs; CD4+CD25+Foxp3+) in the thymus and also contributes to their survival and homeostasis in the periphery. We studied whether and how CD28 and ICOS control the differentiation of Tregs from naive T cells. By using wild-type, CD28-, ICOS-, or CD28/ICOS-double knockout mice on C57BL/6 background as T cell sources, we found that CD28 is essential, whereas ICOS is dispensable, for the development and homeostasis of Tregs. Furthermore, the differentiation of Tregs from naive CD4+CD25− T cells in vivo also depends on CD28. The requirement of CD28 for Treg differentiation was mediated by IL-2, because neutralization of IL-2 with its specific mAb-blocked Treg differentiation from wild-type CD4+CD25− T cells and addition of IL-2 restored Treg differentiation from CD28−/− T cells. Other common γ-chain cytokines, IL-4, IL-7, or IL-15, do not share such a role with IL-2. Although CD28 is required for the differentiation of Tregs from naive T cells, already generated Tregs do not depend on CD28 to exert their suppressive function. Our study reveals a new aspect of CD28 function in regulating T cell response.
Among many types of regulatory T cells (Tregs)3, the naturally occurring CD4+CD25+ Tregs (nTregs) have been well characterized and have promising potential as immunotherapy for the induction of tolerance in autoimmunity or transplantation (1). The development of nTregs in the thymus requires Foxp3, a member of transcription factors characterized by their winged helix-forkhead DNA-binding domain (2). Although it is widely accepted that CD4+CD25+Foxp3+ Tregs developed in the thymus, accumulating evidence suggests that Tregs with an identical phenotype can be induced in the periphery from CD4+ non-Treg precursors under certain conditions. For example, all CD4+ cells from RAG−/− TCR transgenic (Tg) mice are CD25−, but a small proportion of these cells convert to a CD25+ Treg phenotype after adoptive transfer into Ag-bearing mice or mice that have been administered a tolerizing dose of peptide Ag (3, 4). De novo generation of CD4+CD25+ Tregs from CD4+CD25− cells also occurs in thymectomized mice, and B7 costimulation is required for this conversion (5). These Tregs induced in the periphery were also termed as induced Tregs (iTregs).
Evidence has emerged that the CD28 family members are not only critical costimulatory molecules but also crucial regulators of nTregs. The first clue to the critical role of the CD28 family in nTreg function was the observation that prevention of CD28 ligation with CTLA4-Ig exacerbated autoimmune disease in NOD mice (6). Mice deficient in CD28 or its ligands (B7, CD80, and CD86) have a substantially reduced number of nTregs (6, 7). As a consequence, NOD mice develop more rapid and severe autoimmune diabetes in CD28 knockout (KO) background as compared with CD28 wild type (WT). Recent studies clearly indicate that CD28 is essential for nTreg development in the thymus and for nTreg survival and homeostasis in the periphery (7, 8). Thus, although these mice are lacking a potent costimulation (CD28) for T effector cells, they are also lacking nTregs, the most effective mediators of self-tolerance, yielding a balanced deficit that results in the preservation of Ag-mediated activation (9). A potential role of CD28 in the generation of iTreg has not been rigorously investigated. Experimental evidence points out that CTLA4 or ICOS may play an important role in the effector function rather than development of nTregs (10–13).
Signals mediated by IL-2/IL-2R binding have been linked to the development, maintenance, survival, and expansion of Tregs (14). Consistent with critical involvement of IL-2 signals in nTreg biology, IL-2-, or IL-2R-deficient mice suffer from lymphoproliferative autoimmune disorders (15, 16). Furthermore, administration of neutralizing anti-IL-2 mAb to normal mice selectively reduces the number of nTregs in thymus and periphery, resulting in the development of organ-specific autoimmune disease (17). However, more recent studies firmly conclude that IL-2 is dispensable for the induction of Foxp3 expression and nTreg development in the thymus, but it is essential for the survival and homeostasis of nTregs in periphery (18, 19).
In the current study, we asked whether CD28 and ICOS have redundant functions on Tregs, and found that CD28 but not ICOS is critical for their generation and/or homeostasis. Our study focused on whether or how CD28 affects Treg generation in the periphery. We found that CD28 is critical for the generation of CD4+CD25+Foxp3+ Tregs from CD4+CD25− T cells both in vitro and in vivo. The requirement of CD28 in iTreg generation is IL-2 dependent, and other common γ-chain cytokines cannot replace this function of IL-2.
Materials and Methods
Mice
C57BL/6 (B6) and BALB/c mice were purchased from the National Cancer Institute. Foxp3gfp knock-in (KI) strain was obtained from Rudensky's laboratory at University of Washington (Seattle, WA) (19, 20). Ly5.1 congenic, CD28 KO, and B7 KO (CD80 and CD86 double deficient) on B6 background were purchased from The Jackson Laboratory. Founders of CD28 WT and mutation Tg mice on B6 background were provided by Dr. A. Singer at the National Cancer Institute (Bethesda, MD) (7). B6 ICOS KO strain was provided by C. Dong at the MD Anderson Cancer Center (Houston, TX) (21), and CD28/ICOS double KO mice by Dr. T. W. Mak at Advanced Medical Discovery Institute (Toronto, Canada) (22). These KO strains were bred and all mice used in this study were housed in H. Lee Moffitt Cancer Center and Research Institute. Experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee.
Reagents and Abs
Recombinant mouse IL-2, IL-4, IL-7, IL-15, and human TGF-β1 were purchased from R&D Systems. Anti-mouse CD3 (clone 145.2C11) and CD28 (clone 37.51) mAbs were produced and purified in our laboratory. Purified anti-mouse IL-2 (clone JES6−5H4), PE-conjugated anti-mouse/rat Foxp3 (clone FJK-16s) were purchased from eBioscience. FITC-, PE-, PE/CY7-, and APC- conjugated anti-CD4 or CD8 mAbs were purchased from BD Biosciences.
T cell purification and adoptive cell transfer
CD4+CD25− T cells were purified by negative selection using a magnetic cell separation system according to the manufacturer's protocol (Miltenyi Biotec) with the exception that biotin-labeled anti-CD25 mAb was added to the mixture to remove CD25+ cells. The purity of CD4+CD25− cells ranged from 85 to 95%, but the percentage of CD4+CD25+ cells was always less than 1% among total CD4+ cells. For adoptive cell transfer experiments, 7- to 8-wk-old B6 Ly5.1 mice were exposed to 500 cGy at a dose rate of 120 cGy/min, a dose that is immunosuppressive but not lethal. CD4+CD25− cells from B6 WT, CD28-KO, ICOS-KO, or CD28/ICOS KO donors were suspended in PBS and injected via the tail vein into the irradiated B6 Ly5.1 recipients.
In vitro generation of Tregs
CD4+CD25− T cells were seeded at 2.5 × 105/well in 48-well plates and stimulated with 0.5 μg/ml anti-CD3 mAb in the presence of 1.25 × 106 irradiated syngeneic T cell-depleted splenocytes as APCs, with or without 2 ng/ml TGF-β1. After stimulation for various times, cells were harvested for the measurement of Foxp3 expression. Student's t test was used to compare percentage of Foxp3+ generated from CD4+CD25− T cells under various conditions.
CFSE labeling and flow cytometry analysis
For measurement of proliferative response in vitro, T cells were labeled with CFSE (Molecular Probes) as described previously (23), stimulated, and the CFSE dilution in T cells was analyzed by flow cytometry. Two-, three-, or four-color flow cytometry was performed to measure the expression of surface molecules and intracellular Foxp3 according to the manufacturer's instruction (eBiosciences). Analysis was performed by using a FACScan or FACSCalibur and CellQuest (BD Biosciences) or FlowJo software (Tree Star).
Assay for Treg function
After the generation of Tregs with TGF-β1 in vitro, CD4+CD25high cells were isolated by enriching CD25+ cells using magnetic beads followed by FACS sorting of CD4+CD25high to reach 98−99% purity. To test the suppressive function, purified CD4+CD25− cells from B6 mice at 50 × 103/well were stimulated with 0.5 μg/ml anti-CD3 mAb in the presence of irradiated T cell-depleted splenocytes as APCs at 2.5 × 105/well. Graded numbers of CD4+CD25high cells in vitro generated from WT, ICOS, or CD28 KO CD4+CD25− T cells were added into the culture. CD4+CD25high cells isolated directly from B6 mice were used as positive controls. Proliferation was measured by [3H]thymidine incorporation after 3 days of culture.
Results
CD28 and ICOS affect the generation and homeostasis of Tregs in vivo
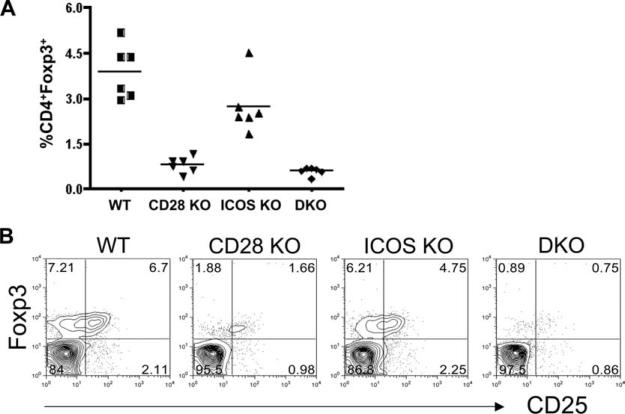
To determine the role of CD28 and ICOS in Treg generation and homeostasis, initial experiments were conducted to compare the percentage of CD4+Foxp3+ cells in WT, CD28-, ICOS-, and CD28/ICOS-KO mice. In the peripheral blood, the frequency of CD4+Foxp3+ cells was moderately reduced in ICOS KO (p = 0.03) but severely reduced in CD28 KO (p = 0.0001) as compared with WT mice. The frequency of CD4+Foxp3+ cells was not significantly different in CD28/ICOS KO and in CD28 KO mice (p = 0.06) (Fig. 1A). The data were confirmed in pooled spleen and lymph node cells (Fig. 1B), indicating that CD28 plays a critical role, but ICOS is less important, in the generation and/or homeostasis of Tregs.
FIGURE 1.
CD28 and ICOS affect the number of Tregs in vivo. A, Percentage of CD25+Foxp3+ cells is shown in gated PBMC from individual mouse of WT, CD28-, ICOS-, and CD28/ICOS-KO strains on B6 background. The data represent each of six mice per group from one of the two replicate experiments. B, Percentage of CD25+Foxp3+ cells in gated CD4+ cells are shown in pooled spleen and lymph nodes from B6 mice with indicated genotypes. The data show the expression pattern of one mouse per group that represents an average value of seven to nine mice in each group from three replicate experiments.
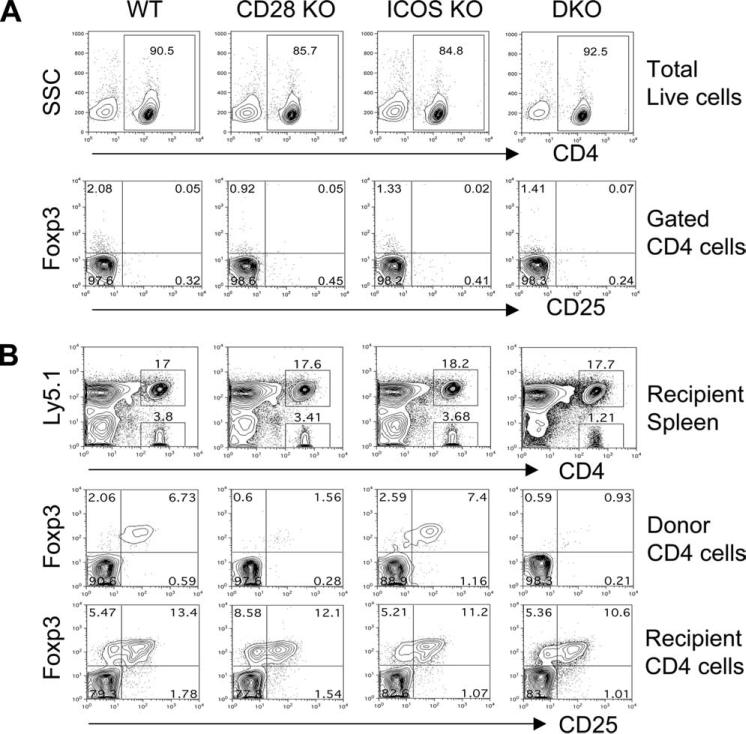
Because Foxp3+ Tregs can be generated both in the thymus and in the periphery and CD28 is known to be essential in nTreg generation in the thymus, we next tested the effects of CD28 and ICOS on the generation of iTregs in the periphery using an adoptive transfer model established by others (5). CD4+CD25− T cells were purified from B6 WT, CD28 KO, ICOS KO, or DKO donors (Fig. 2A) and then transferred into sublethally irradiated congenic B6 Ly5.1 recipients. Six weeks after cell transfer, the percentage of CD25+Foxp3+ cells among total CD4+ cells was compared in the recipient spleen. Although comparable iTregs (CD25+Foxp3+) were generated from ICOS KO and WT CD4+CD25− T cells (9.2 ± 2.5 vs 8.0 ± 1.7), significantly fewer iTregs were generated from CD28 KO (2.7 ± 0.7) or CD28/ICOS double KO (1.6 ± 0.5) CD4+CD25− cells (Fig. 2B). As internal controls, the percentage of iTregs among recovered host T cells (CD4+Ly5.1+) was not different in recipients regardless of donor cell type (Fig. 2B). These data indicate that CD28 but not ICOS is required for de novo generation of CD4+CD25+Foxp3+ iTregs from CD4+CD25− naive T cells in vivo outside the thymus.
FIGURE 2.
CD28, not ICOS, is required for de novo generation of Tregs from naive CD4 T cells in the periphery. A, CD4+CD25− cells were purified by negative selection with magnetic beads as described in Materials and Methods. The upper panel shows percentage of CD4+ cells on gated live cells, and the lower panel shows Foxp3 and CD25 expression on gated CD4+ cells after isolation and before adaptive transfer. B, B6.Ly5.1 mice were irradiated (500 cGy) and transplanted with 5 × 106 CD4+CD25− cells from B6 donors with indicated genotypes. Six weeks after cell transfer, recipient spleens were harvested and measured for CD4, Ly5.1, and Foxp3 expression. The top panel shows Ly5.1 expression in total splenocytes: Ly5.1+ indicating host cells while Ly5.1− indicating donor cells. The middle panel shows Foxp3 and CD25 expression among gated donor CD4+ cells (Ly5.1−), and the bottom panel shows Foxp3 and CD25 expression among gated host CD4+ cells (Ly5.1+). The data represent one of six mice in each group from two replicate experiments.
CD28 is required for the generation of iTregs from CD4+CD25− T cells in vitro
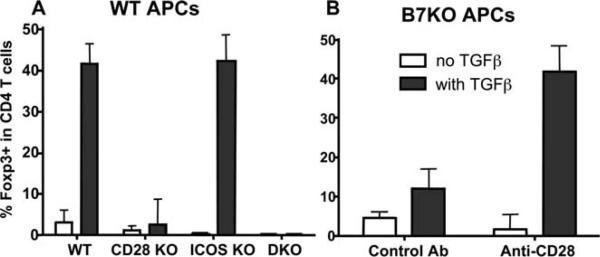
To study the mechanism by which CD28 controls the generation of iTregs from naive T cells, we adapted an in vitro culture system where naive T cells can differentiate into Foxp3+ Tregs upon anti-CD3 stimulation in the presence of TGF-β1 (24). As previously reported, Foxp3+ cells (42%) can be differentiated from WT CD4+CD25− T cells only in the presence of TGF-β1 (Fig. 3A). ICOS KO T cells differentiated into iTregs at the same rate (43%) as WT cells. However, the lack of CD28 blocked the T cell ability to differentiate into Tregs, because only 3.8 and 0.3% of Foxp3+ cells were generated from CD4+CD25− T cells deficient for CD28 or for CD28 and ICOS (Fig. 3A). Furthermore, WT CD4+CD25− T cells failed to differentiate into Tregs when stimulated in the presence of B7 KO (CD80 and CD86 double deficient) APCs, and the addition of anti-CD28 mAb restored Treg differentiation of WT T cells by B7 KO APCs (Fig. 3B). These results clearly demonstrate that CD28 is required for the differentiation of Tregs from CD4+CD25− T cells.
FIGURE 3.
CD28 is required for the differentiation of iTregs from naive CD4 T cells in vitro. A, CD4+CD25− cells from indicated strains were stimulated with anti-CD3 mAb at 0.5 μg/ml and irradiated B6 APCs in the absence or presence of TGF-β1 at 2 ng/ml. At 72 h, cultured cells were harvested and measured for surface CD4 and intracellular Foxp3 expression. B, WT CD4+CD25− cells were cultured under the same conditions as in A, except that B6 B7 KO (CD80 and CD86 double deficient) APCs were used. Control Ab (hamster IgG) or anti-CD28 mAb at 2 μg/ml was also added into the culture. The data are presented as mean ± 1 SD of % Foxp3+ in CD4+ cells pooled from three replicate experiments.
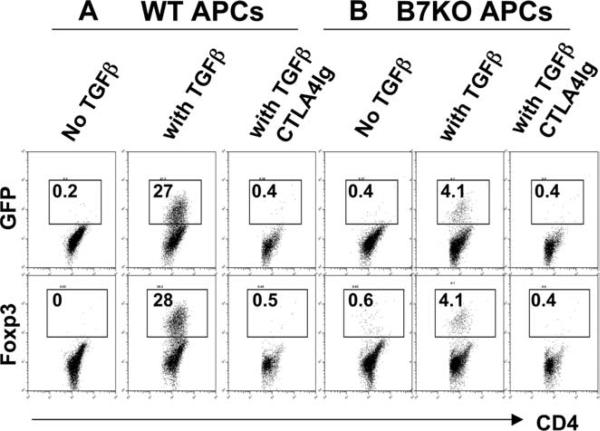
Because a small subset of Foxp3+ cells are CD25− (Fig. 2A) (19), Foxp3+ cells cannot be removed completely by depleting CD25+ cells. Thus, it is possible that after a 3-day culture, Foxp3+ cells resulted from expansion of residual Foxp3+ nTregs, and the expansion was dependent on CD28. To exclude this possibility, we took advantage of a strain of mice containing the Foxp3gfp KI allele encoding a GFP-Foxp3 fusion protein that enables identification of Tregs based on GFP expression (19). CD4+GFP− cells were sorted by FACS to 99% purity and then stimulated with anti-CD3 mAb in the presence of WT or B7 KO APCs for 72 h. Without additional TGF-β1, there were virtually no GFP+ or Foxp3+ cells regardless of APC type (Fig. 4). With TGF-β1, there were 27.3% of GFP+ cells or 28.2% Foxp3 + cells, confirming that the GFP+ or Foxp3+ iTregs were de novo generated from non-Tregs (GFP−) cells. In contrast to WT APCs, only 4.1% iTregs were generated from CD4+Foxp3− cells when stimulated with B7 KO APCs. Furthermore, when B7 was blocked with CTLA4-Ig, iTregs were completely absent regardless of APC type (Fig. 4). We interpret that the low level of iTreg generation (4%) in the presence of B7 KO APCs resulted from CD28-ligation by a low level of B7 expressed on activated T cells. Together with the data in Fig. 3, these results clearly indicate that CD28 is required for the de novo generation of iTregs from naive CD4 precursors.
FIGURE 4.
CD28 is required for de novo generation of iTregs from CD4+Foxp3− precursors in vitro. FACS-sorted CD4+GFP− cells from Foxpgfp KI mice were stimulated with anti-CD3 mAb at 0.5 μg/ml plus irradiated B6 WT or B7KO APCs in the absence or presence of TGF-β1 at 2 ng/ml. CTLA4-Ig was added at 50 μg/ml in cultures where indicated to block B7 ligands. After 72 h, cultured cells were harvested and measured for CD4, GFP and intracellular Foxp3 expression. The numbers present the mean of % GFP+ or Foxp3+ cells in CD4+ cells in duplicate wells, and the data represent one of four replicate experiments.
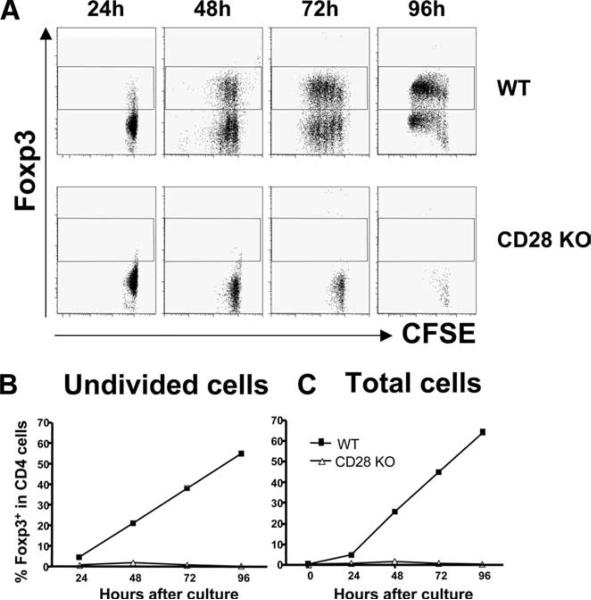
CD28 can promote cell cycle progression through inhibiting p27kip1 activation and enhance T cell expansion through IL-2 production (25). Thus, it is possible that failure of iTreg generation from CD28−/− T cells was due to limited cell division or expansion in the absence of CD28 costimulation. To address this question, CD4+CD25− T cells from WT or CD28 KO B6 mice were labeled with CFSE and stimulated with anti-CD3 mAb plus WT B6 APCs in the presence of TGF-β1. After stimulation for various time points, cells were harvested and tested for division by CFSE dilution and for Foxp3 expression. As demonstrated in Fig. 5, WT cells started to express Foxp3 (4.7%) before any cell division as early as 24 h after stimulation, and 21, 38, and 55% of these undivided cells were Foxp3+ at 48, 72 and 96 h, respectively (Fig. 5C). The percentages of Foxp3+ cells were comparable among cells at different cell division (Fig. 5B). CD28 KO T cells barely divided, and the proportion of Foxp3+ cells was minimal (Fig. 5, A and B). These data demonstrate that iTregs can arise before cell division, suggesting that the failure of iTreg generation from CD28−/− T cells may not be due to the limited cell divisions.
FIGURE 5.
Treg differentiation occurs before cell division. A, CFSE labeled CD4+CD25− cells from WT and CD28 KO mice were stimulated with WT B6 APCs in the presence of anti-CD3 and TGF-β1. Cells were collected at indicated time points and stained for CD4 and Foxp3 expression. B, The summary of average percentage of Foxp3+ in non-dividing CD4+ cells in each time point in the same experiment as in A. C, A summary of average percentage of Foxp3+ in total CD4+ cells in the same experiment as in A. The represent one of three replicate experiments.
CD28 mediates iTreg generation through IL-2 production
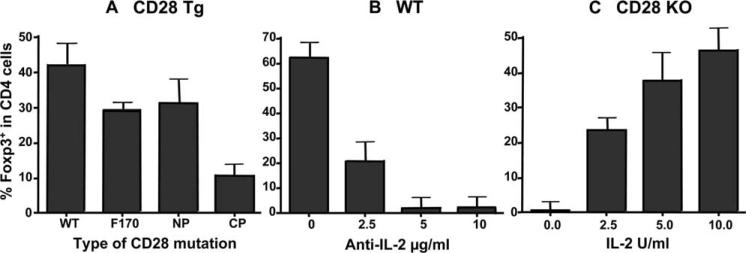
Intracellular domain of CD28 contains multiple motifs that can recruit and activate many kinases, including PI3K, IL-2-inducible T cell kinase (Itk), and Lck, which are responsible for various functions of CD28 receptor (26). We next asked which pathway is accountable for the requirement of CD28 in Treg differentiation. To address this question, we took advantage of a set of transgenes that encoded CD28 molecules with different mutations in the CD28 cytoplasmic tail (7): CD28 with an unmutated cytoplasmic tail (CD28-WT); CD28 with a mutation in Y170F that abrogated PI3K binding (CD28−170); CD28 with mutations in P175A and P178A (N-terminal proline residues) that abrogated Itk binding (CD28-NP); and CD28 with mutations in P187A and P190A (C-terminal proline residues) that abrogated Lck kinase binding (CD28-CP). Each of these transgenes was introduced into CD28 KO mice so that Tg CD28 molecules were the only CD28 molecules expressed by transgenic T cells. Under the culture condition in favor of iTreg differentiation in vitro, we found that CD28-WT CD4+CD25− T cells were equally capable of differentiating into Tregs as WT T cells from normal mice. Slightly reduced Tregs were generated from CD28−170 or CD28-NP T cells compared with those generated from CD28-WT cells. In contrast, significantly reduced Tregs were generated from CD28-CP T cells under the same culture condition (p < 0.001) (Fig. 6A). These results indicate that recruitment and activation of Lck, not PI3K or Itk, is accountable for CD28 function in iTreg generation.
FIGURE 6.
CD28-mediated Treg differentiation is dependent on IL-2 production. A, CD4+CD25− cells from B6 mice that bear CD28-WT, CD28−170, CD28-NP, or CD28-CP mutation were stimulated with anti-CD3 mAb plus irradiated B6 APCs in the presence of TGF-β1. B, WT CD4+CD25− cells were stimulated under the same conditions as described in A, and anti-IL-2 mAb was added into the culture at the concentrations indicated. C, CD28−/− CD4+CD25− cells were stimulated under the same conditions as described in A, and IL-2 was added into the culture at the concentrations indicated. In all the experiments, CD4 and Foxp3 expression was measured 72 h after stimulation. Percentage of Foxp3+ in CD4+ cells were always less than 5% in the absence of TGF-β1 (data not shown). The data are presented as mean ± 1 SD of % Foxp3+ in CD4+ cells pooled from three replicate experiments.
Because Lck activation is responsible for CD28-mediated IL-2 production (7), the data presented above suggest that IL-2 may play an essential role in promoting Treg differentiation from naive CD4 T cells. To further test this possibility, we evaluated whether neutralizing IL-2 with its specific mAb would block Treg differentiation in the presence of CD28, and whether adding exogenous IL-2 could restore Treg differentiation in the absence of CD28. As shown in Fig. 6, B and C, anti-IL-2 mAb blocked Treg differentiation from WT CD4+CD25− T cells in a dose-dependent manner, while additional IL-2 restored Treg differentiation from CD28−/− CD4+CD25− T cells in a dose-dependent manner (Fig. 6C). These data clearly demonstrate that CD28-mediated Treg differentiation requires IL-2 production.
IL-2 shares a common γ-chain with other cytokines including IL-4, IL-7, and IL-15. We tested whether other common γ-chain cytokines have redundant function of IL-2 in Treg differentiation, and found that none of these cytokines could replace IL-2 to restore Treg differentiation from CD28−/− naive T cells (Table I). These results indicate that IL-2 has a unique role in the induction of Foxp3 expression or Treg differentiation, which is not shared with other common γ-chain cytokines.
Table I.
Percentage of CD4+Foxp3+ cells after stimulation with common γ-chain cytokinesa
| Stimulation | Non | IL-2 (ng/ml) | IL-4 (ng/ml) | IL-7 (ng/ml) | IL-15 (ng/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 1.5 | 7.5 | 0.2 | 1 | 5 | 0.25 | 5 | 100 | 2 | 10 | 50 | ||
| % | 2.0 | 26.8 | 60.5 | 83.3 | 0.6 | 1.2 | 1.2 | 8.1 | 15.1 | 14.5 | 0.83 | 0.69 | 1.2 |
CD4+CD25− cells from CD28 KO mice were stimulated with anti-CD3 mAb plus irradiated B6 APCs in the presence of TGF-β1. Mouse recombinant IL-2, IL-4, IL-7, or IL-15 was added into the cell culture at the concentrations indicated, and CD4 and Foxp3 expression was measured 72 h after stimulation. The data present percentage of Foxp3+ in CD4+ cells in each culture condition and represent one of four replicate experiments.
De novo-generated iTregs exhibit suppressive function regardless of CD28
We tested de novo generation of iTregs and assessed whether ICOS or CD28 is required for their suppressive function. CD4+CD25− T cells from WT, ICOS−/−, or CD28−/− mice were stimulated with anti-CD3 mAb in the presence of TGF-β1 plus IL-2, an optimum culture condition for Treg differentiation from naive CD4 T cells. Four days after in vitro culture, CD4+CD25high cells were purified by positive selection with magnetic beads, followed by FACS sorting, and then cocultured with freshly isolated CD4+CD25− cells as responders that were stimulated with anti-CD3. As shown in Fig. 7, in vitro differentiated Tregs, regardless of ICOS or CD28 expression, were equally capable in suppressing proliferation of CD4 T responder cells. Thus, de novo generated Tregs possess suppressive function that is independent from ICOS or CD28 KO.
FIGURE 7.
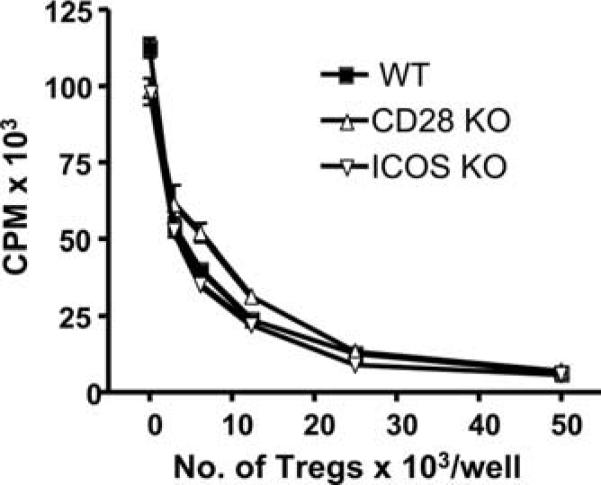
De novo generated Tregs have suppressive function independent of CD28. Freshly isolated CD4+CD25− cells from B6 mice were stimulated at 50 × 103/well with 0.5 μg/ml anti-CD3 in the presence of irradiated APCs in U-bottom, 96-well plates. Sorted CD4+CD25high cells generated from WT, ICOS−/− or CD28−/− naive CD4+CD25− T cells were added into the cell culture at the indicated numbers. Cell proliferation was determined by [3H]thymidine incorporation 72 h after stimulation. Data present the mean ± 1 SD cpm of triplicate wells in each culture condition, and three replicate experiments were done with similar results.
Discussion
Regulation by Tregs is an important mechanism of peripheral tolerance responsible for controlling autoreactive T cells (14). CD4+CD25+Foxp3+ Tregs develop not only in the thymus, and but they can also be generated from naive T cells in the periphery (3, 4). In vitro, the generation of iTregs from peripheral T cells depends on TCR signaling and TGF-β1 (24), whereas in vivo generation requires costimulation via B7.1/B7.2 (CD80/CD86) (5). Our data extend previous findings and demonstrates that CD28, but not CTLA4 or ICOS, is essential for generation of Tregs in the periphery (Fig. 1 and 2), and that CD28 controls the generation of Tregs from CD4+ non-Tregs via IL-2 production.
CD28 is a dominant costimulatory receptor that positively regulates T cell response. CD28, however, also plays critical role in Treg development in the thymus and in Treg survival and homeostasis in the periphery (9). By using CD28−/− T cells or B7−/− APCs, we found that the CD28 signal is indispensable for the generation of CD4+CD25+Foxp3+ Tregs from naive CD4+CD25− T cells both in vitro or in vivo. Because there is a small population of Foxp3+ cells among CD4+CD25− T cells (19), a possible explanation is that the apparent differentiation of Foxp3+ Tregs from CD25− naive T cells was simply the result of selective expansion of Foxp3+ cells under the culture condition. However, we do not believe this was the case for the following observations. Upon stimulation with anti-CD3 mAb in the presence of TGF-β1, cells increased Foxp3 expression within 24 h before cell division, and those undivided cells continuously increased Foxp3 expression along with incubation time (Fig. 5C). Furthermore, we used highly purified CD4+GFP− cells from Foxp3gfp KI mice and clearly demonstrated that iTregs were generated from non-Treg precursors (Fig. 4).
Several lines of evidence indicate that CD28-mediated Treg differentiation depends on IL-2 production. The CD28 cytoplasmic motif that binds Lck, but not PI3K or Itk, is required for CD28-mediated Treg differentiation (Fig. 6A). Given that Lck-binding motif is responsible for CD28-induced IL-2 production (7), the data suggested that IL-2 may contribute to CD28-mediated Treg differentiation. This assumption was confirmed by the observations that neutralizing IL-2 completely blocked Treg differentiation from WT CD4+CD25− T cells (Fig. 6B), whereas providing exogenous IL-2 completely restored Treg differentiation from CD28−/− CD4+CD25− T cells (Fig. 6C). These observations are consistent with recent reports that IL-2 is essential for TGF-β1 to convert naive CD4+CD25− cells to CD25+Foxp3+ Tregs (27, 28). It has been reported that Lck-binding motif of CD28 receptor is indispensable for nTreg development in the thymus, but IL-2 is not required for this process (7), which led to the conclusion that CD28 independently promotes IL-2 production and nTreg development in the thymus. Interestingly, our data provide the evidence that CD28 promotes these two processes using a shared signaling pathway through Lck in the periphery. Collectively, these data suggest that although naturally derived Tregs (in the thymus) and differentiated Tregs (in the periphery) are phenotypically (Foxp3 expression) and functionally (suppressive activity) indistinguishable, their generation is likely attributed to separate mechanisms: IL-2 independent vs IL-2 dependent pathway. It is worthy to point out that the amount of IL-2 required for Treg differentiation is very low because 2−4 Unit/ml was able to restore Foxp3 expression for CD28−/− T cells (Fig. 6C) or IL-2−/− T cells (27, 28). The IL-2 is often under the detection level in the culture supernatant in which CD4+CD25− T cells were prone to differentiate into iTregs, i.e., low level of TCR stimulation and with TGF-β1 (data not shown). We observed that iTregs could be generated before cell division (Fig. 5), which is consistent with a recent report that TGFβ and anti-CD3-induced Foxp3 expression was independent of cell cycle (29). That the CD28 signal induces iTreg generation through an IL-2-dependent but cell cycle-independent manner seems contradictory. We surmise that the time and/or IL-2 dose can distinguish these two processes, i.e., Foxp3 expression may occur earlier and require lower levels of IL-2 compared with those needed for cell cycle progression.
Liang et al. demonstrated that CD4+CD25− cells can be converted into CD4+CD25+ Tregs in vivo through a thymic independent but B7-dependent pathway. Because B7 are the ligands for both CD28 and CTLA4, their data did not distinguish whether either or both of these two receptors are responsible for the conversion. Using CD28−/− T cells, we showed that CD28 was necessary for this process in vitro (Fig. 3) and in vivo (Fig. 2). In addition, supplemental anti-CD28 mAb could completely restore the Treg differentiation in the absence of B7 (Fig. 3B), indicating that the CD28 signal alone was sufficient for this process. In conclusion, CD28 is necessary and sufficient to promote the differentiation of CD4 T cells into iTregs through IL-2 production under suboptimal TCR stimulation. This study reveals a new aspect of CD28 function, which may provide critical information that leads to potential applications in manipulating T cell immunity and tolerance through CD28 in clinic.
Acknowledgments
We thank Alfred Singer for providing CD28 WT and mutation Tg mice, Chen Dong and Richard Flavell for ICOS KO, and Tak Mak for CD28/ICOS KO mice. We are grateful for the technical assistance provided by the Flow Cytometry Core Facility at the Moffitt Cancer Center.
Footnotes
This work was supported in part by National Institutes of Health Grants AI 63553, CA 118116 (to X.-Z. Y.), and AI 51693 (to C. A.). X.-Z.Y. is a recipient of New Investigator Award supported by American Society for Blood and Bone Marrow Transplantation.
Abbreviations used in this paper: Treg, regulatory T cell; nTreg, naturally occurring Treg; Tg, transgenic; iTreg, induced Treg; KO, knockout; WT, wild type; B6, C57BL/6 mice; KI, knock-in; Itk, IL-2-inducible T cell kinase.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Albert MH, Anasetti C, Yu XZ. T regulatory cells as an immunotherapy for transplantation. Expert Opin. Biol. Ther. 2006;6:315–324. doi: 10.1517/14712598.6.4.315. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 3.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 5.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25− cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J. Exp. Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 7.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 8.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 9.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 10.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor β (TGF-β) production by murine CD4+ T cells. J. Exp. Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 13.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 15.Kramer S, Schimpl A, Hunig T. Immunopathology of interleukin (IL) 2-deficient mice: thymus dependence and suppression by thymus-dependent cells with an intact IL-2 gene. J. Exp. Med. 1995;182:1769–1776. doi: 10.1084/jem.182.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 17.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 22.Suh WK, Tafuri A, Berg-Brown NN, Shahinian A, Plyte S, Duncan GS, Okada H, Wakeham A, Odermatt B, Ohashi PS, Mak TW. The inducible costimulator plays the major costimulatory role in humoral immune responses in the absence of CD28. J. Immunol. 2004;172:5917–5923. doi: 10.4049/jimmunol.172.10.5917. [DOI] [PubMed] [Google Scholar]
- 23.Yu XZ, Albert MH, Martin PJ, Anasetti C. CD28 ligation induces transplantation tolerance by IFN-γ-dependent depletion of T cells that recognize alloantigens. J. Clin. Invest. 2004;113:1624–1630. doi: 10.1172/JCI20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J. Immunol. 2000;164:144–151. doi: 10.4049/jimmunol.164.1.144. [DOI] [PubMed] [Google Scholar]
- 26.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signaling. Nat. Rev. Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 27.Davidson TS, Dipaolo RJ, Andersson J, Shevach EM. Cutting edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 28.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25−cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 29.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-β. J. Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]