Abstract
Objective
Poor penetration of anti-retroviral therapy across the blood brain barrier (BBB) poses an impediment on control of HIV-1 infection in brain macrophages. PPARγ, a member of the nuclear receptors family, regulates important physiological functions (including anti-inflammatory effects) in response to ligand-mediated activation. Since PPARγ agonists are rapidly absorbed by oral administration and efficiently permeate the BBB we hypothesized that PPARγ stimulation may suppress HIV-1 replication.
Design & Methods
We investigated the effect of PPARγ ligand (rosiglitazone) on HIV-1 replication in human monocyte-derived macrophages (MDM) and in vivo using a murine model (immunodeficient mice reconstituted with human lymphocytes and intracerebrally inoculated with HIV-1 infected macrophages) of HIV-1 encephalitis (HIVE).
Results
Treatment with rosiglitazone caused a significant decrease of virus infection in macrophages. PPARγ stimulation inhibited virus replication by modulating NF-κB activation in a receptor-depended manner, leading to down-regulation of HIV-1 LTR promoter activity and suppression of HIV-1 replication. These effects were PPARγ specific as PPARγ silencing or addition of PPARγ antagonist abolished effects of PPARγ stimulation on HIV-1 LTR and virus replication. Using a murine model for HIVE, we demonstrated that PPARγ ligand suppressed HIV-1 replication in macrophages in brain tissue and reduced viremia by 50%.
Conclusion
In vitro data delineated the novel mechanism by which PPARγ activation suppresses HIV-1 replication, and in vivo findings underscored the ability of PPARγ agonists to reduce HIV-1 replication in lymphocytes and brain macrophages offering new therapeutic intervention in brain and systemic infection.
Keywords: PPARγ, Suppression of HIV-1 replication, Rosiglitazone, Animal model of HIV-1 encephalitis, Brain macrophages
Introduction
Despite wide use of anti-retroviral therapy (ART), poor penetration of anti-retroviral drugs across the blood brain barrier (BBB) undermines the control of HIV-1 replication in brain macrophages, the primary targets of HIV-1 infection in the central nervous system (CNS) [1]. Mononuclear phagocytes (brain macrophages and microglia) serve as viral reservoirs in the brain and are potential sources of HIV-1 re-seeding after ART interruptions. Given inefficient penetration of anti-retroviral drugs across the BBB and chronic neuro-inflammation as a cause of HIV-1-associated dementia, the nuclear receptor, peroxisome proliferators-activated receptor gamma (PPARγ) could be an attractive therapeutic target.
PPARγ is ubiquitously expressed in many tissues and cell types, including monocytes, macrophages [2–5] and is up-regulated upon immune activation [6]. PPARs mediate transcriptional regulation of target genes by two mechanisms, transduction and transrepression. In the former, co-activators interact with nuclear receptors in a ligand-dependent manner affecting the gene transcription, while in the transrepression, gene transcription occurs via negative interference of PPARs with other signal-transduction pathways [3, 4]. After activation by specific agonists, these receptors form dimers and translocate to the nucleus, where they act as agonist-dependent transcription factors and regulate gene expression by binding to specific promoter regions of target genes (mechanism of up-regulated genes). However, for negative regulation, ligand activation of PPARγ antagonizes the activation of transcription factors (like activator protein −1, AP-1 and NF-κB), which results in suppression of gene expression [i.e. nitric oxide, tumor necrosis factor - α, TNFα, and interleukin −1β, IL-1β) [3, 4]. The potential therapeutic efficacy of PPARγ ligands in neuro-inflammatory and neurodegenerative diseases is highlighted by a large body of work in animal models for multiple sclerosis, Parkinson's and Alzheimer's diseases [7–14].
While PPARγ agonists were shown to inhibit HIV-1 replication in macrophages and T lymphocytes in vitro [15, 16], the mechanisms of PPARγ ligand effects remain elusive. The current study (1) delineated the pathway by which PPARγ ligands block HIV-1 replication and (2) explored the idea that PPARγ stimulation can efficiently block HIV-1 replication in vivo. Using a potent PPARγ synthetic ligand, rosiglitazone, we showed significant reduction of HIV-1 replication in human monocyte-derived macrophages (MDM). We found that the decrease in virus replication was in response to the down-modulation of HIV-1 LTR promoter activity by PPARγ via inhibition of NF-κB. Lastly, using a well-developed animal model for HIV-1 encephalitis, HIVE [17], we demonstrate efficient suppression of HIV-1 replication in brain macrophages and lymphocytes in blood suggesting that PPARγ activation can ameliorate HIV-1 CNS and systemic infection.
Materials and Methods
Cell isolation and culture
Monocytes and peripheral blood lymphocytes (PBL) were obtained by countercurrent centrifugal elutriation of leukopheresis packs from HIV-1, 2 and hepatitis B seronegative donors and monocytes were cultured as described [18].
HEK 293 and TZM-bl cells were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, from J. C. Kappes, X. Wu, and Tranzyme (Research Triangle Park, NC).
Virus infection, PPARγ ligand treatment and HIV-1 p24 ELISA
Monocytes or MDM (after 7 days in culture in presence of macrophage-colony stimulating factor) were pretreated with PPARγ ligand (rosiglitazone, 50 µM, Cayman Chemical, Ann Arbor, MI), PPARγ antagonist (GW9662, 50 µM) or 3'-azido-2', 3’-dideoxythymidine (AZT, 10µM) and infected for 18 hrs with macrophage-tropic strain, HIV-1ADA at multiplicity of infection of 0.1 [19]. MDM viability was evaluated by live/dead cell assay (Invitrogen, data not shown), and HIV-1 p24 levels were measured by ELISA (Beckman Coulter™, Miami, FL) according to manufacturer’s instructions.
DNA and short hairpin (sh) RNA against PPARγ
HIV-1 LTR and pCEP4 transactivation activator (Tat) expression plasmids constructs were obtained from AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. shRNA were designed against human PPARγ transcript (GenBankTM accession number NM138711) for knock down of PPARγ expression as described elsewhere[20]. Oligonucleotides coding for shRNA and scrambled were cloned into pRNAT-H1.1/Adeno vector and obtained from GenScript (Piscataway, NJ). The shRNA constructs were bicistronic for GFP expression, allowing for assessment of transfection efficiency (data not shown).
Transfection and luciferase assay
HEK 293 cells transient transfection was carried out using Lipofectamine 2000 (Invitrogen). Transfections of primary cells (monocytes or macrophages) were performed by Nucleofection according to the manufacturer’s instruction (Amaxa Biosystem, Gaithersburg, MD). In brief for each transfection, 9 × 106 cells were resuspended in 100 µl of Nucloefector™ solution either with the PPARγ response elements (PPRE, Panomics, Fremont, CA) linked to the luciferase reporter (1µg), HIV-1 LTR luciferase reporter (1µg) alone or together with PPARγ or scrambled shRNA (1µg). The instrument program (Y-01 for monocytes and Y-10 for macrophages) were used and the transfection efficiency determined by FACS was 85–89% and 50–60% for moncytes and macrophages repectively (data not shown). The luciferase reporter assays were performed on a fluorescence plate reader with luminometer function (M5, Molecular Devices, Sunnyvale, CA) according to the manufacturer’s protocol.
Western blotting
Protein lysates from human monocytes or macrophages (~9×106/per condition) 48 hrs post-transfection were resolved by SDS-PAGE. PPARγ or α-actin was detected by Western blotting using antibodies to PPARγ (SC-7273; Santa Cruz Biotech) at 1:1000 and α-actin (Chemicon, Temecula, CA) at 1:2000 dilutions, respectively.
PPAR γand NF-κB binding assay
PPARγ trancriptional activation and HIV-1 induced NF-κB p65 activation was determined using the TransAM kits (Active Motif, Carlsbad, CA). Nuclear extracts from macrophages or monocytes were prepared by using a nuclear extraction kit (Sigma). For HIV-1 induced NF-κB p65 activation monocytes/macrophages were transfected (HIV-1 LTR luciferase reporter) and pretreated with rosgilitazone (50µM) and infected for 30 min (maximum activation of NF-κB p65 was observed at 30 min post-infection). The assays were performed as per the manufacturer’s protocol and the transcriptional activation was quantified using a fluorescence plate reader (M5, Molecular Devices).
Generation of hu-PBL-NOD/SCID HIVE mice and drug administration
Four-week old male NOD/C.B-17 SCID mice purchased from Jackson Laboratory (Bar Harbor, ME) were maintained in sterile microisolator cages under pathogen-free conditions in accordance with ethical guidelines for care of laboratory animals at the University of Nebraska Medical Center (UNMC) and National Institutes of Health (NIH). The hu-PBL-NOD/SCID HIVE mice were generated as described [17]. Rosiglitazone pills were purchased from the UNMC pharmacy, pulverized, mixed with carboxymethylcellose and almond paste, and administered orally (10mg/kg/day). Drug treatment started one day prior to HIV-1 MDM intracerebral (i.c.) injection and continued for the entire duration of the experiment. Mice were sacrificed at 7, 14 and 21 d after i.c. injection with human MDM. In vivo experiments were repeated two times.
Flow cytometric analysis
FACS analysis was performed on mononuclear cells from blood and spleen at week 1 to 3 after i.c. injection of human MDM as described [18]. Data analysis was performed with a FACS Calibur™ using CellQuest software (Becton Dickinson Immunocytometry System, San Jose, CA). Fluorochrome-conjugated monoclonal Abs (mAbs) to human CD4, CD8, and CD3 were used and FITC-conjugated anti-mouse CD45 mAbs were included to exclude murine cells. Control and rosiglitazone treated MDM were labeled with anti-CXCR4 and anti-CCR5 fluorescent conjugates and sorted on a flow cytometer. Alexa Flour conjugated anti-PPARγ antibody (Santa Cruz Biotech) was used to detect PPARγ expression on CD14 positive gated macrophages. Otherwise mentioned, all the antibodies were obtained from eBioscience, San Diego, CA.
Histopathology and image analysis
Mice were sacrificed at 7, 14, and 21 d after i.c. injection of human MDM, tissues were fixed in 4% phosphate-buffered paraformaldehyde and paraffin embedded. Immunohistochemistry, histopathologic evaluation and image analyses were carried out as described [17]. Primary antibodies were purchased from Dako Carpentaria, CA (CD68, CD8, vimentin and HIV-1 p24). The numbers of CD8+, CD68+ MDM, and HIV-1 p24+ cells in each section were counted in a blinded fashion by three independent observers.
Statistical analysis
Results were presented as mean plus or minus (±) SD, and P values < 0.05 were considered significant. Data were analyzed using Prism (Graph Pad) and statistical significance for multiple comparisons was assed by one-way ANOVA with Newman-Keuls post-test. All in vitro assays were repeated at least two to three times (each condition in triplicate).
Results
Rosiglitazone inhibits HIV-1 replication in human MDM
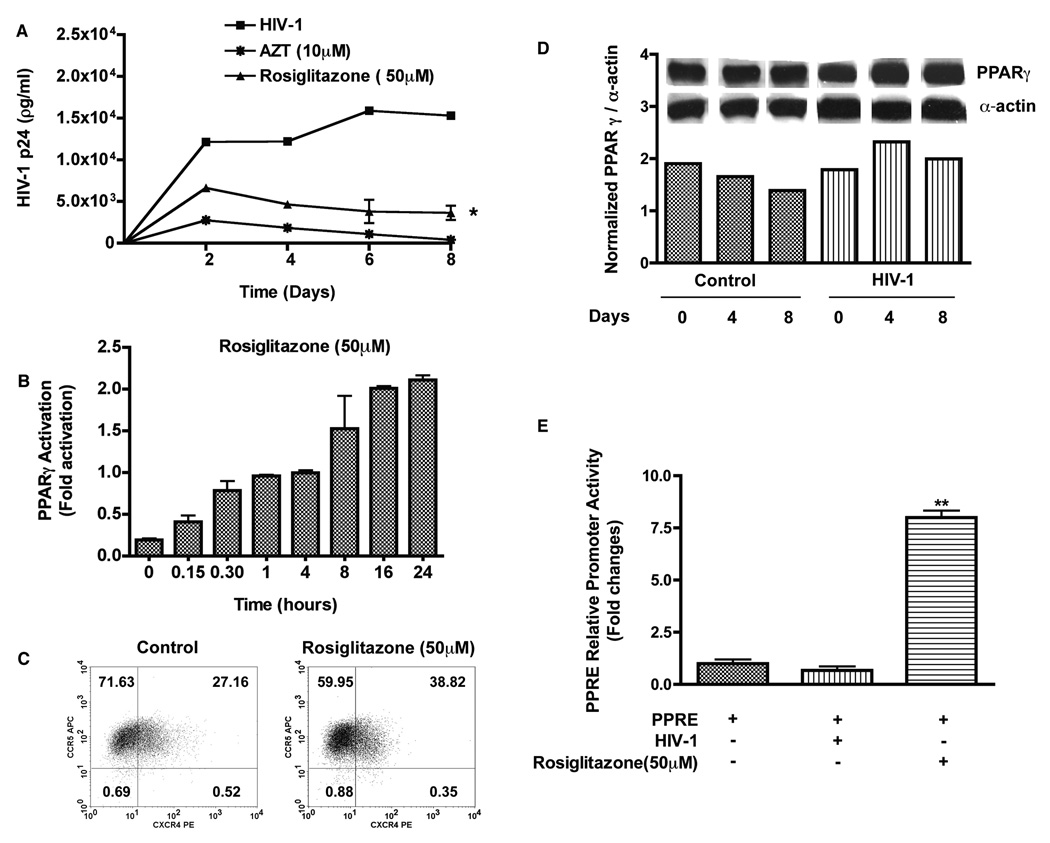
PPARγ activation by ligands such as thiazolidinediones (TZDs) have been shown to play a role in cellular proliferation, differentiation, inflammation [21, 22], and inhibition of HIV-1 replication [15]. Rosiglitazone (50 µM) treated macrophages produced lower level of HIV-1 p24 after infection (P < 0.05, Fig.1 A). Rosiglitazone (50 µM) activated PPARγ as indicated by stimulation of PPARγ transcriptional activity (Fig. 1 B). Given the complex biological effects of PPARγ ligand [23] and decreased PPARγ expression in HIV-1-infected patients [24], we tested whether rosiglitazone had any affect on HIV-1 co-receptors expression and whether HIV-1 infection modulated PPARγ protein expression in macrophages. Expression levels of CXCR4/CCR5 receptors (Fig. 1 C) and PPARγ protein levels (Fig. 1 D) were not down regulated in macrophages treated with rosiglitazone or infected with HIV-1. Similar experiments using luciferase reporter assay for PPRE further established that infection alone had no effect on PPARγ activity whereas macrophage treatment with rosiglitazone (50 µM) induced a 7.5 fold increase in PPARγ reporter activity (Fig. 1 E). Taken together, these data suggest that rosigiltazone induced PPARγ activation and that this stimulation may be involved in inhibition of HIV-1 infection in macrophages.
Figure 1. Rosiglitazone activates PPARγ and suppresses HIV-1 replication in MDM.
MDM were pre-treated with rosiglitazone (50 µM) for 18 hrs. Cells were infected with macrophage-tropic HIV-1ADA (at a multiplicity of infection of 0.1) for 12–18 hrs following which cultures were washed to remove any residual virus and replenished with fresh media. Half media exchange was performed every 2–3 days. (A) Levels of HIV-1 p24 antigen in culture supernatants. (B) Macrophages were treated with rosiglitazone (50 µM) for 0–24 hrs and the levels of PPARγ activity was measured by the TransAM PPARγ Kit. Results are expressed as fold changes in activation compared to control. (C) Representative flow cytometer scatter plots depicting CXCR4 and CCR5 expression in macrophages. (D) Expressions of PPARγ protein in control and HIV-1 infected human macrophages. (E) MDM were transfected with 1µg of PPRE luciferase reporter plasmid. Cells were harvested 24 hrs following infection or after treatment with rosiglitazone (50 µM) and luciferase activity of cell lysates was determined. Results are expressed as fold changes compared to basal luciferase activity. Data shown are mean ± SD of three independent experiments performed with three different MDM donors. *; P< 0.05 (PPARγ ligand treated vs. untreated HIV-1 infected MDM, **; P< 0.05 (Rosiglitazone treated vs. HIV-1 infected MDM.
PPARγ activation suppresses HIV-1 LTR promoter activity
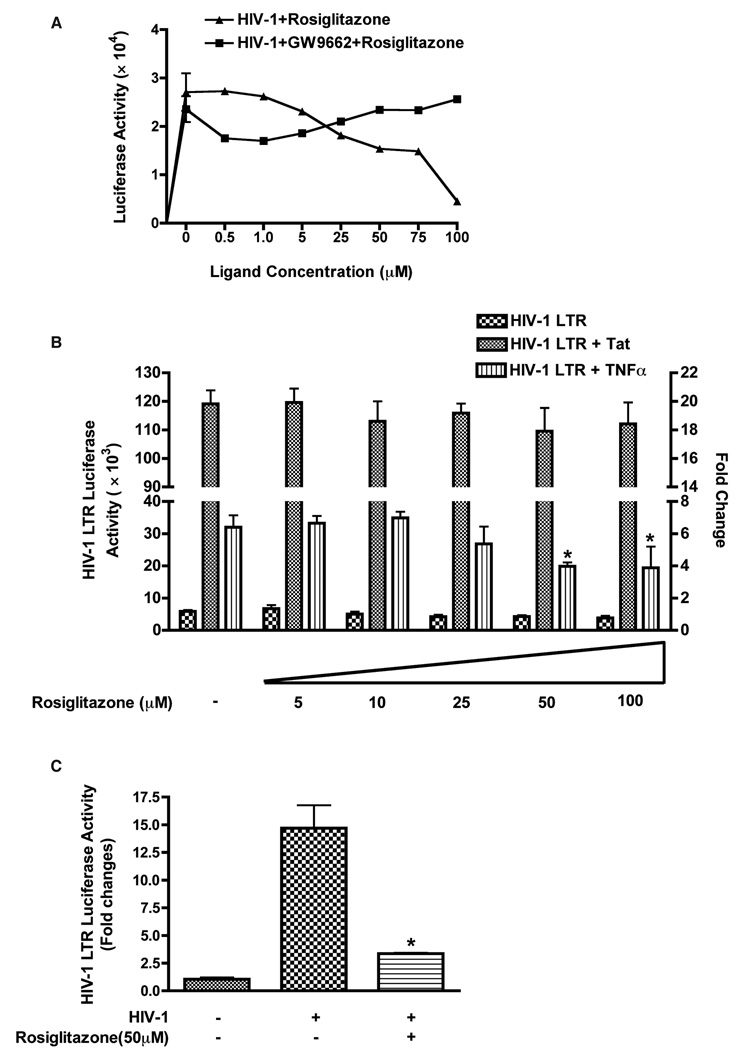
Expression of HIV-1 provirus is regulated by interaction of cellular and viral transcription factors that bind to HIV-1 LTR [25–27]. Therefore, we explored whether activation of PPARγ could suppress HIV-1 LTR activity. To test this idea, TZM-bl cells were infected with HIV-1ADA, and 48 hrs post-infection luciferase levels (indicating HIV-1 LTR activity) were determined. Rosiglitazone treatment (0–100 µM) resulted in a dose dependent suppression of HIV-1 LTR activity and PPARγ antagonist (GW9662, 50 µM) abolished the effects of rosiglitazone (Fig. 2 A). Since Tat is a powerful viral transactivator and plays a central role in viral replication, we tested whether activation of PPARγ could suppress HIV-1 LTR activity. Rosiglitazone at various concentrations (0–100 µM) did not significantly affect induction of HIV-1 LTR by Tat. However, Tat-independent transactivation of the HIV-1 LTR by tumor necrosis factor α (TNFα, 100 ng/ml) was suppressed (Fig. 2 B). Since transcriptional activation of the HIV LTR by TNFα is associated with the induction of a nuclear factor(s) binding to the NF-kB sites of the LTR, it is likely that the PPARγ mediated LTR suppression is mediated by cellular factors rather than viral elements.
Figure 2. PPARγ activation suppressed HIV-1 LTR promoter activity.
(A) Promoter activity in HIV-1 infected TZM-bl cells either treated with various concentration of PPARγ ligand (rosiglitazone, 0–100 µM) alone or in combination of PPARγ ligand (rosiglitazone, 0–100 µM) and antagonist (GW9662, 50 µM). (B) HEK 293 cells were co-transfected with HIV-1 LTR luciferase (10 ng) and Tat construct, pCEP4 Tat (50 ng). Untransfected cells as well as cells transfected with empty vectors (PUC 19) served as negative controls. Cells were infected with HIV-1ADA (at a multiplicity of infection of 0.1) for 4 hrs and cells lysates were collected 48 hrs later. In separate experiments, HIV-1 LTR luciferase transfected 293 cells were incubated with or without TNFα (100 ng) in absence or presence of various concentrations (0–100 µM) of rosiglitazone. Cells were harvested and luciferase assay was performed and normalized for renella luciferase activity. Results are expressed as fold changes compared to basal luciferase activity. Data shown are mean ± SD of five independent experiments. (C) Primary human monocytes transfected with HIV-1 LTR-luciferase in absence or presence of rosiglitazone (50 µM) were infected with HIV-1ADA (at a multiplicity of infection of 0.1) and 48 hrs post-infection luciferase activity was determined. The mean value of the relative promoter activity is shown mean ± SD from three independent experiments. *; P< 0.05 (rosiglitazone treated vs. untreated TNFα incubated HIV-1 LTR transfected 293 cells). *; P< 0.01 (rosiglitazone treated vs. untreated HIV-1 infected primary monocytes).
Monocytes are the cells presumably carrying HIV-1 to the brain [28] where they establish viral reservoirs differentiating into macrophages. Therefore, we investigated effects of PPARγ stimulation in primary human monocytes. Infection of monocytes transfected with HIV-1 LTR luciferase induced fifteen-fold increase in LTR activity, and rosiglitazone treatment resulted in its five-fold inhibition (Fig. 2 C). Collectively, these results suggested that PPARγ stimulation led to suppression of HIV-1 LTR activation.
PPARγ silencing impairs the effects of rosiglitazone on HIV-1 suppression and binding of NF-κB to HIV-1 LTR
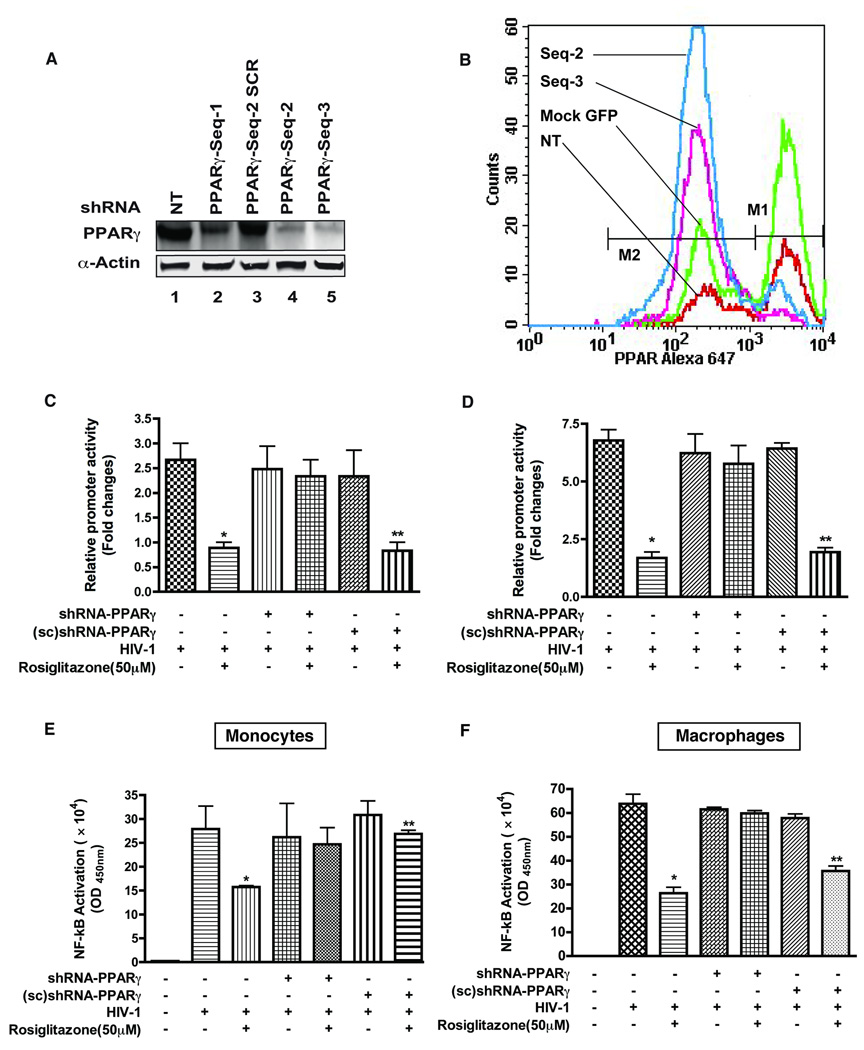
To investigate the specificity of rosiglitazone effects on HIV-1 LTR activation, we sought to knock down expression of PPARγ by shRNA. Multiple shRNA constructs targeting PPARγ nuclear receptors were designed and the sequence (shPPARγ-2) that provided most optimal knockdown of endogenous level of PPARγ in primary human monocytes (Fig. 3 A) and macrophages (Fig. 3 B) was selected. HIV-1 LTR activation was efficiently suppressed by rosiglitazone in HIV-1 infected cells and also in those that were transfected with the scramble shRNA (control). In contrast, expression of PPARγ shRNA in cells infected and treated with rosiglitazone resulted in lack of HIV-1 LTR suppression both in monocytes (Fig. 3 C) and macrophages (Fig. 3 D). These results indicated that rosiglitazone mediated effect were PPARγ specific and rule out the possible involvement of other PPAR subtypes in mediating the effect of rosiglitazone on HIV-1 suppression.
Figure 3. Decreased expression of PPARγ by shRNA knockdown abolished the effect of PPARγ ligand activation.
(A) Analysis of shRNA knock-down of PPARγ protein (labeled shPPARγ-seq) in primary human monocytes expressing three different RNA hairpins directed at PPARγ (lanes 2, 4 and 5) along with control shRNA (scrambled sequence 2, lane 3). (B) Evaluation of PPARγ knockdown by flow cytometery in macrophages expressing RNA hairpins to PPARγ. Shift in greater number of macrophages transfected with shPPARγ-seq-2 towards PPARγ low intensity mean fluorescence is indicative of decreased expression of PPARγ. Primary human monocytes (C and E) and macrophages (D and F) were transfected with either HIV-1 LTR-lucifrease alone or co-transfected with shRNA-PPARγ/ (sc) shRNA-PPARγ by nucleofection. Luciferase assay was measured 48 hrs post-infection and expressed as the relative fold changes compared to mock-control. Nuclear extracts from cells were assayed for NF-κB p65 activation using the TransAM NF-κB p65 Chemi Kit. PPARγ ligand treatment inhibits HIV-1 induced NF-κB activation and subsequently NF-κB binding. Results are shown as mean ± SD of three independent experiments. *; P< 0.001 (Rosiglitazone treated HIV-1 infected vs. Rosiglitazone treated HIV-1 infected shRNA-PPARγ), **; P< 0.05 (Rosiglitazone treated HIV-1 infected (sc) shRNA-PPARγ vs. HIV-1 infected (sc) shRNA-PPARγ).
Because transrepression or negative interaction with NF-κB are suspected to underlie the effects of PPARγ stimulation [29–36] and NF-κB is one of the central transcription factors that bind the HIV-1 LTR, we investigated the effect of rosiglitazone on the DNA-binding activity of NF-κB. Rosiglitazone treatment of monocytes (Fig. 3 E) or macrophages (Fig. 3 F) diminished HIV-1 induced NF-κB DNA-binding activity as compared to control (P < 0.05). Transfection of shRNA vectors, to knock down endogenous PPARγ expression abolished the effect of rosiglitazone on the DNA-binding activity while irrelevant shRNA had no effect. Together, these results suggest that PPARγ ligand could suppress HIV-1 replication via modulation of binding of NF-κB to HIV-1 LTR promoter.
Rosiglitazone suppressed viremia in animal model for HIV-1 infection
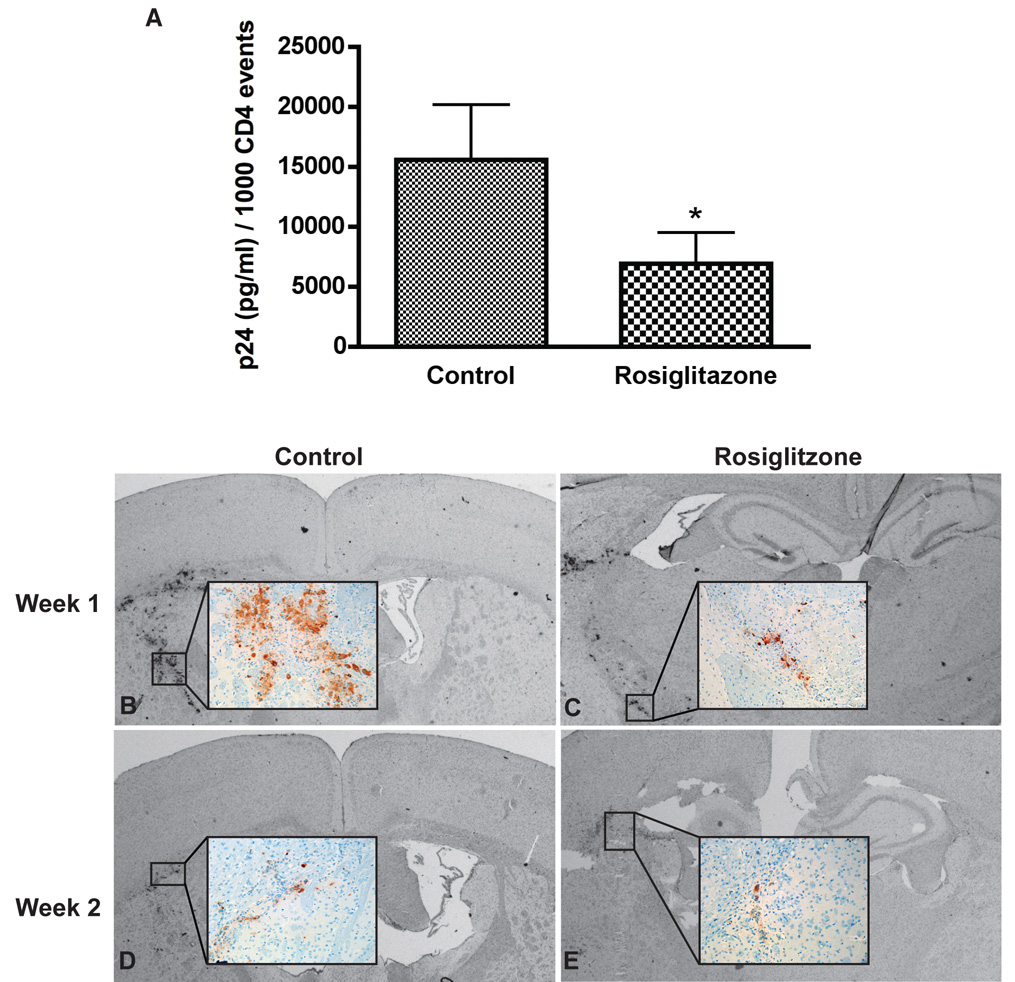
To determine whether PPARγ ligand can suppress HIV-1 replication in vivo, we investigated the effect of rosiglitazone in our mouse model for HIVE [17, 18]. NOD/SCID mice were reconstituted with human PBL (hu-PBL-NOD/SCID) and inoculated i.c. with autologous HIV-1 infected MDM. Animals (n = 5–8 mice/group/week) were orally fed daily with rosiglitazone (10mg/kg) or placebo and sacrificed at weeks 1, 2, and 3 after MDM inoculation. Similar percentage of CD4+, CD8+, and CD3+ cells observed between the two groups (data not shown) assessed by flow cytometry analysis on cells derived from blood and spleen of hu-PBL-NOD/SCID HIVE mice assured comparability of the results between groups. Viremia was measured by ELISA (HIV-1 p24) and expressed in pg/ml per 1000 CD4+ cells. Two weeks following the i.c. injection of HIV-1 infected MDM, both rosiglitazone and control animals (33.7 ± 9. 5 versus 37.3 ± 15.7) had equally low levels of HIV-1 p24. At week 3, when infection spread from HIV- 1 infected MDM to circulating CD4+ lymphocytes control demonstrated high levels of viremia while rosiglitazone treated mice had 50% reduction in HIV-1 p24 levels (P < 0.02, Fig. 4 A).
Figure 4. Viral replication in control and rosiglitazone-fed mice.
(A) Viremia levels were measured by ELISA assays (Beckman Coulter™, Miami, FL) according to manufacturer’s instructions and expressed in pg/ml per 1000 CD4+ cells. Animals were orally fed daily with rosiglitazone (10mg/kg) or placebo. Rosiglitazone-fed mice showed 50% reduction of viremia compared with control group. Rosiglitazone treated mice (B and D) contained fewer HIV-1 p24 MDMs as compared with controls (C and E). Vectastain Elite Kit was used to detect Primary Ab and visualized by using DAB as a substrate. Original magnification for panels A and B: 10 (insets, 200). Results are presented as mean ± SD *; P< 0.02 (rosiglitazone vs. control).
Rosiglitazone suppressed HIV-1 replication in brain macrophages in hu-PBL-NOD/SCID HIVE mice
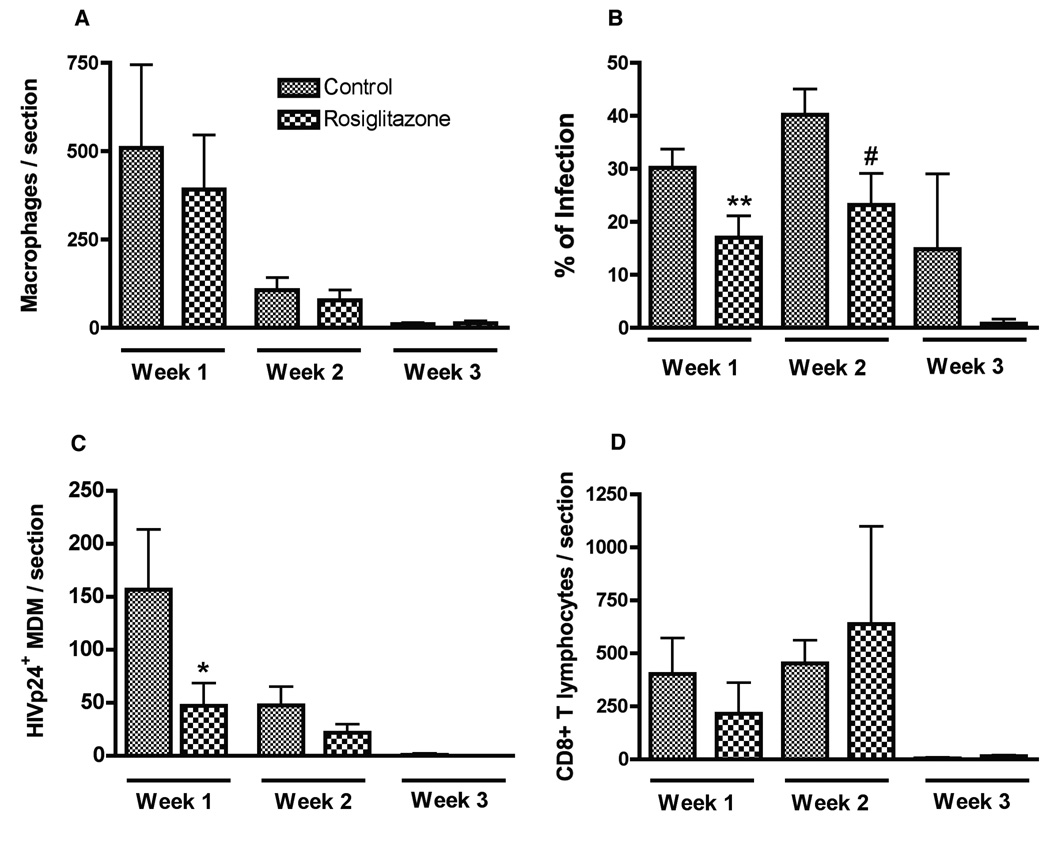
To examine whether rosiglitazone treatment can affect HIV-1 replication in brain macrophages, serial brain sections were immunostained for human CD68 (marker for human MDM), HIV-1 p24 (viral antigen), and human CD8 (T lymphocytes indicating anti-viral responses). A prominent reduction of HIV-1 p24+ MDM was noted in rosiglitazone treated mice (Fig. 4 C) at week 1 as compared with controls (Fig. 4 B). Similarly, at week 2 rosiglitazone treated mice (Fig. 4 E) featured fewer HIV-1 p24+ MDM (Fig. 4 D). The level of virus replication, elimination of virus-infected macrophages and CD8 cell infiltration were quantitatively analyzed by counting CD68+, HIV-1 p24+, and CD8+ lymphocytes in brain tissue sections in a blind fashion by three independent observers. As shown in (Fig. 5 A), the mean numbers of CD68+ MDM in rosiglitazone and control group were similar at weeks 1–3. The percentage of infected macrophages was significantly lower in rosiglitazone as compared with controls at week 1 and 2 (P < 0.05, Fig. 5 B), and a similar trend was found at week 3. At week 1, fewer HIV-1 p24+MDM were found in rosiglitazone mice as compared with controls (130 ± 34.8 vs. 47.2 ± 21.5, P < 0.05, Fig. 5 C). A similar trend existed at week 2, however, differences failed to reach statistical significance (47.9 ± 17.2 vs. 22 ± 7.8, P > 0.05). Comparable amounts of CD8+ T cells were detected at weeks 1 and 2 in rosiglitazone and control mice (Fig. 5 D). These finding suggest that oral administration of rosiglitazone was effective in diminishing viral replication in brain macrophages and blood CD4+ lymphocytes of hu-PBL-NOD/SCID HIVE mice.
Figure 5. PPARγ ligand suppresses HIV-1 replication in brain macrophages in hu-PBL-NOD rosiglitazone /SCID HIVE mice.
Serial brains sections were immunostained for human macrophages (CD68+), HIV-1 p24+ MDM (viral antigen), and human lymphocytes (CD8+). Coronal brain sections were cut first to identify the injection site containing MDM. Serial 5-µm-thick sections of the brain (30 to 100) covering the entire area of human MDM injection were cut for each mouse brain and matched 3 to 7 slides (10 sections apart) were analyzed. Mean numbers of stained cells per section within the injected hemisphere were calculated for each mouse (3–7 sections/mouse), and a total of 5–8 mice per group were examined. Number of CD68+ MDM (A), percentage of infected MDM (B), number of infected MDM (C), and CD8+ lymphocytes (D) were counted in brain tissue section of control and rosiglitazone-fed hu-PBL-NOD/SCID HIVE mice. Rosiglitazone-fed mice demonstrated fewer HIV-1 p24+ MDM as compared to controls at week 1 (C). PPARγ treatment significantly decreased percentage of HIV-1 p24+ MDM rosiglitazone-fed mice at weeks 1 and 2 (B). Value represents mean ± SD per 5-µm section (n=5–8 mice/group). *,**, #; P< 0.05 (rosiglitazone vs. control).
Discussion
Several lines of evidence indicate that PPARγ natural and synthetic ligands such as TZDs control brain inflammation by inhibiting microglial activation, and synthesis of nitric oxide, prostaglandins, chemokines and inflammatory cytokines [12, 14, 37, 38]. CNS inflammation during HIV-1 infection is driven in part by HIV-1 replication, which utilizes cellular transcription factors. Hence, we sought to evaluate whether PPARγ, via inhibition of transcription factors, important in pro-inflammatory responses, could attenuate HIV-1 replication. PPARγ ligand treatment resulted in a significant decrease of virus infection in primary human MDM, the major virus reservoir in the brain. TZDs are rapidly absorbed by oral administration and efficiently permeate the BBB [39]. Capitalizing on this ability of TZD, we evaluated the effect of PPARγ activation on HIV-1 infection in a mouse model of HIVE. Animals fed with PPARγ ligand, rosiglitazone showed 50% reduction of viremia and diminished HIV-1 replication in human MDM inoculated into mice brains.
PPARγ, a member of the family of nuclear factors is expressed in abundance in macrophages and lymphocytes [4, 40]. In addition to up-regulation of target genes, PPARγ also forms repressor complexes (transrepression) to down-regulate genes induced by other transcription factors (reviewed in [41] [42]). It is through transrepression that PPARγ ligands act as anti-inflammatory agents via inhibition of AP-1, NF-κB, and STAT-1 either by direct interaction or sequestration of essential cofactors [4, 36, 43, 44]. Although previously Hayes and colleagues [15] speculated that transcriptional and post-transcriptional effects could be responsible for inhibited virus replication in macrophages by PPARγ ligands, the mechanism of virus suppression remained elusive. Our study found that activation of PPARγ receptor with ligand rosiglitazone suppressed HIV-1 LTR activity both in a HIV-1 LTR stable transfected cell line and in primary mononuclear cells. Moreover, the replication of HIV-1 was inhibited by rosiglitazone in human macrophages. We also showed that the effect of the ligand was specific to PPARγ, since rosiglitazone failed to attenuate the induction of HIV-1 LTR promoter activity (HIV-1 infection) in monocytes expressing PPARγ specific shRNA or in presence of antagonist.
The role of NF-κB in activation of HIV-1 transcription was analyzed [45] which is of particular importance as it is stimulated by several cytokines involved in immune and inflammatory responses [25]. NF-κB is one of the several transcription factors that strongly induces HIV-1 LTR. PPARγ activation in HIV-1 infected monocytes reduced the NF-κB-DNA binding activity similar to findings in other studies where PPARγ stimulation down-regulated NF-κB-DNA binding in a variety of cell types [46, 47]. Consequently, ligand treatment had no effect on NF-κB binding in infected monocytes with diminished PPARγ receptor expression. There is a possibility that PPARγ could inhibit LTR activity by interfering with HIV-1 protein, Tat. Tat is a key regulatory protein of HIV-1 and its ability to stimulates HIV transcription from the LTR [48] via RNA rather than DNA promoter elements is unique amongst other transcriptional activators. While HIV-1 LTR contains binding sites for multiple cellular transcription factors, it is primarily targeted by HIV-1 Tat. Interestingly, PPARγ had no effect on HIV-1 Tat mediated induction of the LTR, which explains the partial inhibition of HIV-1 replication (via NF-κB inhibition). These findings indicated that suppression of HIV-1 replication in monocytes/macrophages is achieved via suppression of the NF-κB activation.
Our results suggested that PPARγ ligand activation in HIV-1 infected monocytes down-regulated NF-κB DNA activity through a transrepression that involves p65 subunit of NF-κB and PPARγ resulting in decreased HIV-1 LTR transcription and HIV-1 virus replication. Nevertheless, the possibility of PPARγ ligand mediated suppression of HIV-1 virus independent of NF-κB transrepression cannot be completely ruled out. For instance, PPAR ligands can activate kinases that lead to phosphorylation cascades that trigger transcriptional changes leading to several levels of downstream regulation (reviewed by Diradourian et al. [49] and Gardner et al. [50]), can alter ligand binding, co-repressor/co-activator recruitment, or heterodimerization with retinoid X receptor α all influencing PPAR-mediated transcription (reviewed in Peraza et al. [51]). Whether these interactions play any role in suppression of HIV-1 still remains to be elucidated.
Beneficial effects of PPARγ stimulation were demonstrated in a variety of animal models of inflammatory and neurodegenerative disorders (reviewed by Moreas et al. [52], Bernado and Minghetti [37]). Our in vivo model of HIVE recapitulates adaptive and innate immune responses, immunopathological features of HIV-1 CNS infection (including neuro-inflammation and neuronal demise), and viremia, [17, 53]. These features are relevant for chronic immune activation seen in HIV-1 infection driven by activation of NF-κB and increased viral transcription [54–56].
In vivo results supported the in vitro observations showing that PPARγ receptor stimulation reduced viremia and suppressed HIV-1 infection in MDM in the brain. As CNS macrophages are a major viral reservoir poorly accessible by ART [57], these findings are of particular interest since effects of PPARγ ligand on HIV-1 replication might not be prevailed by viral mutations. The dose of rosiglitazone used in our animal studies did not affect PBL engraftment levels and was within the range used for patient treatment. Antigen-specific CD8+ T lymphocytes serve a prominent role in the control of HIV-1 infection of brain macrophages in the hu-PBL-NOD/SCID mouse model of HIVE [17, 18]. PPARγ treatment did not affect the number of brain infiltrating CD8+ T lymphocytes. Whether PPARγ stimulation provides additional immunomodulatory or anti-inflammatory effects awaits future investigation.
In summary, our results suggest that the suppressive mechanism of PPARγ ligand involves transrepression of NF-κB and decreased HIV-1 LTR promoter transcription. In vitro and in vivo data strongly support potential use of PPARγ for treatment of HIVE and suppression of HIV-1 replication in lymphocytes and MDM thus offering new therapeutic intervention in brain and systemic infection.
Acknowledgment
The authors thank Drs. Sanjay Maggirwar and Tsuneya Ikezu for critical reading of the manuscript. The authors appreciate the excellent administrative support from Ms. Robin Taylor and Ms. Pamela Ferrick.
This work was supported by NIH grants RO1 AA15913, PO1 NS043985, RO1 MH65151 to Y.P
Footnotes
Conflict of Interest: The authors have no conflicting financial interests.
References
- 1.Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- 2.Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 3.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 4.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 5.Denning GM, Stoll LL. Peroxisome proliferator-activated receptors: potential therapeutic targets in lung disease? Pediatr Pulmonol. 2006;41:23–34. doi: 10.1002/ppul.20338. [DOI] [PubMed] [Google Scholar]
- 6.Ricote M, Huang J, Fajas L, Li A, Welch J, Najib J, et al. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klotz L, Schmidt M, Giese T, Sastre M, Knolle P, Klockgether T, Heneka MT. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J Immunol. 2005;175:4948–4955. doi: 10.4049/jimmunol.175.8.4948. [DOI] [PubMed] [Google Scholar]
- 8.Kiaei M, Kipiani K, Chen J, Calingasan NY, Beal MF. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2005;191:331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Hume DA, Fairlie DP. Therapeutic targets in inflammatory disease. Curr Med Chem. 2005;12:2925–2929. doi: 10.2174/092986705774462923. [DOI] [PubMed] [Google Scholar]
- 10.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 11.Niino M, Iwabuchi K, Kikuchi S, Ato M, Morohashi T, Ogata A, et al. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by an agonist of peroxisome proliferator-activated receptor-gamma. J Neuroimmunol. 2001;116:40–48. doi: 10.1016/s0165-5728(01)00285-5. [DOI] [PubMed] [Google Scholar]
- 12.Kielian T, Drew PD. Effects of peroxisome proliferator-activated receptor-gamma agonists on central nervous system inflammation. J Neurosci Res. 2003;71:315–325. doi: 10.1002/jnr.10501. [DOI] [PubMed] [Google Scholar]
- 13.Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J Neurosci. 2004;24:10908–10917. doi: 10.1523/JNEUROSCI.3987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Hayes MM, Lane BR, King SR, Markovitz DM, Coffey MJ. Peroxisome proliferator-activated receptor gamma agonists inhibit HIV-1 replication in macrophages by transcriptional and post-transcriptional effects. J Biol Chem. 2002;277:16913–16919. doi: 10.1074/jbc.M200875200. [DOI] [PubMed] [Google Scholar]
- 16.Skolnik PR, Rabbi MF, Mathys JM, Greenberg AS. Stimulation of peroxisome proliferator-activated receptors alpha and gamma blocks HIV-1 replication and TNFalpha production in acutely infected primary blood cells, chronically infected U1 cells, and alveolar macrophages from HIV-infected subjects. J Acquir Immune Defic Syndr. 2002;31:1–10. doi: 10.1097/00126334-200209010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, et al. Inhibition of Indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in animal model of HIV-1 encephalitis. Blood. 2005;106:2383–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potula R, Haorah J, Knipe B, Leibhart J, Chrastil J, Heilman D, et al. Alcohol abuse enhances neuroinflammation and impairs immune responses in an animal model of human immunodeficiency virus-1 encephalitis. Am J Pathol. 2006;168:1335–1344. doi: 10.2353/ajpath.2006.051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of Peroxisome Proliferator-Activated Receptor {gamma} (PPAR{gamma}) Suppresses Rho GTPases in Human Brain Microvascular Endothelial Cells and Inhibits Adhesion and Transendothelial Migration of HIV-1 Infected Monocytes. J Immunol. 2008;180:1854–1865. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klappacher GW, Glass CK. Roles of peroxisome proliferator-activated receptor gamma in lipid homeostasis and inflammatory responses of macrophages. Curr Opin Lipidol. 2002;13:305–312. doi: 10.1097/00041433-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 23.Lemay DG, Hwang DH. Genome-wide identification of peroxisome proliferator response elements using integrated computational genomics. J Lipid Res. 2006;47:1583–1587. doi: 10.1194/jlr.M500504-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Bastard JP, Caron M, Vidal H, Jan V, Auclair M, Vigouroux C, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359:1026–1031. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 25.Rohr O, Marban C, Aunis D, Schaeffer E. Regulation of HIV-1 gene transcription: from lymphocytes to microglial cells. J Leukoc Biol. 2003;74:736–749. doi: 10.1189/jlb.0403180. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y. HIV-1 gene expression: lessons from provirus and non-integrated DNA. Retrovirology. 2004;1:13. doi: 10.1186/1742-4690-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira LA, Bentley K, Peeters A, Churchill MJ, Deacon NJ. A compilation of cellular transcription factor interactions with the HIV-1 LTR promoter. Nucleic Acids Res. 2000;28:663–668. doi: 10.1093/nar/28.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genolet R, Wahli W, Michalik L. PPARs as drug targets to modulate inflammatory responses? Curr Drug Targets Inflamm Allergy. 2004;3:361–375. doi: 10.2174/1568010042634578. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto H, Iwamoto T, Kotake S, Momohara S, Yamanaka H, Kamatani N. Inhibition of NF-kappaB signaling by fenofibrate, a peroxisome proliferator-activated receptor-alpha ligand, presents a therapeutic strategy for rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:323–330. [PubMed] [Google Scholar]
- 31.Hisada S, Shimizu K, Shiratori K, Kobayashi M. Peroxisome proliferator-activated receptor gamma ligand prevents the development of chronic pancreatitis through modulating NF-kappaB-dependent proinflammatory cytokine production and pancreatic stellate cell activation. Rocz Akad Med Bialymst. 2005;50:142–147. [PubMed] [Google Scholar]
- 32.Fan W, Yanase T, Morinaga H, Mu YM, Nomura M, Okabe T, et al. Activation of peroxisome proliferator-activated receptor-gamma and retinoid X receptor inhibits aromatase transcription via nuclear factor-kappaB. Endocrinology. 2005;146:85–92. doi: 10.1210/en.2004-1046. [DOI] [PubMed] [Google Scholar]
- 33.Chen F, Harrison LE. Ciglitazone induces early cellular proliferation and NF-kappaB transcriptional activity in colon cancer cells through p65 phosphorylation. Int J Biochem Cell Biol. 2005;37:645–654. doi: 10.1016/j.biocel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Vanden Berghe W, Vermeulen L, Delerive P, De Bosscher K, Staels B, Haegeman G. A paradigm for gene regulation: inflammation, NF-kappaB and PPAR. Adv Exp Med Biol. 2003;544:181–196. doi: 10.1007/978-1-4419-9072-3_22. [DOI] [PubMed] [Google Scholar]
- 35.Chen F, Wang M, O'Connor JP, He M, Tripathi T, Harrison LE. Phosphorylation of PPARgamma via active ERK1/2 leads to its physical association with p65 and inhibition of NF-kappabeta. J Cell Biochem. 2003;90:732–744. doi: 10.1002/jcb.10668. [DOI] [PubMed] [Google Scholar]
- 36.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, et al. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 37.Bernardo A, Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- 38.Park EJ, Park SY, Joe EH, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278:14747–14752. doi: 10.1074/jbc.M210819200. [DOI] [PubMed] [Google Scholar]
- 39.Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung. 1997;47:29–35. [PubMed] [Google Scholar]
- 40.Ricote M, Valledor AF, Glass CK. Decoding transcriptional programs regulated by PPARs and LXRs in the macrophage: effects on lipid homeostasis, inflammation, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:230–239. doi: 10.1161/01.ATV.0000103951.67680.B1. [DOI] [PubMed] [Google Scholar]
- 41.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 42.Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, Stemerman MB. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- 44.Yang XY, Wang LH, Chen T, Hodge DR, Resau JH, DaSilva L, Farrar WL. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 45.Hiscott J, Kwon H, Genin P. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J Clin Invest. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 47.Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, Kim TS. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J Biol Chem. 2000;275:32681–32687. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- 48.Barboric M, Peterlin BM. A new paradigm in eukaryotic biology: HIV Tat and the control of transcriptional elongation. PLoS Biol. 2005;3:e76. doi: 10.1371/journal.pbio.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diradourian C, Girard J, Pegorier JP. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie. 2005;87:33–38. doi: 10.1016/j.biochi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Gardner OS, Dewar BJ, Graves LM. Activation of mitogen-activated protein kinases by peroxisome proliferator-activated receptor ligands: an example of nongenomic signaling. Mol Pharmacol. 2005;68:933–941. doi: 10.1124/mol.105.012260. [DOI] [PubMed] [Google Scholar]
- 51.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol Sci. 2006;90:269–295. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- 52.Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Poluektova LY, Munn DH, Persidsky Y, Gendelman HE. Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J Immunol. 2002;168:3941–3949. doi: 10.4049/jimmunol.168.8.3941. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Perkins ND, Schmid RM, Nabel GJ. Specific NF-kappa B subunits act in concert with Tat to stimulate human immunodeficiency virus type 1 transcription. J Virol. 1992;66:3883–3887. doi: 10.1128/jvi.66.6.3883-3887.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West MJ, Lowe AD, Karn J. Activation of human immunodeficiency virus transcription in T cells revisited: NF-kappaB p65 stimulates transcriptional elongation. J Virol. 2001;75:8524–8537. doi: 10.1128/JVI.75.18.8524-8537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunt G, Tiemessen CT. Occurrence of additional NF-kappaB-binding motifs in the long terminal repeat region of South African HIV type 1 subtype C isolates. AIDS Res Hum Retroviruses. 2000;16:305–306. doi: 10.1089/088922200309412. [DOI] [PubMed] [Google Scholar]
- 57.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]