Abstract
The failure to reject the semiallogenic fetus by maternal T lymphocytes suggests that potent mechanisms regulate these cells. PDCD1 is a CD28 family receptor expressed by T cells, and its ligand CD274 is strongly expressed by trophoblast cells of the human placenta. In this study, we examined whether human maternal T cells express PDCD1. Immunofluorescence examination of uterine tissues revealed PDCD1 expression on CD3+ cells was low in nonpregnant endometrium but increased in first-trimester decidua and remained elevated in term decidua (P < 0.05). In addition, higher relative proportions of term decidual CD8bright, CD4+, and regulatory T cells expressed PDCD1 in comparison to autologous peripheral blood (P < 0.05). Term decidual T cells also expressed full-length and soluble PDCD1 mRNA isoforms more abundantly than their peripheral blood counterparts (P ≤ 0.05). We also examined the effects of PDCD1:CD274 interactions in decidual T cells. Jar choriocarcinoma cells were transfected with CD274 and cocultured with activated decidual CD4+ or CD8bright T cells for 72 h followed by analysis of cytokine concentration and decidual T cell apoptosis. Compared with empty vector-transfected cells, CD274-transfected Jar cells caused a significant suppression of interferon gamma and tumor necrosis factor alpha production by CD4+ (P < 0.05) but not CD8bright T cells, while having no effect on secretion of IL10 or T cell apoptosis. These results suggest that the PDCD1:CD274 pathway functions in modification of maternal decidual lymphocyte cytokine secretion during pregnancy.
Keywords: B7-H1, CD274, CD279, decidua, PD-1, PDL1, PDCD1, placenta, T cells
The PDCD1 immunoinhibitory receptor is preferentially expressed on decidual T cell subpopulations throughout pregnancy, and its ligand, CD274, causes selective inhibition of cytokine production by term decidual T cells
INTRODUCTION
The fetus expresses both maternal and paternal genes, and is therefore partially foreign, or semiallogenic, in relation to the mother's immune system. Because a central feature of the immune system is to discriminate between self and nonself tissues, it is paradoxical that the semiallogenic fetus develops in the mother without normally being rejected by her leukocytes. Maternal T cells are among the immune cell populations at the maternal-fetal interface, constituting 15%–30% of decidual leukocytes in first trimester [1–3], and remain in proximity to the placenta until term [4, 5]. Because their presence in the decidua puts them in contact with the semiallogenic trophoblast cells throughout gestation, it has been thought that maternal T cell activity, including cytokine production, is modulated in order to maintain normal pregnancy.
Because cytokines can cause either harmful or beneficial outcomes in pregnancy, their production needs to be carefully controlled. For example, an overproduction of interferon γ (IFNG) and tumor necrosis factor α (TNFA) may be associated with recurrent spontaneous abortions in humans and fetal loss in mice [6–8]. However, these cytokines are also found in normal decidual and placental tissues, and accumulating evidence suggests physiological roles for these factors in regulating trophoblast invasion, spiral artery modification, and placental morphogenesis [9–11].
Although decidual natural killer cells contribute significantly to the cytokine environment at the maternal-fetal interface [11], cytokine production by decidual T cells may also be involved in the normal functions of pregnancy [10]. However, because of the potential for T cells to mediate allograft rejection [12], activation and cytokine production by these cells are likely to be held in check to ensure a favorable balance between physiological function and pathological consequences. One mechanism by which decidual T cells might be controlled is through the CD28 family of immune cell receptors. These cell surface proteins transduce either positive or negative signals following ligation of their B7-family ligands. One of these receptors, programmed cell death-1 (PDCD1, also called PD-1) is induced on activated lymphocytes and has been shown to negatively regulate T cells in vitro through its ligand, CD274 (also called B7-H1 or PD-L1) [13–17]. In addition, in vivo studies have demonstrated that targeted mutation or blockade of PDCD1 results in development of spontaneous tissue-specific autoimmune disease or T cell cytotoxicity against ectopically expressed tissue antigens in vivo [18–21]. In addition, this inhibitory receptor promotes allograft acceptance [22–25]. PDCD1 thus appears to have a key role in maintaining not only immunological self-tolerance to peripheral tissues, but also tolerance to foreign grafts. Interestingly, five alternatively spliced isoforms of the PDCD1 mRNA transcript have recently been identified [26], including an isoform (PDCD1 Δ Ex3) that lacks a transmembrane domain while retaining an intact CD274-binding domain, suggesting that it is a secreted but inactive form of PDCD1. Soluble PDCD1 has been detected in the synovial fluid and plasma of patients with rheumatoid arthritis and may act as a decoy receptor to prevent CD274:PDCD1-mediated T cell inhibition [27].
Trophoblast cells, including those invading the gravid uterus and those exposed to maternal blood, abundantly and constitutively express the CD274 protein throughout pregnancy [28, 29]. In addition, CD274 has been suggested to be necessary for the survival of semiallogenic fetuses in murine pregnancy [30]. The role of PDCD1 on T cells in peripheral tolerance and allograft acceptance, together with the abundance of CD274 at the maternal-fetal interface suggest an important function for this receptor:ligand complex in the immunological maintenance of pregnancy in women. The goal of this study was to examine the expression of the PDCD1 receptor and its soluble isoforms on T cells at the human maternal-fetal interface. In addition, we examined the effect of the PDCD1:CD274 interactions on maternal T cell subpopulations during pregnancy.
MATERIALS AND METHODS
Tissue Collection
All tissue samples were collected in accordance with human subjects protocols approved by the University of Kansas Medical Center Institutional Research Board (HSC no. 3037). Archival midsecretory phase (n = 1) and late secretory phase (n = 5) endometrial tissues were obtained from the University of Kansas Medical Center tissue bank and staged by a board-certified pathologist. Late secretory phase endometrial samples were identified by predecidual changes and variable apical vacuolation of glandular epithelium, characteristic of the time at which implantation occurs. Samples were collected from premenopausal women ages 24–49 years undergoing surgery for reasons other than uterine pathology; patients were negative for birth control or replacement hormone therapies at the time of biopsy, although intrauterine device status was unknown. First-trimester tissues, including decidua from voluntary pregnancy terminations and peripheral blood, were collected from patients at 6–11 wk of gestation. First-trimester decidual samples were also collected from the Tissue Collection Core (HD049480) at the University of Chicago. Four of seven first-trimester decidual samples contained decidua basalis and extravillous trophoblast cells. Normal autologous term decidua and peripheral blood were obtained on the day of elective or repeat cesarean delivery. Tissue collected for histology was either snap frozen in liquid nitrogen and subsequently fixed in 4% paraformaldehyde for 20 min after sectioning, or was prefixed for 4 h in 4% paraformaldehyde and soaked in 18% sucrose for 18–24 h prior to freezing. In addition, blood and decidual tissue were processed for lymphocyte isolation as described below.
Immunofluorescence
Ten-micrometer-thick tissue sections were cut on a cryostat and placed in duplicate onto lysine-coated slides. After blocking of nonspecific antibody-binding sites in 2% rabbit serum, 50% SuperBlock (Pierce, Rockford, IL), and 0.2% Triton X-100 (Fisher Scientific, Pittsburgh, PA), tissue sections were incubated with an anti-PDCD1 antibody (10 μg/ml; clone J116; eBioscience, San Diego, CA) or its isotype control (mouse IgG1κ; BD Pharmingen, San Jose, CA) overnight at 4°C, followed by incubation with Alexa 568-conjugated rabbit anti-mouse secondary antibody (Invitrogen, Carlsbad, CA) for 1 h at room temperature. Tissues were then incubated with CD3-fluorescein isothiocyanate (FITC; 10 μg/ml; clone UCHT-1; BD Pharmingen) or its isotype (mouse IgG1κ-FITC; eBioscience) at 37°C for 1 h. Absence of binding of the secondary antibody to anti-CD3 was confirmed by omission of the PD-1 primary antibody. Slides were coverslipped with Prolong Gold (Invitrogen) and cured overnight before imaging. Immunofluorescence was visualized on a Nikon 90i upright microscope via mercury fluorescent excitation then confirmed via confocal scanning (Nikon C1 series confocal scan head; Nikon, Melville, NY). Lasers used for emission detection were a 488 Multiline Argon (green IR) and 561 Diode laser (red IR), acquisition via the Nikon EZ-C1 series software (3.60). For quantification of single- and double-positive cells, images were captured from 10 randomly chosen, nonoverlapping viewing fields (40× objective) for each tissue. Double-positive cells were confirmed by comparison of single-color scans for each image. Samples from six (secretory endometrium) or seven (all other tissues) different patients were examined for each tissue group.
Isolation of Decidual and Peripheral Blood Mononuclear Cells
Term decidua was obtained by either peeling the fused decidua capsularis/parietalis (decidua) and chorion from the amnion for flow cytometric studies or by scraping the decidua from the chorion for mRNA and in vitro cocultures. Decidual tissues were then dissociated using 200 U/ml type IV collagenase, 1 mg/ml type 1-S hyaluronidase, and 150 μg/ml type IV DNAse in a shaking 37°C water bath [31]. All enzymes were purchased from Sigma-Aldrich. Both first-trimester and term cell suspensions were layered over Histopaque (Sigma-Aldrich) and centrifuged. The mononuclear cell fraction was collected and counted to assess cell viability and yield. For mRNA analysis and in vitro assays, the mononuclear cell fraction was collected and plated for 2–3 h at 37°C to allow adherence of nonlymphocytes. For preparation of term autologous peripheral blood lymphocytes, samples were diluted in PBS, layered over Histopaque, collected, and counted to assess cell viability and yield.
Flow Cytometry
Dispersed decidual lymphocytes, chorionic trophoblast cells, and peripheral blood lymphocytes were labeled for specific markers using the following anti-human antibodies from eBioscience: CD4- FITC (clone RPA-T4), CD8-phycoerythrin (PE; clone RPA-T8), CD25- allophycocyanin (APC; clone BC96), FoxP3-PE (clone PCH101), PDCD1-biotin (clone J116), and CD274-biotin (clone M1H1). Biotinylated antibodies were detected using a conjugate of PE-Cy5 bound to streptavidin (BD Pharmingen). Fluorophore-conjugated mouse IgG1 isotype controls were used for CD4, CD8, and CD25; PE-conjugated rat IgG-2a control was used for FoxP3 (eBioscience). A minimum of 20 000 lymphocytes were gated based on forward and side scatter characteristics as well as T cell subpopulation marker expression, and data were collected for each desired population. Samples were processed on a BD LSRII instrument and analyzed using FACS Diva software (BD Pharmingen).
Fluorescence-Activated Cell Sorting
Peripheral blood mononuclear cells and nonadherent decidual mononuclear cells were labeled for specific markers using the following anti-human antibodies from eBioscience: anti-CD3 allophycocyanin (clone UCHT-1), anti-CD4-FITC (clone RPA-T4), anti-CD8-PE (clone RPA-T8). Lymphocytes were sorted into CD3+/CD4+ or CD3+/CD8bright populations using a BD FACS Aria using FACS DIVA software (Becton Dickinson). Cell population purity for five samples was analyzed after sorting and was an average of 96.0% ± 1.22% for CD3+/CD4+ cells and 97.2% ± 0.61% for CD3+/CD8bright cells.
Isolation, Reverse Transcription, and PCR Analysis of RNA
After sorting of specific T cell subpopulations, total cellular RNA was isolated from each group of cells using Trizol (Invitrogen) according to the manufacturer's instructions. Following quantification by spectrophotometry, 1 μg RNA was reverse transcribed using MMLV reverse transcriptase and oligo-dT primers (Invitrogen) in a 40-μl reaction volume. RNA was also added to a reaction without MMLV reverse transcriptase as a control to ensure the absence of genomic DNA amplification. Polymerase chain reaction amplification of cDNA products was performed using Taq DNA polymerase (Invitrogen) in conjunction with primers (forward: 5′ GCGGCCAGGATGGTTCTTA-3′; reverse: 5′-TACTCCGTCTCGTCAGGA-3′), which correspond to positions 125–143 and 793–811 of the PDCD1 mRNA transcript, respectively (GenBank accession no. NM-005018) [26]. Polymerase chain reaction products (20 μl) were subjected to electrophoresis on a 2% 3:1 agarose gel (Amresco, Solon, OH) and were visualized using ethidium bromide (Sigma-Aldrich) to examine product size. Following visualization, products were excised and sequenced to confirm identity.
Real-Time PCR
The following PDCD1 isoform-specific primers were used: full-length PDCD1 (forward: 5′-CTCAGGGTGACAGAGAGAAG-3′, positions 492–511 of PDCD1 mRNA transcript; reverse: 5′-GACACCAACCACCAGGGTTT-3′, positions 568–587); PDCD1 Δ Exon 2 (forward: 5′-GGTTCTTAGAGAGAAGGGCA-3′, positions 135–144 and 505–515; reverse: 5′-AGGGTGACAGGGACAATAGG-3′, positions 568–587); PDCD1 Δ Exon 3 (forward: 5′-AGGGTGACAGGGACAATAGG-3′, positions 495–504 and 661–670; reverse: 5′-CCATAGTCCACAGAGAACAC-3′, positions 720–739); PDCD1 Δ Exon 2, 3 (forward: 5′-TGGTTCTTAGGGACAATAGG-3′, positions 135–144 and 661–670; reverse 5′-TCTTCTCTCGCCACTGGAAA-3′, positions 749–768); and PDCD1 Δ Exon 2, 3, 4 (forward: 5′-TGGTTCTTAGAAGGAGGACC-3′, positions 135–144 and 696–705; reverse 5′-TCTTCTCTCGCCACTGGAAA-3′, positions 749–768) [26]. The β-actin (ACTB) primers were purchased from Applied Biosystems (Foster City, CA). These primers were used along with Power SYBR Green PCR Master Mix (Applied Biosystems) and 1 μl reverse-transcribed RNA. As a negative control, 1 μl water was substituted for cDNA. Reactions were run for 40 cycles in an ABI Prism 7900 Sequence Detection System (Applied Biosystems). Threshold cycle (Ct) values were obtained for each reaction and compared to those of a standard curve generated from decidual T cell cDNA to determine the relative abundance of the amplified product. All values were normalized to relative abundance values of ACTB products. The identity of PDCD1 isoform products was confirmed by sequence analysis.
Jar Cell Transfectants
The full-length coding sequence for CD274 [32] was subcloned into a pcDNA3.1 expression vector (Invitrogen) containing a neomycin resistance cassette. Expression vectors with or without coding sequences were then stably transfected into Jar choriocarcinoma cells, which lack endogenous CD274 expression [29]. Jar/V (vector only) and Jar/B7 (CD274 expressing) cell lines were maintained under selective pressure 300 μg/ml neomycin in RPMI containing 10% fetal bovine serum and antibiotics. To ensure consistency of the presence (Jar/B7) and absence (Jar/V) of CD274 expression, both cell lines were routinely evaluated by flow cytometry using a PE-conjugated anti-CD274 antibody (clone MIH1) and PE-conjugated Mouse IgG1κ isotype control (eBioscience).
Trophoblast/Lympocyte Coculture
Transfected Jar cells were irradiated (2000 rad) and plated overnight in neomycin-containing selective medium at a density of 5000 cells/well in a 48-well tissue culture plate. The following day, Jar cells were washed with fresh culture media to remove neomycin from the culture wells, and sorted CD4+ or CD8bright decidual T cells were plated at a density of 275 000 cells/well in 500 μl culture media containing 3 μg/ml phytohemagglutinin (Sigma-Aldrich). Cocultures were placed at 37°C for 72 h, after which cell culture supernatants were collected and stored at −80°C. To evaluate the effect of CD274 on cell proliferation in separate cocultures, sorted decidual T cells were labeled with 5 μM of carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) according to the manufacturer's instructions and then placed into culture with transfected Jar cells as described above.
Analysis of Cytokine Production
Analysis of IFNG, TNFA, interleukin 2 (IL2), and IL10 concentrations in cell culture supernatants was performed using a multiplex Beadlyte Human Multi-Cytokine Detection System (Upstate/Millipore, Billerica, MA) according to the manufacturer's instructions. Samples were processed on a Luminex 200 instrument, and data were analyzed using Luminex IS software version 2.3 (Upstate).
Evaluation of Apoptosis and Proliferation
In experiments evaluating apoptosis or cell proliferation after the 72-h coculture, decidual T cells in the supernatant were collected and labeled with APC-conjugated anti-human CD3 antibody (eBioscience), and LIVE/DEAD Fixable Violet Dead Cell Stain Kit (Invitrogen). For samples to be analyzed for apoptosis, cells were also stained with PE-conjugated annexin V (BD Pharmingen). All samples were processed on a BD LSRII instrument and analyzed using FACS Diva software (BD Pharmingen). For apoptosis evaluation, CD3+ cells with a violetbright and annexin-positive phenotype were designated as apoptotic. In separate experiments, cell proliferation was quantified by evaluating the CFSE profiles of CD3+ cells.
Statistics
Statistical significance was determined using either a one-way analysis of variance (ANOVA) with a Student-Newman-Keul posthoc analysis (cell counts from immunohistochemistry) or two-way ANOVA with either a Fisher LSD (flow cytometry) or Tukey posthoc analysis (semiquantitative real-time PCR, multiplex cytokine analysis). Differences were considered significant at α = 0.05.
RESULTS
PDCD1 Is Expressed on Decidual T Cells Throughout Pregnancy
Because of the high level of CD274 expression on human fetal trophoblast cells, we examined whether CD3+ T cells at the maternal-fetal interface express its cognate receptor, PDCD1. Using double immunofluorescence histochemistry, we examined both PDCD1 and CD3 expression in normal secretory phase endometrium (n = 6), first-trimester decidua, and term extraplacental membranes and basal plate (n = 7 per group; Fig. 1). Cells staining for one or both markers were enumerated (Fig. 2). In agreement with other observations [1–5], CD3+ cells were present in secretory phase endometrium as well as first-trimester and term decidua (Fig. 1, A–E). Although the number of CD3+ T cells per image varied, the overall number of CD3+ cells counted did not differ significantly between tissue groups (P = 0.849).
FIG. 1.
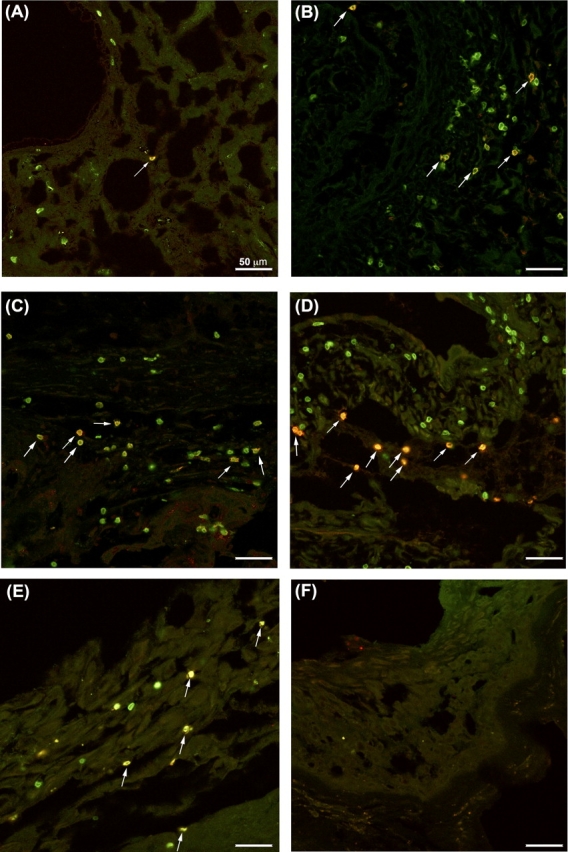
PDCD1 expression in endometrial and decidual tissues. Double-immunofluorescent immunohistochemistry of normal secretory endometrial tissues (A), first-trimester decidua (B), term basal plate (C), and term extraplacental membranes (D, E) with antibodies against CD3 (green) and PDCD1 (red). A–E) Photomicrographs are representative images of CD3 and PDCD1 immunolabeling for each group of tissues. F) Representative isotype control image from extraplacental membranes. Arrows indicate double-positive cells. Bars = 50 μm.
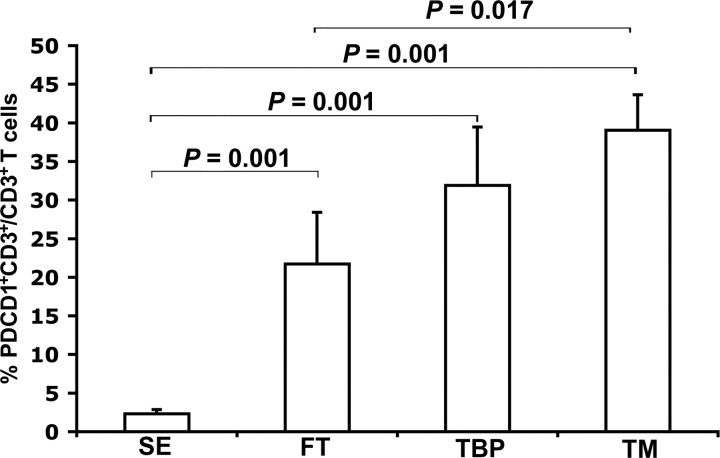
FIG. 2.
Quantification of PDCD1-expressing T cells in endometrial and decidual tissues. Graph represents mean percentages of PDCD1+CD3+ cells in each tissue type (SE, n = 6; FT, TBP, and TM, n = 7/group). Error bars indicate SEM. SE, Secretory endometrium; FT, first-trimester decidua; TBP, term basal plate; TM, term extraplacental membranes. Comparisons for which no P value is given were not significantly different (P > 0.05).
When examining PDCD1 expression, we next observed that >96% of all PDCD1+ cells were also CD3+ (Fig. 1), suggesting that the majority of the PDCD1-expressing cells were T cells. Although signal intensity for both CD3 and PDCD1 varied within individual sections of all tissues, no consistent pattern was noted. Quantitation of these cells revealed that the percentage of CD3+ cells expressing PDCD1 was low in nonpregnant secretory endometrium (Figs. 1A and 2), but increased significantly in first-trimester decidua (Figs. 1B and 2), with no apparent difference between first-trimester decidua parietalis and basalis (data not shown). The abundance of PDCD1+CD3+ cells remained elevated in late gestation (Figs. 1, C–E, and 2) and was significantly higher in term decidua associated with fetal membranes compared with first-trimester decidua (Fig. 2). We also examined PDCD1 expression on peripheral blood T cells from patients in first trimester (n = 4) and term pregnancy (n = 8) by flow cytometry; the proportion of PDCD1+ T cells was low in both groups and did not differ among CD4+, CD8+, and TReg subpopulations (P > 0.05; Fig. 3 and data not shown). In summary, these data suggest that PDCD1 expression is induced on T cells that localize to the decidua during pregnancy.
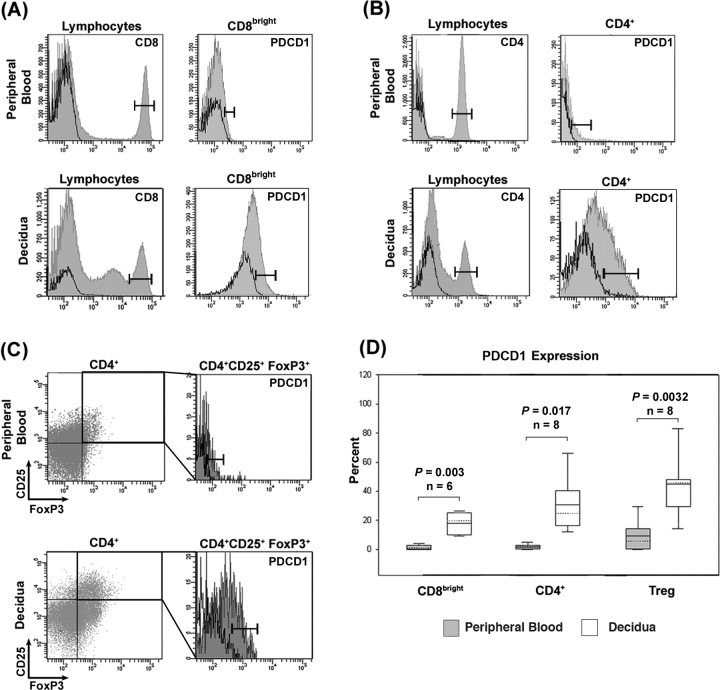
FIG. 3.
Flow cytometry of PDCD1-expressing T cell subpopulations in term peripheral blood and decidua. Histograms are representative images of flow cytometry in autologous peripheral blood (top panels of A–C) and decidual samples (bottom panels of A–C). Bars represent boundaries of gating for each T cell subpopulation marker (left panels of A and B) and PDCD1 (right panels of A–C). Solid lines represent isotype controls for CD4 and CD8 and fluorescence minus one controls for PDCD1. A) Left panels: CD8 expression on lymphocytes; right panels: PDCD1 expression on CD8bright populations. B) Left panels: CD4 expression on lymphocytes; right panels: PDCD1 expression on CD4+ population. C) Upper right quadrant of dot plot represents gated CD4+CD25+FoxP3+ population for PDCD1 expression analysis. Left panels: CD25 and FoxP3 expression on CD4+ lymphocytes; right panels: PDCD1 expression on the CD4+CD25+FoxP3+ population. D) Combined data from a minimum of six different patients. Boxes, 10th to 90th percentile values of the data set; solid lines in box, mean value; dotted lines in box, median value; bars, range of values for each data set; Treg, regulatory T cells.
PDCD1 Expression on T Cell Subpopulations During Pregnancy
We further examined the distribution of PDCD1 on T cells in peripheral blood and decidual samples from the same patients at term pregnancy. Figure 3 shows the flow cytometric analysis of CD8bright (n = 6), CD4+ (n = 8), and CD4/CD25/FoxP3+ (TReg; n = 8) lymphocyte populations in peripheral blood just before normal term cesarean delivery and autologous term decidual tissue collected after delivery. Each of these populations was easily identifiable in both peripheral blood and decidua. As expected [33], TReg were more abundant in decidual tissue than in peripheral blood (6.65% vs. 0.64%, respectively; P = 0.007). Further analysis by flow cytometry consistently revealed that cell surface-associated PDCD1 was higher on decidual T cells than on peripheral blood T cells from the same patient; this was true for each of the three populations examined (Fig. 3, A–C). Figure 3D shows the combined data for all patients, and statistical analysis revealed that differences in percentages of PDCD1-expressing T cells between decidua and blood were highly significant.
To confirm the specificity of our findings of PDCD1 expression on T cells, we stained decidual and peripheral blood samples with antibodies against CD3, CD4, CD8, and a second clone of an anti-PDCD1 antibody (clone MIH4; eBioscience). Through flow cytometric analysis, we observed a similar preferential expression of PDCD1 in decidual CD3+/CD4+ and CD3+/CD8bright T cell populations compared with peripheral blood (n = 2, data not shown). Also, to verify that decidual cell populations examined were resident within the decidua, we routinely labeled cells with an anti-human CD19 antibody (clone HIB19; eBioscience) using peripheral blood as a positive control. As expected [2], purified decidual lymphocytes completely lacked CD19+ B cells, confirming little or no peripheral blood contamination of decidual tissues (data not shown).
PDCD1 mRNA Isoforms Are Preferentially Expressed in Decidual T Cell Subpopulations
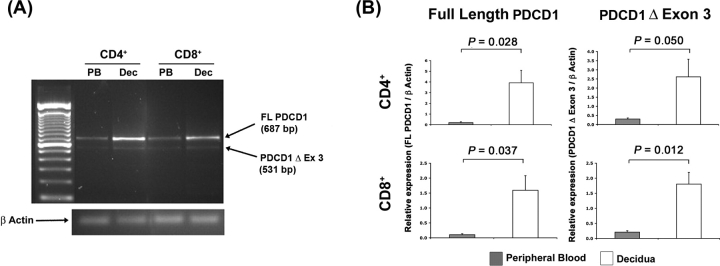
Because these data revealed that decidual T cells express the PDCD1 surface protein (Figs. 1–3), we next examined whether these cells express alternatively spliced PDCD1 mRNA isoforms. Conventional RT-PCR analysis followed by gel electrophoresis of mRNA from peripheral blood and decidual CD4+ or CD8+ T cell subpopulations showed two amplicons in both types of sample. Sequence analysis identified these products as the full-length (FL PDCD1) and soluble (PDCD1Δ Ex 3) isoforms of the PDCD1 mRNA transcript (Fig. 4A). In separate experiments using semiquantitative real-time PCR and isoform-specific primers, we found that the full-length PDCD1 mRNA transcript was more abundantly expressed in decidual T cells compared with autologous peripheral blood T cells at term pregnancy (n = 5; Fig. 4B). This was true of both CD4+ and CD8bright T cell subpopulations, and was consistent with our observations for PDCD1 surface expression in decidual T lymphocytes (Fig. 3). The Δ Ex3 PDCD1 isoform was also expressed more abundantly in decidual CD4+ and CD8bright T cells. Three other PDCD1 mRNA isoforms were detectable by real-time PCR in both peripheral blood and decidual lymphocytes, and although the sequence of each isoform was verified [26], the abundance of all transcripts was too low to confidently assess quantity (data not shown).
FIG. 4.
Reverse transcription-polymerase chain reaction analysis of PDCD1 mRNA isoforms in autologous term peripheral blood and decidual T cells. A) Agarose gel analysis of RT-PCR using PDCD1- and ACTB-specific primers. ACTB is 171 bp. PB, Peripheral blood; Dec, decidua. B) Graphs represent semiquantatitive real-time PCR analysis of cDNA (n = 5 patients) using PDCD1 mRNA isoform-specific primers. Samples were normalized to ACTB expression. Error bars indicate SEM.
Effects of CD274 on Term Decidual T Cells
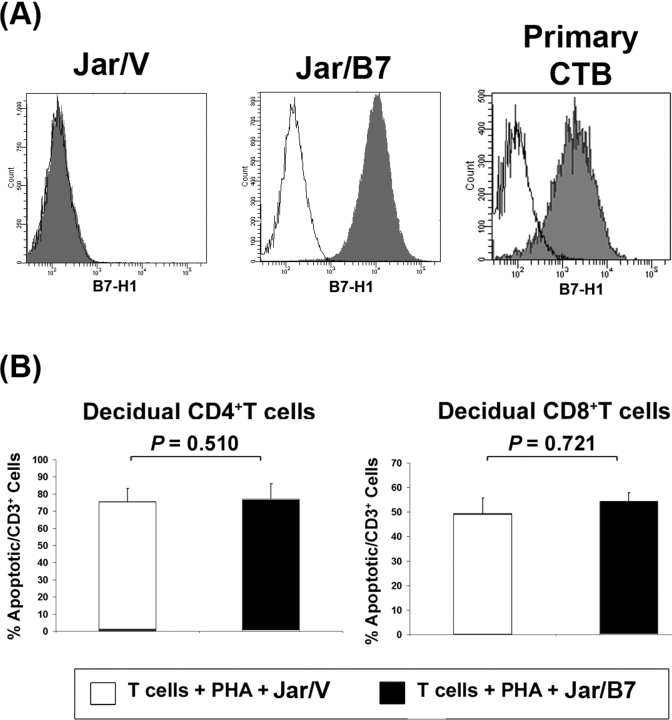
Because CD274 is abundantly expressed on placental trophoblast cells [28, 29] and PDCD1 is preferentially expressed by decidual T cells at term pregnancy (Figs. 2–4), we examined the effects of CD274-expressing cells on term decidual T lymphocyte apoptosis, proliferation, and cytokine production. We established a coculture system with CD274-transfected Jar choriocarcinoma cells and decidual CD4+ and CD8bright T cells. CD274 expression was routinely monitored on control transfected (Jar/V) or CD274 transfected (Jar/B7) cells by flow cytometry and found to be highly expressed on the surface of Jar/B7 cells, as it is on primary chorionic trophoblast cells (n = 5; Fig. 5A) [29].
FIG. 5.
A) Representative histograms from flow cytometric analysis of B7-H1 expression in control (Jar/V) or CD274-transfected (Jar/B7) cells and primary chorionic trohpoblast cells (Primary CTB). B) Analysis of CD274 effects on decidual T cell apoptosis. Graphs represent mean percentages of apoptotic T cells from six different patients. Error bars indicate SEM. PHA, phytohemagglutinin.
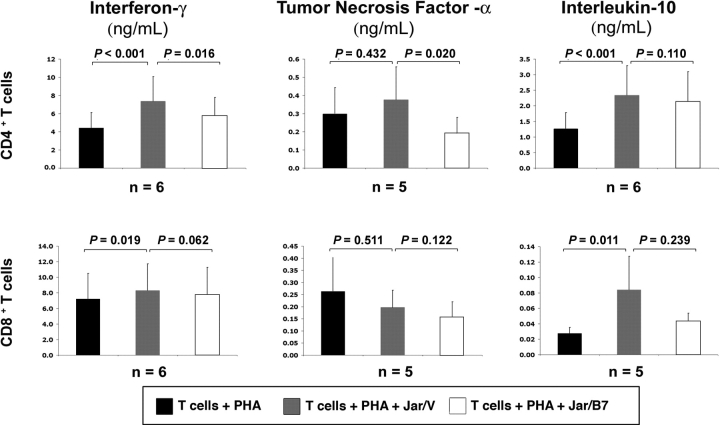
When evaluating the influence of CD274 on decidual T cell death, Jar/B7 cocultures, when compared with Jar/V, did not increase the percentage of either CD4+ or CD8bright T cells undergoing apoptosis (n = 6; Fig. 5B). Further, there was no apparent effect of Jar/B7 on decidual T cell proliferation as quantified by CFSE analysis (n = 2, data not shown). On the other hand, examination of cytokine production revealed that secretion of IFNG and IL10 by CD4+ and CD8+ decidual T cells was increased in the presence of Jar/V (n = 6; Fig. 6). CD274-transfected Jar cells reduced IFNG and TNFA secretion by CD4+ T lymphocytes in comparison with vector-transfected cells, whereas it had no significant effect on the production of IL10 from these cells (Fig. 6). Jar/B7 cells did not significantly influence the secretion of any cytokines from CD8+ T cells (Fig. 6). Interleukin 2 secretion was also evaluated, but its concentration was below detectable limits in all cocultures examined, suggesting that decidual T cells were not induced to secrete this cytokine under these conditions. Additionally, Jar/V or Jar/B7 cells cultured without T cells did not secrete any detectable IFNG, TNFA, IL10, or IL2 (n = 4, data not shown).
FIG. 6.
Cytokine assay analysis of CD274-mediated effects of decidual T cell cytokine production. Graphs represent mean values of decidual T cell cytokine secretion from a minimum of five different patients. Error bars indicate SEM. PHA, phytohemagglutinin.
DISCUSSION
Through these studies we have identified a novel location and potential function of the CD28 family molecule, PDCD1, at the maternal-fetal interface. While we found PDCD1 to be expressed on a small percentage of T cells in the secretory-phase endometrium, there was a significant increase in the proportion of PDCD1+ T cells in first-trimester and term decidua (Figs. 1 and 2). This induction of PDCD1 expression was specific to the decidua, as autologous term pregnancy decidual samples contained significantly higher percentages of PDCD1+ T cells than their peripheral blood counterparts (Fig. 3). Indeed, peripheral blood samples from patients in both first-trimester and term pregnancy contained low percentages of PDCD1+ T cells (Fig. 3 and data not shown), similar to levels found in nonpregnant peripheral blood lymphocytes [34]. Taken together, these data suggest that pregnancy-specific factors induce the expression of this receptor preferentially at the maternal-fetal interface. Surface PDCD1 can be upregulated on T cells by antigen-specific stimulation [35], thus exposure to fetal antigen may induce PDCD1 expression in decidual T cells during pregnancy. In addition, PDCD1 upregulation has been reported to occur under hormonal influence [36]; regulation of decidual T cell expression of this receptor by elevated hormone levels in gestation is also possible.
The preferential expression of PDCD1 receptor by decidual T cells in both first-trimester and term pregnancy complements our previous studies in which we observed expression of the its ligand, CD274, on trophoblast cell populations throughout gestation and hypothesized that placental CD274 serves as a molecular shield against a possible maternal alloresponse to the fetus [28, 29]. The expression of PDCD1 on both decidual CD8bright and CD4+ T cells in first-trimester and term pregnancy samples supports the hypothesis that these cells could be targets for trophoblast CD274-mediated regulation during pregnancy. Indeed, in vitro and in vivo studies have shown CD274:PDCD1-mediated inhibition of allogeneic immune responses and amelioration of allograft rejection, including antifetal responses [15, 25, 30].
Along with CD8bright and CD4+ T cells, PDCD1 was preferentially expressed on decidual TReg (Fig. 3, C and D). PDCD1 expression by TReg has been previously documented [37], and two recent studies have suggested that CD274 expression on parenchymal cells may induce differentiation of naïve CD4+ T cells into TReg outside of the thymus [38, 39]. TReg are a significant population in the decidua, are associated with the success of human pregnancy, and are required for allogenic murine pregnancies [33, 40–43]. While the function of PDCD1 on TReg is unclear, its presence could be a reflection of their differentiation and raise the interesting possibility that trophoblast CD274 may induce naïve CD4+ T cells to differentiate into TReg in order to promote immune tolerance of the fetal allograft.
The preferential expression of PDCD1 by decidual CD4+ and CD8bright T cells was confirmed at the mRNA level, where the full-length PDCD1 transcript was upregulated in decidual T cells compared with peripheral blood. Interestingly, the PDCD1 Δ Ex3 transcript was also more abundantly expressed in decidual T cells (Fig. 4B). Because the PDCD1 Δ Ex3 transcript encodes a soluble form of the receptor that may prevent T cell inhibition by acting as a decoy [27], its upregulation by decidual T cells was somewhat unexpected. While expression of this isoform may be a safeguard against oversuppression of decidual T cells in order to maintain their physiological activity [10, 44], it will be interesting to examine PDCD1 Δ Ex3 in pregnancy pathologies, such as recurrent spontaneous abortion, where endometrial/decidual T cells may be activated [45–47].
In regard to the function of PDCD1 during pregnancy, we also examined whether its ligand, CD274, could alter cytokine secretion or apoptosis of decidual T cells as a potential means of modifying maternal immune activation [16, 48]. In coculture experiments, we observed that the Jar/V cells caused increased cytokine production in the decidual T cells (Fig. 6), which parallels a previous study showing that Jar cells can induce production of IL10 and TNFA in uterine natural killer cells [49]. We also observed that CD274 expression on Jar cells selectively inhibited secretion of IFNG and TNFA in CD4+ but not CD8bright decidual T cells (Fig. 6). CD4+ T helper cells control activation of antigen-presenting cells and CD8+ T cells through cytokine secretion, and therefore it may be particularly important to modify the cytokine repertoire of these cells in order to control maternal antifetal immune reactions. The lack of effect of CD274 on IL-10 production in either cell type (Fig. 6) suggests that CD274 is causing selective inhibition, rather than a global suppression, of decidual T cytokines. Consistent with this finding, CD274 also did not increase the percentage of T cells undergoing apoptosis (Fig. 5B).
A balance of cytokine production appears to be critical in promoting pregnancy. For example, overproduction of IFNG and TNFA is associated with preterm labor and recurrent spontaneous abortions in humans as well as fetal rejection in mice [7, 8, 50]. On the other hand, the same cytokines may also control trophoblast invasion in early pregnancy and play a role in parturition at term [9, 10, 51–54]. Anti-inflammatory cytokines also have a critical role at the maternal-fetal interface, as a reduction or absence of IL10 is associated with chronic human pregnancy failure and potentiation of inflammation-induced murine fetal loss [44, 55, 56]. The CD274:PDCD1 pathway may selectively modify decidual T cell cytokine secretion in order to maintain a proper immunological environment at certain times during pregnancy while still allowing for appropriate physiological functions throughout gestation.
In conclusion, these studies identify a novel location of the PDCD1 receptor at the maternal-fetal interface, highlight potential targets of CD274-mediated regulation, and demonstrate that the CD274:PDCD1 pathway is present and functional for modification of the maternal immune system during pregnancy.
Acknowledgments
The authors would like to thank the following individuals: L. Chen (Johns Hopkins) for CD274 vector, L. Holets and E. Kharatyan for technical assistance, E. Roach for imaging assistance, J. Ballenger (Department of Pathology) for endometrial samples, J. Vu (Department of Obstetrics and Gynecology) for patient sample collection, and C. Ober, C. Billstrand, M. Gilliam, L. Bennett, and E. Quansah at the University of Chicago for assistance with collection of first trimester decidual samples.
Footnotes
Supported by National Institutes of Health grants HD45611 to M.G.P. and HD049480 to M.G.P., director of Project II; C. Ober, director of Tissue Collection Core, and J.S. Hunt, principal investigator. E.S.T. was supported by a fellowship from the University of Kansas Medical Center (KUMC) Biomedical Research Training Program. Further resources and services were provided by the KUMC for Reproductive Sciences (NICHD), the KUMC Flow Cytometry Core (NCRR), the KUMC COBRE program in Cell Development and Differentiation (P20RR024214), the Kansas State University COBRE program in Epithelial Function (P20RR017686), the Kansas Idea Network of Biomedical Research Excellence (P20RR016475), and the Smith Intellectual and Developmental Disabilities Research Center (HD02528).
REFERENCES
- Morii T, Nishikawa K, Saito S, Enomoto M, Ito A, Kurai N, Shimoyama T, Ichijo M, Narita N.T-cell receptors are expressed but down-regulated on intradecidual T lymphocytes. Am J Reprod Immunol 1993; 29: 1–4. [DOI] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Baranov V, Yeung MM, Hammarstrom S, Hammarstrom ML.Immunomorphologic studies of human decidua-associated lymphoid cells in normal early pregnancy. J Immunol 1994; 152: 2020–2032. [PubMed] [Google Scholar]
- Vassiliadou N, Bulmer JN.Quantitative analysis of T lymphocyte subsets in pregnant and nonpregnant human endometrium. Biol Reprod 1996; 55: 1017–1022. [DOI] [PubMed] [Google Scholar]
- Haller H, Radillo O, Rukavina D, Tedesco F, Candussi G, Petrovic O, Randic L.An immunohistochemical study of leucocytes in human endometrium, first and third trimester basal decidua. J Reprod Immunol 1993; 23: 41–49. [DOI] [PubMed] [Google Scholar]
- Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH.Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol 2004; 62: 125–137. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Roberts J, Wilson R, MacLean MA, Shilito J, Walker JJ.Evidence of a T(H) 1 type response associated with recurrent miscarriage. Fertil Steril 2000; 73: 1206–1208. [DOI] [PubMed] [Google Scholar]
- Mjihdi A, Truyens C, Detournay O, Carlier Y.Systemic and placental productions of tumor necrosis factor contribute to induce fetal mortality in mice acutely infected with Trypanosoma cruzi. Exp Parasitol 2004; 107: 58–64. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee D, Watanabe K, Furuoka H, Suzuki H, Watarai M.Interferon-gamma promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol 2005; 5: 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Meisser A, Haenggeli L, Bischof P.Involvement of MAPK pathway in TNF-alpha-induced MMP-9 expression in human trophoblastic cells. Mol Hum Reprod 2006; 12: 225–232. [DOI] [PubMed] [Google Scholar]
- Scaife PJ, Bulmer JN, Robson SC, Innes BA, Searle RF.Effector activity of decidual CD8+ T lymphocytes in early human pregnancy. Biol Reprod 2006; 75: 562–567. [DOI] [PubMed] [Google Scholar]
- Hanna J, Mandelboim O.When killers become helpers. Trends Immunol 2007; 28: 201–206. [DOI] [PubMed] [Google Scholar]
- Rocha PN, Plumb TJ, Crowley SD, Coffman TM.Effector mechanisms in transplant rejection. Immunol Rev 2003; 196: 51–64. [DOI] [PubMed] [Google Scholar]
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubat T, Yagita H, Honjo T.Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8: 765–772. [DOI] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001; 2: 261–268. [DOI] [PubMed] [Google Scholar]
- Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH.PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A 2004; 101: 10691–10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T.Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11: 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T.Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11: 141–151. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T.Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001; 291: 319–322. [DOI] [PubMed] [Google Scholar]
- Keir ME, Freeman GJ, Sharpe AH.PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol 2007; 179: 5064–5070. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Wang YH, Yagita H, Dong C.Cutting edge: programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol 2006; 177: 8291–8295. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol 2007; 179: 5204–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Han R, Hancock WW.Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol 2007; 37: 2983–2990. [DOI] [PubMed] [Google Scholar]
- Yang J, Popoola J, Khandwala S, Vadivel N, Vanguri V, Yuan X, Dada S, Guleria I, Tian C, Ansari MJ, Shin T, Yagita H, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation 2008; 117: 660–669. [DOI] [PubMed] [Google Scholar]
- Ozkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O'Keefe T, Duong T, Smith T, Gutierrez-Ramos JC, Rottman JB, Coyle AJ, Hancock WW.Programmed death-1 targeting can promote allograft survival. J Immunol 2002; 169: 6546–6553. [DOI] [PubMed] [Google Scholar]
- Nielsen C, Ohm-Laursen L, Barington T, Husby S, Lillevang ST.Alternative splice variants of the human PD-1 gene. Cell Immunol 2005; 235: 109–116. [DOI] [PubMed] [Google Scholar]
- Wan B, Nie H, Liu A, Feng G, He D, Xu R, Zhang Q, Dong C, Zhang JZ.Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol 2006; 177: 8844–8850. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Chen L, Phillips TA, Hunt JS.B7 family molecules: novel immunomodulators at the maternal-fetal interface. Placenta 2003; 23: S95–S101. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS.B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod 2003; 68: 1496–1504. [DOI] [PubMed] [Google Scholar]
- Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH.A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med 2005; 202: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince G, Starkey P, Jackson M, Sargent IL, Redman C.Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods 1990; 132: 181–189. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L.B7-H1, a third member of the B7 family, co-stimulates T cell proliferation and interleukin 10 secretion. Nat Med 1999; 5: 1365–1369. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, Kanhai HHH, Claas FHJ, Scherjon SA.Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(-) T-cells in decidua and maternal blood during human pregnancy. Placenta 2006; 27: S47–S53. [DOI] [PubMed] [Google Scholar]
- Hatachi S, Iwai Y, Kawano S, Morinobu S, Kobayashi M, Koshiba M, Saura R, Kurosaka M, Honjo T, Kumagai S.CD4+ PD-1+ T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol 2003; 30: 1410–1419. [PubMed] [Google Scholar]
- Isogawa M, Furuichi Y, Chisari FV.Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity 2005; 23: 53–63. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H.Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res 2006; 84: 370–378. [DOI] [PubMed] [Google Scholar]
- Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW.Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol 2006; 176: 2808–2816. [DOI] [PubMed] [Google Scholar]
- Krupnick AS, Gelman AE, Barchet W, Richardson S, Kreisel FH, Turka LA, Colonna M, Patterson GA, Kreisel D.Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol 2005; 175: 6265–6270. [DOI] [PubMed] [Google Scholar]
- Beswick EJ, Pinchuk IV, Das S, Powell DW, Reyes VE.Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after helicobacter pylori exposure promotes development of CD4(+) CD25(+) FoxP3(+) regulatory T cells. Infect Immun 2007; 75: 4334–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen J, Mottonen M, Alanen A, Lassila O.Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol 2004; 136: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S.Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 2004; 10: 347–353. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M, Betz AG.Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5: 266–271. [DOI] [PubMed] [Google Scholar]
- Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL.CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett 2006; 102: 106–109. [DOI] [PubMed] [Google Scholar]
- Piccinni M-P, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S.Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med 1998; 4: 1020–1024. [DOI] [PubMed] [Google Scholar]
- Quack KC, Vassiliadou N, Pudney J, Anderson DJ, Hill JA.Leukocyte activation in the decidua of chromosomally normal and abnormal fetuses from women with recurrent abortion. Hum Reprod 2001; 16: 949–955. [DOI] [PubMed] [Google Scholar]
- Vassiliadou N, Searle RF, Bulmer JN.Elevated expression of activation molecules by decidual lymphocytes in women suffering spontaneous early pregnancy loss. Hum Reprod 1999; 14: 1194–1200. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Makino T, Sugi T, Matsubayashi H, Ozawa N, Nozawa S.Flow-cytometric analysis of immune cell populations in human decidua from various types of first-trimester pregnancy. Hum Immunol 1992; 34: 212–218. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800. [DOI] [PubMed] [Google Scholar]
- Rieger L, Kammerer U, Hofmann J, Sutterlin M, Dietl J.Choriocarcinoma cells modulate the cytokine production of decidual large granular lymphocytes in coculture. Am J Reprod Immunol 2001; 46: 137–143. [DOI] [PubMed] [Google Scholar]
- El-Shazly S, Makhseed M, Azizieh F, Raghupathy R.Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am J Reprod Immunol 2004; 52: 45–52. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA.Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 2000; 192: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Miyamoto S, Komatsu H, Tsukimori K, Kobayashi H, Seki H, Takeda S, Nakano H.TNFalpha-induced apoptosis and integrin switching in human extravillous trophoblast cell line. Biol Reprod 2003; 68: 1771–1778. [DOI] [PubMed] [Google Scholar]
- Buonocore G, De Filippo M, Gioia D, Picciolini E, Luzzi E, Bocci V, Bracci R.Maternal and neonatal plasma cytokine levels in relation to mode of delivery. Biol Neonate 1995; 68: 104–110. [DOI] [PubMed] [Google Scholar]
- Pollard JK, Mitchell MD.Tumor necrosis factor alpha stimulates amnion prostaglandin biosynthesis primarily via an action on fatty acid cyclooxygenase. Prostaglandins 1993; 46: 499–510. [DOI] [PubMed] [Google Scholar]
- Murphy SP, Fast LD, Hanna NN, Sharma S.Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol 2005; 175: 4084–4090. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Care AS, Skinner RJ.Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod 2007; 76: 738–748. [DOI] [PubMed] [Google Scholar]