Abstract
To figure out the epidemiological status and relevance with other diseases in toxoplasmosis, we checked serum IgG antibody titers of 1,265 patients and medical records of seropositive patients. Seropositive rates were 6.6% by latex agglutination test (LAT) and 6.7% by ELISA. No significant differences were detected between sexes and age groups. The peak seroprevalence was detected in the 40-49-year-old age group. According to clinical department, Toxoplasma-positive rates were high in patients in psychiatry, ophthalmology, health management, emergency medicine, and thoracic surgery. Major coincidental diseases in seropositive cases were malignant neoplasms, diabetes mellitus, arthritis, chronic hepatitis B, chronic renal diseases, schizophrenia, and acute lymphadenitis, in the order of frequency. In particular, some patients with chronic hepatitis B and malignant neoplasms had high antibody titers. These results revealed that the seroprevalence of toxoplasmosis in a general hospital-based study was similar to that in a community-based study, and T. gondii seropositivity may be associated with neoplasms, diabetes, and other chronic infections.
Keywords: Toxoplasma gondii, comorbidity, general hospital, seroprevalence, Daejeon
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan parasite with a global distribution in humans and other warm-blooded animals. Transmission to humans occurs through ingestion of T. gondii oocysts shed into the environment by cats, or by eating meat of infected animals. Under normal immune conditions, T. gondii infection is frequently asymptomatic, but in individuals who are immunocompromised, such as in patients with AIDS, the parasites can become widely disseminated, causing severe toxoplasmosis and encephalitis. Primary infections acquired during pregnancy may also result in severe damages to the fetus, manifested as mental retardation, seizures, blindness, and death [1].
T. gondii is a common infection in South America, France, Turkey, and Brazil, and the prevalence is also high in people living under poor socioeconomic conditions. T. gondii positivity is up to 54% in southern European countries, and is 15.8% in the 12-49-year-old age group in USA [1]. Some studies have reported on the correlation between T. gondii infection and other diseases. T. gondii may be associated with schizophrenia [2], and an association likely exists between T. gondii infection and some kinds of cancers [3]. In Korea, T. gondii has been isolated from humans [4], and several clinical cases have been reported [5,6]. The recent seroprevalence of T. gondii infection ranges from 0.79% to 12.9% in the Korean population [7-13]. Although some studies have examined the seroprevalence of T. gondii among Koreans, there have been few data regarding seropositive patients. Therefore, we evaluated the rate of T. gondii seropositivity in randomly selected patients in 2 general hospitals in Daejeon, Korea, and sought to clarify the characteristics of seropositive individuals.
MATERIALS AND METHODS
Preparation of Toxoplasma lysate antigen (TLA)
Infected fibroblasts were scraped, forcibly passed through a 27-gauge needle, and centrifuged at 900 × g for 10 min using Percoll (Sigma Chemical Co., St. Louis, Missouri, USA) to pellet parasites. The parasites were sonicated on ice and centrifuged at 100,000 × g for 40 min. The supernatant was pooled and sterile filtered, and the protein content was determined by the Bradford method using bovine serum albumin (BSA) as the standard. TLA was stored in aliquots at -20℃ until use.
Collection of patients' sera
We collected sera, which had already been used for various tests besides toxoplasmosis, from 2 general hospitals in Daejeon, Korea, in February 2008. In total, 1,265 sera were randomly selected for this study and stored at -70℃ until use. The age range of the subject population was 0-96 years old, and the average age was 49.9 ± 19.7 years. The number of patients according to clinical department included 46.2% in internal medicine, 8.1% in healthmanagement, 7.1% in orthopedic surgery, 5.8% in pediatrics, and others (5.8%). This study was approved by the Ethics Committee of the College of Medicine, Chungnam National University, Daejeon, Korea.
Latex agglutination test (LAT)
LAT for toxoplasmosis was performed using the Toxotest-MT Kit (Eiken Chemical Co., Tokyo, Japan), according to the manufacturer's instructions. Briefly, serum samples and positive sera were diluted 2-fold serially in U-shaped 96-well microtiter plates using dilution buffer (0.2 M amino-2-methyl-1-propanol) and reacted with Toxoplasma antigen-adsorbed polyethylene latex suspension, overnight at room temperature. Antibody titers were determined by the last dilution number of sera which precipitated latex in the middle class dispersion. Based on the manufacturer's recommendation, agglutination at a dilution of 1 : 32 or higher was regarded as positive.
IgG antibody titers by ELISA
The ELISA was performed by the procedure described by Lee et al. [8]. Each well of a 96-well microtiter plate (Nunc, Roskilde, Denmark) was coated with 100 µl TLA (10 µg/ml) in 0.05 M carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at 4℃. Each well was blocked with 1% BSA in phosphate-buffered saline (PBS). Serum samples were diluted 1 : 150 with 0.1% BSA/PBS that contained 0.05% Tween 20 (BSA/PBS/Tween 20). After 2 hr at room temperature, goat antihuman IgG horseradish peroxidase (1 : 30,000 dilution; Sigma Chemical Co.) was applied and the plates were incubated for an additional 2 hr. After washing, freshly prepared o-phenylenediamine dihydrochloride (Sigma Chemical Co.) was added, and the reaction was stopped by adding 4 N H2SO4. The IgG antibody titers were read at OD490 using an automatic ELISA reader (SPECTRA; Molecular Devices, Sunnyvale, California, USA).
Sensitivity and specificity of each test
For each serum sample, the biological interpretation (presence or absence of IgG) was based on the results obtained with LAT and IgG ELISA. The results of the LAT allowed us to classify the sera as positive or negative for IgG. Compared to the results of the LAT, the sensitivity and specificity of the ELISA were calculated.
Statistical analysis
We used SPSS 9.0 software (SPSS Inc., Chicago, Illinois, USA) for analyzing data from these experiments. The chi-square and Student's t-test were used to test statistically significant differences. Differences between 2 groups were considered significant if P < 0.05.
RESULTS
Seroprevalence of Toxoplasma antibody based on the LAT
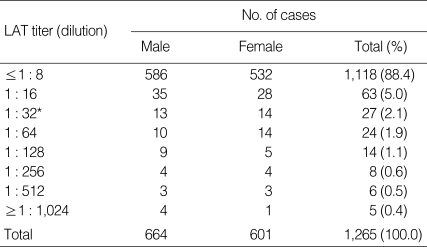
To evaluate the seroprevalence of toxoplasmosis, 1,265 serum samples were examined for T. gondii antibody titer using LAT. IgG ELISA was also performed to increase the accuracy of the diagnosis. When serum samples were screened using the LAT at dilutions from 1 : 8 to 1 : 1,024, 84 samples showed agglutination at a dilution of 1 : 32 or more (Table 1). According to the LAT results, there were 1,118 cases (88.4%) with T. gondii antibody titer of 1 : 8 or less (≤ 1 : 8), 63 cases (5.0%) with 1 : 16, 27 cases (2.1%) with 1 : 32, 24 cases (1.9%) with 1 : 64, 14 cases (1.1%) with 1 : 128, 8 cases (0.6%) with 1 : 256, 6 cases (0.5%) with 1 : 512, and 5 cases (0.4%) with 1 : 1,024 or more. In total, 84 out of 1,265 sera (6.6%) were positive in the LAT, which were agglutinated at a dilution of 1 : 32 or more.
Table 1.
Frequency distribution of Toxoplasma gondii antibody titers in sera of 1,265 cases by latex agglutionation test (LAT)
*LAT titers of 1 : 32 or higher were regarded as positive.
Determination of the cutoff absorbance of ELISA
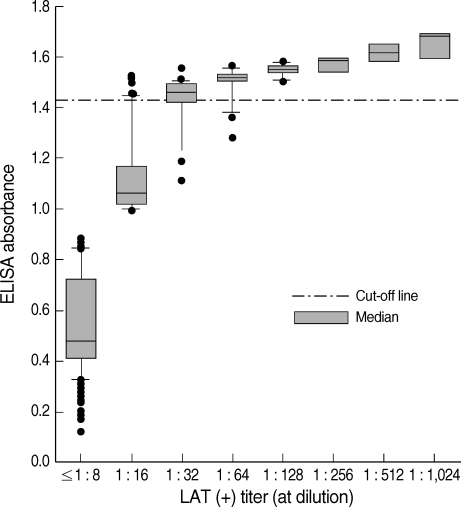
In addition, 1,265 serum samples were tested for anti-T. gondii IgG antibody titers by ELISA. The cutoff value for ELISA was determined by modification of the method of Choi et al. [12]. According to the LAT results, 27 sera showed a LAT titer of 1 : 32 and the mean ELISA value of these sera was 1.374 ± 0.038 (mean ± SD). Therefore, the cutoff absorbance for positive reactions by ELISA was determined to be 1.412, the mean ± 1 SD of the 1 : 32 LAT titer sera; ELISA values for the 1 : 16 and 1 : 32 LAT titer were significantly different (P < 0.05). Among 1,265 serum samples, 85 cases showed positive reactions by ELISA (Table 2; Fig. 1), and this number was similar to that of the LAT. The relationship between antibody titers (absorbance) based on ELISA and LAT is shown in Fig. 1.
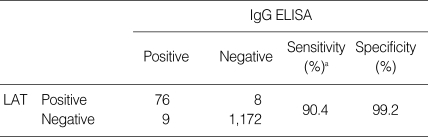
Table 2.
Comparison of two assays used to evaluate the seropositivity of Toxoplasma gondii in 1,265 cases
aWhen compared with the LAT, ELISA had a sensitivity of 90.4% and specificity of 99.2%.
Fig. 1.
Relationship between Toxoplasma gondii seropositive cases by enzyme-linked immunosorbent assay (ELISA) and latex agglutination test (LAT). The cutoff absorbance for seropositivity by ELISA was 1.412.
Seroprevalence of Toxoplasma by ELISA
The distribution of IgG antibody titers of serum samples measured by ELISA is shown in Fig. 1. The mean absorbance of 1,265 cases was 0.643 ± 0.318. Among 1,265 serum specimens, 85 cases showed seropositive antibody titers, with an absorbance of 1.412 or higher. A significant difference of antibody titer was observed between the 85 ELISA seropositive cases (1.523 ± 0.046) and the 1,180 ELISA negative samples (0.594 ± 0.226) (P < 0.01). The seropositive rate by IgG ELISA was 6.7% (Table 2). When compared with the LAT, ELISA had a sensitivity of 90.4% and specificity of 99.2%.
Characteristics of T. gondii-seropositive patients
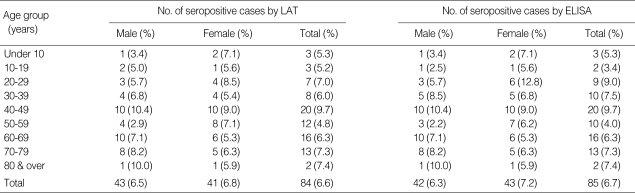
The age and sex distribution of patients positive for Toxoplasma antibody are shown in Table 3. The positive rates for men and women based on the LAT were 6.5% and 6.8%, respectively. No significant differences were observed between the sexes (P = 0.9337). The prevalence tended to increase with age, although this increase was not statistically significant (P > 0.05): < 20 years old, 5.2-5.3%; 20-29 years old, 7.0%; 30-39 years old, 6.0%; and ≥ 40 years old, 4.8-9.7%. The peak prevalence was seen in the 40-49 age group (9.7%). Based on the ELISA results, the positive rates of male and female were 6.3 and 7.2%, respectively. There were not significant differences of seropositive rates between sexes by ELISA (P = 0.551).
Table 3.
Age and sex distribution of Toxoplasma gondii seropositive cases by LAT and ELISA
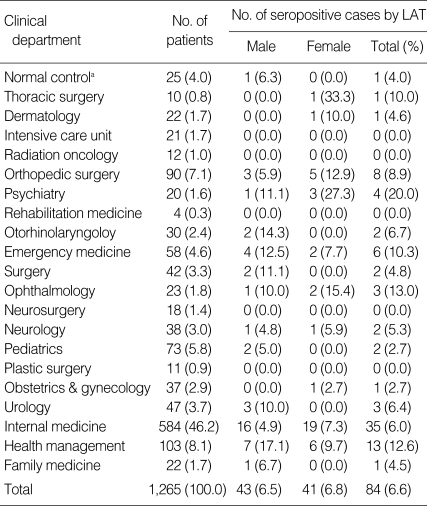
As the normal control group, 25 students at the age between 18 to 24 years from Medical School were subjected in this study. The seropositive rate against T. gondii of the normal control group was 4.0% by LAT or ELISA. To figure out the relevance with other diseases in toxoplasmosis, we checked the medical record of each patient. According to clinical departments, Toxoplasma positive rates were high in patients from psychiatry, ophthalmology, health management, emergency medicine, and thoracic surgery by the results of LAT (Table 4). The seropositive rates within each department also differed in some departments between the sexes. Men had a significantly higher positive rate in the departments of health management, otorhinolaryngology, emergency medicine, pediatrics, surgery, and urology, whereas women had a significantly higher positive rate in the departments of psychiatry, ophthalmology, and orthopedic surgery. The patterns of seropositive rates of clinical departments by ELISA were similar with those of LAT (data not shown).
Table 4.
Distribution of clinical department of Toxoplasma gondii seropositive cases by LAT
aTwenty-five students at the age between 18 to 24 years old from Medical School were referred as "normal control group", and they were not included the total number of patients in this study.
Comorbidities in Toxoplasma-seropositive patients were malignant neoplasms (16 cases), diabetes mellitus (12 cases), arthritis (seven cases), chronic hepatitis B (7 cases), chronic renal diseases (4 cases), schizophrenia (3 cases), and acute lymphadenitis (3 cases). In particular, some patients with chronic hepatitis B and malignant neoplasms revealed antibody titers of 1 : 512 or more based on the LAT.
DISCUSSION
The prevalence of toxoplasmosis is related to several factors, including culture, nutritional habits, age, and a rural or urban setting [1]. It was reported that T. gondii seroprevalence ranges from 0.79% to 12.9% in Korean population [7-13]. In the present study, we have shown that the prevalence of toxoplasmosis in patients from the general hospitals in Daejeon (central part of South Korea) was 6.6% by LAT. This rate was similar to our previous data of Lee et al. [8], who reported a 6.9% positive rate in Okcheon-gun (a provincial area near Daejeon), Korea. However, Song et al. [9] reported a rate of 0.79% in pregnant women, whereas Yang et al. [10] reported 12.9% in adult patients in Jeju, Korea. These differences might be explained by differences in assay systems, the characteristics of the surveyed populations, and the location of the sample populations. According to our data and a previous work [1], T. gondii sereoprevalence in Korea was much lower than those reported in South America, Europe, and other Asian countries. Certain characteristics of the Korean population may contribute to the low prevalence. For example, in Korea, the range of meats that are eaten undercooked or raw is narrow, the frequency of raising a cat is low, many urban people live in apartments that are not directly associated with soil, and water service facilities are well established, especially for those living in cities [5,9,11].
Seroprevalence of toxoplasmosis is known to increase with age [8,14]. In the present study, some tendency was observed that the lowest positive rate was seen in those aged < 20 years, and the rates slowly increased with age, with the peak level being in 40-49 age group. The seropositive rate in the latter group was 9.7%, which was about twice that in the < 20-year-old age group. The reason for the rise in quantitative titers with age is not clear, although the reason might be the increased possibility of an individual coming into contact with one of the transmission routes [1,14]. With respect to sex, the positive rate in women was slightly higher than that in men, but the difference was not significant. Similar results were obtained in the survey performed at Seoul National University Children's Hospital and on Jeju Island [7,10]. In contrast, a report has been made that male patients have a higher infection rate than female patients at Kangnam St. Mary's Hospital [13].
Toxoplasmosis can be diagnosed using several methods. Detection of the organism itself is confirmative but very difficult. Generally, serological tests are used to diagnose toxoplasmosis, and these tests are often helpful for the diagnosis of active infection, as well as indicating previous exposure. Sabin-Feldman dye test was the most specific test for T. gondii, and this test is still considered the gold standard [15,16]. However, it has the major disadvantage of requiring the use of live organism and human serum from healthy individuals as an accessory factor. Indirect fluorescent antibody test is highly specific, but demonstrates low sensitivity and is a complicated test, requiring fluorescently labeled conjugate and special equipment [15,16]. ELISA demonstrates great sensitivity, is quantitative, low cost and may be a good tool for epidemiological studies of Toxoplasma infection [15,16]. Regarding LAT, LAT provided an efficacious method for routine serologic screening for antibodies to T. gondii [17]. In the present study, we used 2 different methods, LAT and IgG ELISA, to increase the accuracy of diagnosis, and the seropositive rates from each assay were 6.5% and 6.8%, respectively. While comparing the seropositivities from LAT and ELISA, it was found that 17 samples were detected positive by only one of the assay systems but not by both of them. The difference in seropositivity according to assay method may have resulted partly from different antigenic epitopes being recognized by each method, and partly from different limits of sensitivity for each test [18].
To clarify the characteristics of T. gondii-seropositive patients in Korea, we checked the patients' medical records. The major coincidental diseases in seropositive patients were malignant neoplasms, diabetes mellitus, arthritis, chronic hepatitis B, chronic renal diseases, schizophrenia, and acute lymphadenitis. It was of note that patients with chronic hepatitis B and malignant neoplasms had an antibody titer of 1 : 512 or more based on the LAT. Since a further clinical evaluation was not performed on these cases, whether they had any symptoms of toxoplasmosis is unclear. These results show a likely association between T. gondii infection and some other diseases. It has been suggested that T. gondii may play some etiological role in a large number of cases of schizophrenia [2], and some correlation is also suggested between toxoplasmosis and congenital anomalies in Korea [7]. Yuan et al. [3] found that the positive rate of T. gondii IgG in nasopharyngeal carcinoma and rectal cancer is significantly higher than that in other cancer groups, which demonstrates a likely association between T. gondii infection and some types of cancer. Perhaps patients with chronic hepatitis B are at a high risk of contracting other infectious diseases such as toxoplasmosis, and patients with malignant neoplasms or diabetes mellitus are easily infected with T. gondii because of immunosuppression induced by radiotherapy or chemotherapy, or metabolic disorders.
In summary, the prevalence of toxoplasmosis in patients in the general hospitals in Daejeon was 6.6% by LAT, and major comorbidities of seropositive patients included malignant neoplasms, diabetes mellitus, arthritis, chronic hepatitis B, chronic renal diseases, schizophrenia, and acute lymphadenitis. Moreover, some patients with chronic hepatitis B and malignant neoplasms showed high T. gondii antibody titers. This survey is important because it is believed to be the first to study the characteristics of T. gondii-seropositive patients in Korea, but further surveys are needed to clarify the exact relationships. Our study, however, has certain limitations. Although general hospitals cover the whole medical field for the general population, some restrictions in the location and distribution of patients may have occurred.
ACKNOWLEDGEMENTS
This study was financially supported by research fund of Chungnam National University in 2006.
References
- 1.Ajioka JW, Soldati D. Toxoplasma-Molecular and Cellular Biology. Norfolk, UK: Horizon Bioscience; 2007. pp. 37–58. [Google Scholar]
- 2.Torrey EF, Yolken RH. Editors' introduction: Schizophrenia and toxoplasmosis. Schizophr Bull. 2007;33:727–728. doi: 10.1093/schbul/sbm026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan Z, Gao S, Liu Q, Xia X, Liu X, Liu B, Hu R. Toxoplasma gondii antibodies in cancer patients. Cancer Lett. 2007;254:71–74. doi: 10.1016/j.canlet.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Chai JY, Lin A, Shin EH, Oh MD, Han ET, Nam HW, Lee SH. Laboratory passage and characterization of an isolate of Toxoplasma gondii from an ocular patient in Korea. Korean J Parasitol. 2003;41:147–154. doi: 10.3347/kjp.2003.41.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi WY, Nam HW, Kwak NH, Huh W, Kim YR, Kang MW, Cho SY, Dubey JP. Foodborne outbreaks of human toxoplasmosis. J Infect Dis. 1997;175:1280–1282. doi: 10.1086/593702. [DOI] [PubMed] [Google Scholar]
- 6.Lee JA, Kim DH, Kim YK, Chung EH, Choi JH, Lee HJ, Chi JG, Chai JY, Lee YH. Two cases of congenital toxoplasmosis diagnosed by polymerase chain reaction. Infect Chemother. 2003;35:45–52. [Google Scholar]
- 7.Kook J, Lee HJ, Kim BI, Yun CK, Guk SM, Seo M, Park YK, Hong ST, Chai JY. Toxoplasma gondii antibody titers in sera of children admitted to the Seoul National University Children' Hospital. Korean J Parasitol. 1999;37:27–32. doi: 10.3347/kjp.1999.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YH, Noh HJ, Hwang OS, Lee SK, Shin DW. Seroepidemiological study of Toxoplasma gondii infection in the rural area Okcheon-gun, Korea. Korean J Parasitol. 2000;38:251–256. doi: 10.3347/kjp.2000.38.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song KJ, Shin JC, Shin HJ, Nam HW. Seroprevalence of toxoplasmosis in Korean pregnant women. Korean J Parasitol. 2005;43:69–71. doi: 10.3347/kjp.2005.43.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang HJ, Jin KN, Park YK, Hong SC, Bae JM, Lee SH, Choi HS, Hwang HS, Chung YB, Lee NS, Nam HW. Seroprevalence of toxoplasmosis in the residents of Cheju island, Korea. Korean J Parasitol. 2000;38:91–93. doi: 10.3347/kjp.2000.38.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han K, Shin DW, Lee TY, Lee YH. Seroprevalence of Toxoplasma gondii infection and risk factors associated with seropositivity of pregnant women in Korea. J Parasitol. 2008;94:963–965. doi: 10.1645/GE-1435.1. [DOI] [PubMed] [Google Scholar]
- 12.Choi WY, Nam HW, Youn JH, Kim DJ, Komg Y, Kang SY, Cho SY. Dectection of antibodies in serum and cerebrospinal fluid to Toxoplasma gondii by indirect latex aggluination test and enzyme-linked immunosorbent assay. Kisaengchunghak Chapchi. 1992;30:83–90. doi: 10.3347/kjp.1992.30.2.83. [DOI] [PubMed] [Google Scholar]
- 13.Choi WY, Nam HW, Youn JH, Kim WS, Kim WK. Toxoplasma antibody titers by indirect latex agglutination test in patients of Kangnam St. Mary's Hospital and Cheju Medical Center. Kisaengchunghak Chapchi. 1989;27:171–175. doi: 10.3347/kjp.1989.27.3.171. [DOI] [PubMed] [Google Scholar]
- 14.Spalding SM, Amendoeira MR, Klein CH, Ribeiro LC. Serological screening and toxoplasmosis exposure factors among pregnant women in south of Brazil. Rev Soc Bras Med Trop. 2005;38:173–177. doi: 10.1590/s0037-86822005000200009. [DOI] [PubMed] [Google Scholar]
- 15.Shaapan RM, El-Nawawi FA, Tawfik MA. Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Vet Parasitol. 2008;153:359–362. doi: 10.1016/j.vetpar.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Evans R, Ashburn D, Chatterton J, Joss A, Ho-Yen D. How to detect current toxoplasma infection. Br J Biomed Sci. 2002;59:4–6. doi: 10.1080/09674845.2002.11783625. [DOI] [PubMed] [Google Scholar]
- 17.Mazumder P, Chuang HY, Wentz MW, Wiedbrauk DL. Latex agglutination test for detection of antibodies to Toxoplasma gondii. J Clin Microbiol. 1988;26:2444–2446. doi: 10.1128/jcm.26.11.2444-2446.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigsby P, Rijpkema S, Guy EC, Francis J, Das RG. Evaluation of a candidate international standard preparation for human anti-Toxoplasma immunoglobulin G. J Clin Microbiol. 2004;42:5133–5138. doi: 10.1128/JCM.42.11.5133-5138.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]