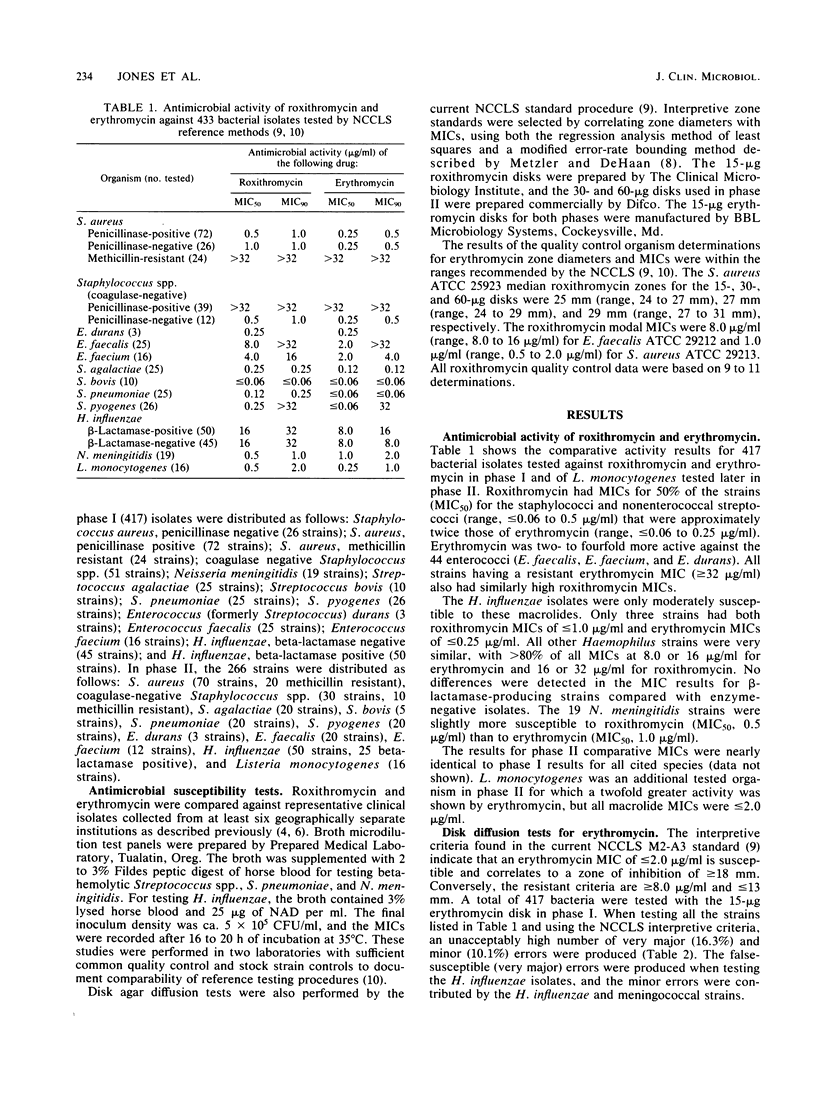
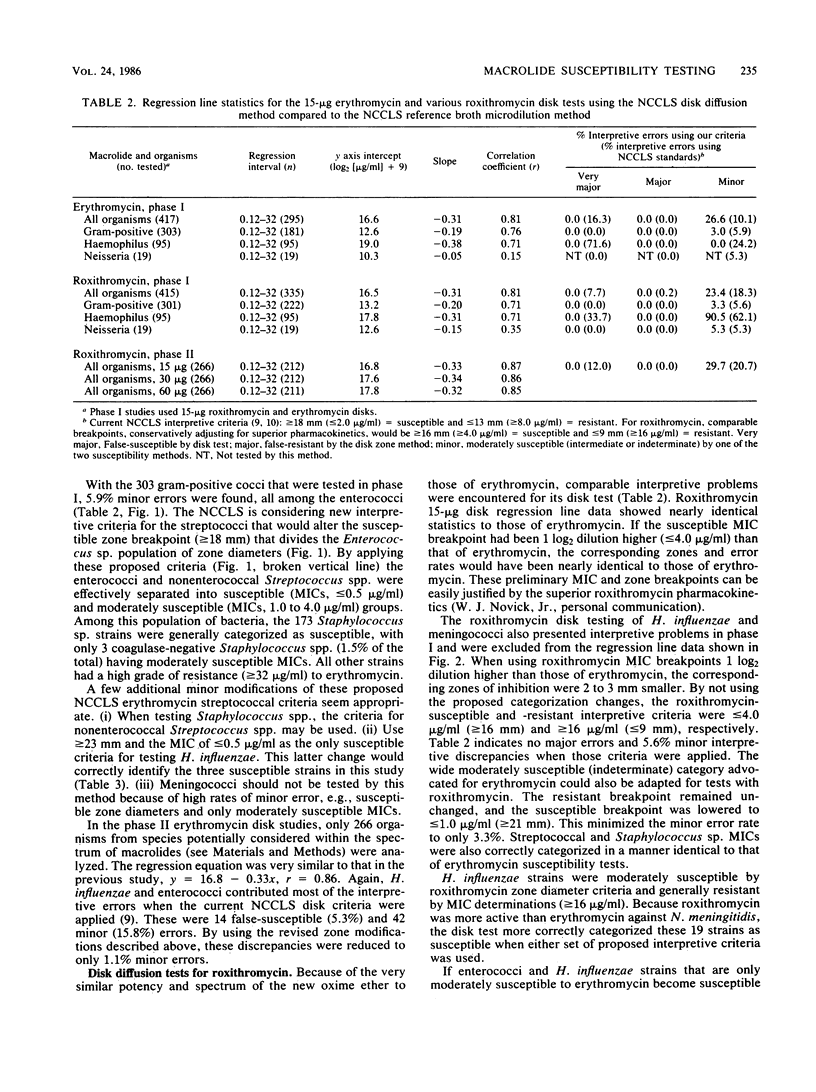
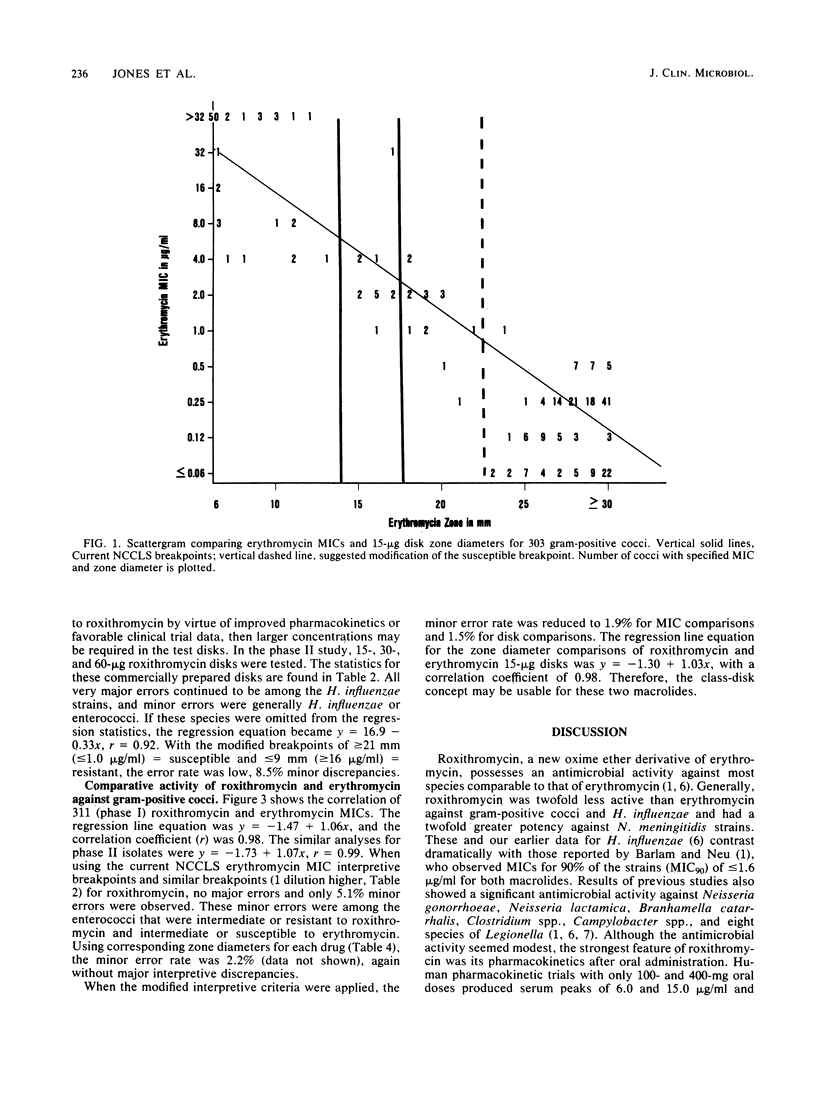
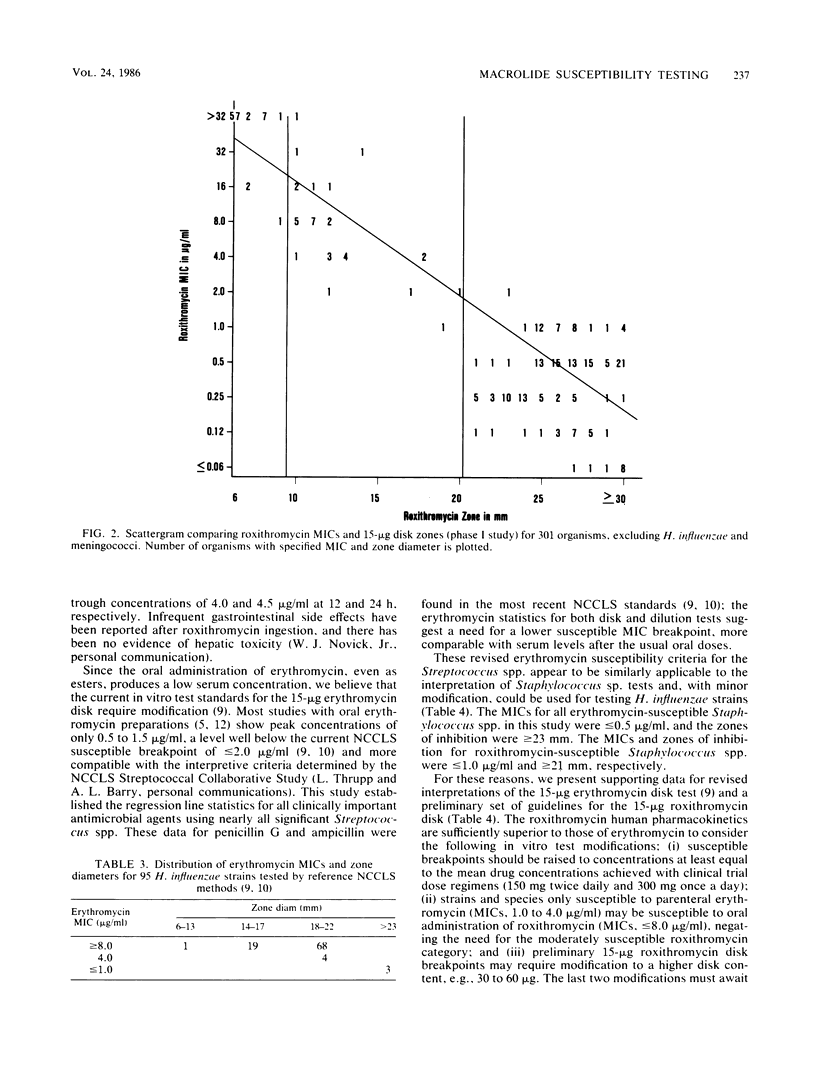
Abstract
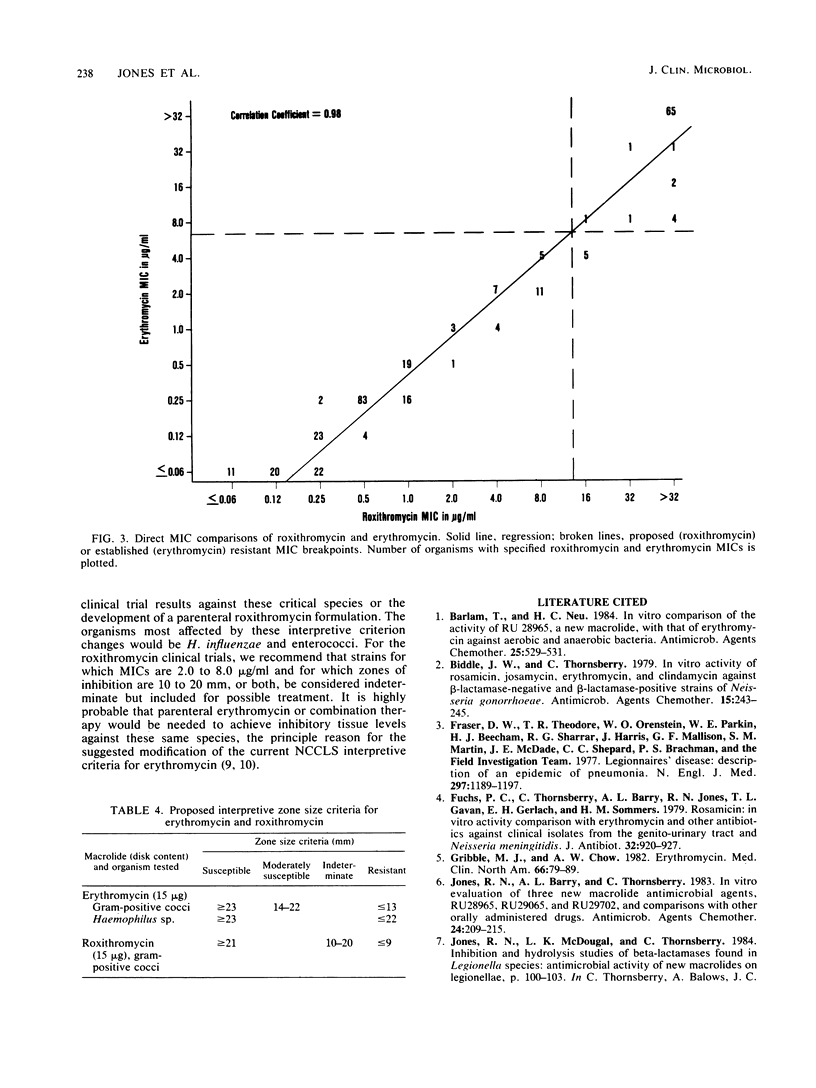
The 15-micrograms erythromycin disk was twice evaluated for interpretive accuracy against 417 and then 266 strains of gram-positive cocci, Neisseria meningitidis, and Haemophilus influenzae by using the criteria suggested by the National Committee for Clinical Laboratory Standards. These studies suggest a revision of streptococcal and Staphylococcus sp. interpretive guidelines to criteria (greater than or equal to 23 mm = susceptible, less than or equal to 13 mm = resistant) that are more compatible with in vivo erythromycin concentrations. It is also recommended that zone diameters be modified for H. influenzae (greater than or equal to 23 mm = susceptible, less than 22 mm = resistant) and that meningococci not be tested. A wide moderately susceptible category (1.0 to 4.0 micrograms/ml) would primarily include enterococcus strains and those organisms that would then respond only to parenteral administration of erythromycin. Roxithromycin (RU 965 or RU 28965), a new oxime ether erythromycin derivative, was also evaluated by investigator-prepared 15-micrograms disks and later with 30- and 60-micrograms commercial disks. Although roxithromycin was comparable to erythromycin in activity and regression line statistics, changes in the susceptible disk criteria were necessary because of superior roxithromycin serum concentrations and a longer serum half-life. Preliminary susceptible breakpoint criteria were greater than 21 mm = susceptible, 10 to 20 mm = indeterminate, and less than or equal to 9 mm = resistant. By using the recommended interpretive criteria for both macrolides, less than 98% absolute agreement was obtained, therefore suggesting the application of a spectrum class concept.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlam T., Neu H. C. In vitro comparison of the activity of RU 28965, a new macrolide, with that of erythromycin against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984 Apr;25(4):529–531. doi: 10.1128/aac.25.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle J. W., Thornsberry C. In vitro activity of rosamicin, josamycin, erythromycin, and clindamycin against beta-lactamase-nagative and beta-lactamase-positive strains of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1979 Feb;15(2):243–245. doi: 10.1128/aac.15.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. W., Tsai T. R., Orenstein W., Parkin W. E., Beecham H. J., Sharrar R. G., Harris J., Mallison G. F., Martin S. M., McDade J. E. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 1977 Dec 1;297(22):1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- Fuchs P. C., Thornsberry C., Barry A. L., Jones R. N., Gavan T. L., Gerlach E. H., Sommers H. M. Rosamicin: in vitro activity comparison with erythromycin and other antibiotics against clinical isolates from the genito-urinary tract and Neisseria meningitidis. J Antibiot (Tokyo) 1979 Sep;32(9):920–927. doi: 10.7164/antibiotics.32.920. [DOI] [PubMed] [Google Scholar]
- Gribble M. J., Chow A. W. Erythromycin. Med Clin North Am. 1982 Jan;66(1):79–89. doi: 10.1016/s0025-7125(16)31443-2. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C. In vitro evaluation of three new macrolide antimicrobial agents, RU28965, RU29065, and RU29702, and comparisons with other orally administered drugs. Antimicrob Agents Chemother. 1983 Aug;24(2):209–215. doi: 10.1128/aac.24.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Neu H. C. In vitro activity of midecamycin, a new macrolide antibiotic. Antimicrob Agents Chemother. 1983 Sep;24(3):443–444. doi: 10.1128/aac.24.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausbaugh L. J., Bolton W. K., Dilworth J. A., Guerrant R. L., Sande M. A. Comparative pharmacology of josamycin and erythromycin stearate. Antimicrob Agents Chemother. 1976 Sep;10(3):450–456. doi: 10.1128/aac.10.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Wilson W. R. Erythromycin: a microbial and clinical perspective after 30 years of clinical use (2). Mayo Clin Proc. 1985 Apr;60(4):271–278. doi: 10.1016/s0025-6196(12)60322-x. [DOI] [PubMed] [Google Scholar]
- Westerman E. L., Williams T. W., Jr, Moreland N. In vitro activity of josamycin against aerobic gram-positive cocci and anaerobes. Antimicrob Agents Chemother. 1976 Jun;9(6):988–993. doi: 10.1128/aac.9.6.988. [DOI] [PMC free article] [PubMed] [Google Scholar]


