Abstract
Background
Vitamin E has been studied extensively in the prevention of atherosclerosis. Cross-sectional population studies as well as randomized controlled intervention trials have demonstrated conflicting results. A recent meta-analysis of these trials has emphasized the ineffectiveness of vitamin E in atherosclerosis prevention with a possibility of harm at higher dosages. However, vitamin E has several isomers, with the alpha form being available via dietary supplements and the gamma form being available via dietary foodstuffs. The gamma form of vitamin E demonstrates several superior properties (such as trapping reactive nitrogen species and detoxifying nitrogen dioxide) compared with alpha vitamin E. All clinical trials have utilized the alpha isomer with little concern that this isomer of vitamin E may actually suppress the gamma isomer of vitamin E.
Objective
We undertook a dose response study in type 2 diabetic volunteers to include all the dosages of alpha vitamin E that have been utilized in cardiovascular prevention trials to determine the effect of alpha vitamin E on gamma vitamin E. We also assessed the effect of alpha vitamin E on several traditional markers of atherosclerotic risk. We added vitamin C to the vitamin E because several clinical trials included this vitamin to enhance the antioxidant effects of alpha vitamin E.
Design
Volunteers received, in randomized order for a two week period, one of the following vitamin dosage arms: 1) no vitamins, 2) low dose supplemental vitamins E plus C, 3) medium dose supplemental vitamins E plus C, and 4) high dose supplemental vitamins E plus C. Blood levels of both alpha and gamma vitamin E were measured as well as surrogate markers of oxidative stress, hypercoagulation, and inflammation during a high fat atherogenic meal (to increase the ambient oxidative stress level during the study).
Results
The results demonstrate that alpha vitamin E levels increased in proportion to the dose administered. However, at every dose of alpha vitamin E, gamma vitamin E concentration was significantly suppressed. No beneficial changes in surrogate markers of atherosclerosis were observed, consistent with the negative results of prospective clinical trials utilizing alpha vitamin E.
Conclusions
Our results suggest that all prospective cardiovascular clinical trials that utilized vitamin E supplementation actually suppressed the beneficial antioxidant gamma isomer of vitamin E. No beneficial effects on several potential cardiovascular risk factors were observed, even when the vitamin E was supplemented with vitamin C. If a standardized preparation of gamma vitamin E (without the alpha isomer) becomes available, the effects of gamma vitamin E on atherosclerotic risk will warrant additional studies.
Keywords: atherosclerosis, oxidative stress, inflammation, hypercoagulation, antioxidant, alpha vitamin E, gamma vitamin E, vitamin C, ascorbic acid
Introduction
The antioxidant vitamin E has been a major subject of controversy in the prevention of atherosclerosis. The initial basic science studies suggested that vitamin E had beneficial effects on several different stages of the atherosclerotic process (1). Vitamin E not only has antioxidant properties, but also non-antioxidant effects, including modulation of signal transduction pathways (2). This was followed by observational, cross-sectional studies in patients with no initial coronary artery disease, which also suggested that vitamin E supplementation lowered the risk of major coronary heart disease (3–5). However, subsequent large randomized controlled trials have shown that vitamin E supplementation had no clear benefit in the primary or secondary prevention of cardiovascular disease, with some trials suggesting harmful effects (6). The recently published Women’s Health Study found that 600 IU of vitamin E, given on alternate days for 10 years, demonstrated no overall benefit in the prevention of major cardiovascular events (7). The HOPE-TOO trial concluded that long-term vitamin E supplementation (400 IU/day for 10 years) did not prevent cardiovascular events, and may have actually increased the risk of heart failure (8,9).
Of the clinical trials showing benefit, most of them studied patients with evidence of increased oxidative stress, and in addition, gave vitamin C supplements with the vitamin E. Vitamin C, a water soluble antioxidant, has been shown to regenerate vitamin E (a lipid soluble antioxidant) (10,11). In patients with increased oxidative stress (i.e. patients with conditions such as diabetes mellitus, end stage renal disease, coronary artery disease, and/or hypercholesterolemia), vitamin C restores endothelium-dependent artery vasodilation, and may reverse endothelial dysfunction in peripheral arteries (10–13). Three recent trials using high dosages of both vitamins E and C together, in populations with high oxidative stress, have shown a delay of coronary arteriosclerosis when compared to placebo (14–17). Supplementation of both vitamins, in individuals with increased oxidative stress, may therefore be more effective than either vitamin taken alone.
All of the above cited clinical trials utilized the alpha tocopherol isomer form of vitamin E (alpha Vitamin E). This is the composition of vitamin E sold in over-the-counter supplements. However, there has been growing interest in another isomer of vitamin E, specifically gamma tocopherol (gamma vitamin E), which is believed to play a distinct and beneficial role in atherosclerosis prevention (2). Several studies have suggested that alpha tocopherol supplementation may decrease serum levels of gamma tocopherol in man (18,19). However, no variable dose response studies of the effects of alpha tocopherol on gamma tocopherol have been published so that the overall effects of alpha tocopherol are unknown. Since a wide range of alpha tocopherol dosages has been utilized in clinical trials, this information is critical to evaluating the effects of vitamin E on atherosclerotic risk.
In addition, surrogate markers of atherosclerosis provide a useful approach to understanding the effects of an intervention on different components of the atherosclerotic process, including oxidative stress, inflammation, and hypercoagulation. Therefore, It was the intent of this study to examine the potential benefits of alpha tocopherol supplementation (plus Vitamin C), or lack thereof, by administering different dosages of these vitamins to type 2 diabetic individuals (a population with high oxidative surrogate markers of atherosclerosis.
Methods
Subjects
The study enrolled 12 adult subjects with well-controlled, non-insulin requiring type 2 diabetes of ≥6 months duration, being treated with diet plus a maximum of two oral diabetes medications. Subjects were excluded if they had known cardiovascular, hepatic, or renal disease, uncontrolled hypertension (>140/90 mm Hg), marked hyperlipidemia (serum LDL >4.1 mmol/L or serum triglycerides > 7.8 mmol/L), a body mass index (BMI) greater than 40 kg/m2, or a hemoglobin A1C (HbA1C) >9%. Eligible patients had a C-peptide stimulation test of at least 6 ng/mL following a Sustecal challenge, and normal hematological, electrolyte, and hepatic laboratory results at screening. Other important exclusion criteria were cigarette smoking, gastroparesis, recent use (within 3 months) of antioxidant vitamin supplements, use of medications affecting coagulation and glucose homeostasis, pregnancy, and current malignancy. The study protocol was approved by the University of New Mexico Human Research Review Committee (HRRC) and all patients gave written informed consent prior to study.
Study Protocol
The study protocol was a randomized, crossover design. Assay technicians and the principal investigator were blinded to the study arms of the volunteers. Studies were performed in randomized sequence with vitamin E (RRR-α-tocopherol) and vitamin C supplementation. Each subject participated in four separate, daylong inpatient studies at the University of New Mexico General Clinical Research Center. Each study represented one of the four arms of the overall study: no vitamins, low dose vitamins (alpha tocopherol = 200 IU, Vit C = 250 mg), medium dose vitamins (alpha tocopherol = 400 IU, Vit C = 500 mg), and high dose vitamins (alpha tocopherol = 800 IU, Vit C = 1000 mg), each dose given once daily. Participants’ usual diabetes medications were withheld only during the inpatient study day. Vitamin supplementation, determined by the arm of the study, was administered daily for 14 days prior to each inpatient study. Participants were contacted three times per week by telephone to encourage compliance with vitamin administration.
At the start of the study, an intravenous catheter was placed in an upper extremity, and participants took the same vitamins, C and E, that they had taken for the two weeks prior to the inpatient study. The patient was then fed a standardized American Diabetes Association (ADA) composition recommended breakfast. After breakfast, participants were administered a continuous intravenous infusion of regular insulin for the purpose of achieving relative euglycemia. Upon achieving a blood glucose concentration between 80 mg/dl and 120 mg/dl, the insulin was stopped, the catheter was removed, and a new catheter placed in the contralateral arm. One hour later, the participant was fed an atherogenic high-fat test lunch equivalent to a McDonald’s® Big Mac meal. The meal was prepared in the GCRC metabolic kitchen and consisted of ground beef, American cheese, white hamburger bun, dill pickles, iceberg lettuce, regular thousand island dressing, onions, French fried potatoes (prepared in reheated 100% vegetable oil), and an ice-cream shake.
Study Measurements
To determine circulating response to the vitamin E supplementation, plasma vitamin E (both alpha tocopherol and gamma tocopherol) was measured. Plasma levels of oxidized LDL (oxy-LDL), malonydialdehyde (MDA), non-esterified fatty acids (NEFA) plus erythrocyte intracellular glutathione peroxidase activity (GPx) were measured as surrogate markers of atherosclerotic risk and oxidative stress. Plasma levels of C-reactive protein (CRP), adiponectin, and interleukin-6 (IL-6) were assessed as surrogate markers of inflammation. PAI-1 and fibrinogen activity were quantified as indices of hypercoagulation. Diabetes control was assessed with levels of fasting blood glucose (FBS) and A1C. Total cholesterol and triglycerides were measured for the purpose of calculating lipid-standardized vitamin E levels (20).
Assays
All blood samples were processed immediately and frozen at minus 70 degrees centigrade until assay. Plasma alpha tocopherol and gamma tocopherol were measured with high performance liquid chromatography by ARUP Laboratories (Salt Lake City, UT) (21). Plasma oxidized LDL levels were assayed with a solid phase two-site ELISA kit from Mercodia (Uppsala, Sweden) (22). Glutathione peroxidase (GPx) activity in erythrocytes was assessed with a kit from Cayman Chemical (Ann Arbor, MI), which measured GPx activity through a coupled redox reaction utilizing GPx and glutathione reductase (23, 24.) A gas chromatography-mass spectrometry method was used to determine plasma free (unbound) MDA (25). Serum non-esterified fatty acids were measured with a kit from Wako Diagnostics (Richmond, VA) (26). High sensitivity C-reactive protein (hsCRP) was determined by an immunoluminometric method, from Diagnostic Products Corporation (Los Angeles, CA) (27). Adiponectin was measured spectrophotometrically after being processed by a sandwich ELISA kit from LINCO Research (St. Charles, MO) (28). Interleukin-6 (IL-6) was measured with a highly sensitive quantitative sandwich ELISA technique and optical density measurements, with a kit from R&D Systems (Minneapolis, MN) (29). Plasminogen activator inhibitor -1 (PAI-1) activity was measured using a Chromolize™ bioimmunoassay from Trinity Biotech, Plc (Bray, Ireland) (30). Fibrinogen levels were assessed with the clot-rate method (31). Plasma glucose was determined with the ACE glucose assay from Alfa Wassermann Diagnostic Technologies, LLC (West Caldwell, NJ) (32). Plasma C-peptide and plasma insulin were each measured with chemiluminescent assays (Immunolite instruments) from Diagnostic Products Corporation (Los Angeles, CA) (33,34). Plasma triglycerides and cholesterol were assayed colorimetrically with ACE reagents from Alfa Wasserman, Inc. (West Caldwell, NJ) (35,36).
Statistical Methods
The primary outcome variables assessed were the changes in plasma levels of both alpha tocopherol and gamma tocopherol. Secondary outcome variables were changes in plasma or serum concentrations of surrogate markers of atherosclerosis. Lipid standardized tocopherol levels were calculated as previously described (20). Tertiary variables were plasma levels of glucose, insulin, C-peptide, triglycerides, and cholesterol. Data were analyzed using the NCSS statistical program (NCSS, Kaysville, UT). Parameters were compared among the various groups by ANOVA for repeated measures with post hoc Student’s t test for paired data where appropriate. Significance was defined by an alpha of P<0.05. All data are reported as mean ± SEM.
A power analysis utilizing the data from Handelman (37) indicated that a sample size of 8 subjects would achieve 86% power to detect a difference of −1.0 between the null hypothesis mean of 0.0 and the alternative hypothesis mean of 1.0 with an estimated standard deviation of 0.8 and an alpha of 0.05 using a two-sided t-test.
Results
Subject Characteristics
Six men and six women (6 Hispanic, 5 non-Hispanic whites, 1 Native-American) were studied. Mean (± SEM) age, body mass index, and duration of diabetes were 53 ± 4 years, 29 ± 1 kg/m2, and 9 ± 3 years, respectively. The subjects had a mean (± SEM) HbA1c of 6.2 ± 0.3% and a mean fasting glucose of 111 ± 6 mg/dL. The mean (± SEM) fasting lipid profile of the subjects consisted of a total cholesterol of 158 ± 9 mg/dL, LDL cholesterol of 77 ± 6 mg/dL, HDL cholesterol of 45 ± 2 mg/dL, and triglycerides of 153 ± 20 mg/dL.
Plasma Levels of Vitamins E
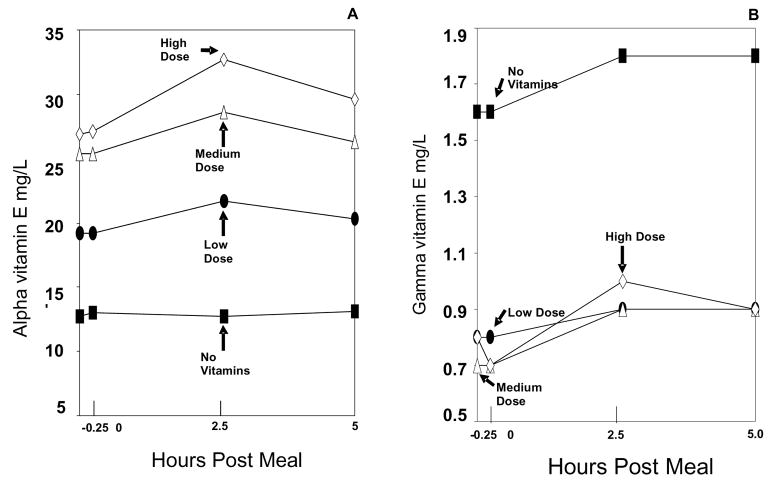
Pre-breakfast administration of vitamins (or no vitamins) showed a dose-dependent elevation in baseline levels of lipid standardized alpha tocopherol, between the four different dosages (study arms). This led to four distinct, statistically different curves (baseline plus test meal) for the study arms (p<0.0001 for each compared to no vitamins) (Figure 1, left panel). In addition, a dose-independent suppression in baseline and test meal gamma tocopherol levels was observed when the low, medium, and high-dose alpha tocopherol arms were compared to the no vitamin arm. This resulted in a no vitamin arm gamma tocopherol curve that was significantly higher in comparison to the gamma tocopherol curves when alpha tocopherol was administered (p<0.0001) (Figure 1, right panel).
Figure 1. Vitamin Levels.
Lipid standardized plasma alpha vitamin E (A) and gamma vitamin E (B) excursions during the no vitamin arm (black square), low dose vitamin arm (black circle), medium dose vitamin arm (open triangle), and high dose vitamin arm (open diamond). All x-axis values (including the −0.25 hr value) were obtained following breakfast and should be interpreted as relative to the lunch meal. The range for the standard error of the mean (SEM) for lipid standardized alpha vitamin E at all data points was: no vitamin arm= 2.7–3.3, low dose vitamin arm= 3.8–4.6, medium dose vitamin arm= 6.0–7.3, and high dose vitamin arm= 5.7–7.2. The range for the SEM for lipid standardized gamma vitamin E at all data points was: no vitamin arm= 0.9–1.1, low dose vitamin arm= 0.2–0.5, medium dose vitamin arm= 0.2–0.3, and high dose vitamin arm= 0.2–0.3. At all doses of vitamin E supplementation, gamma vitamin E was significantly suppressed by alpha vitamin E, p<0.01.
Surrogate Markers of Atherosclerotic Risk and Oxidative Stress
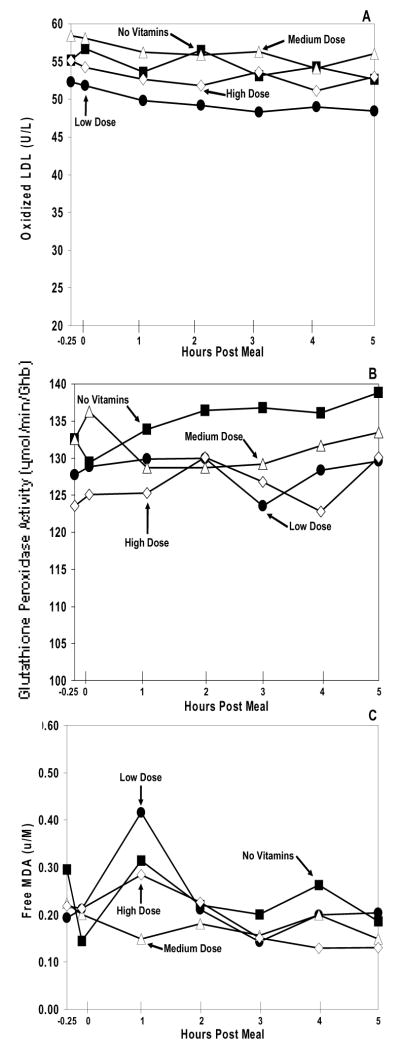
The data showed similar baseline levels of each oxidative stress marker (oxy-LDL, glutathione peroxidase activity, and MDA) (Figure 2) across all study arms (p>0.05 for all). Each marker also had showed similar curves between each of its arms (p>0.05 for all) during the atherogenic high fat meal.
Figure 2. Cardiovascular Risk and Oxidative Stress Markers.
Plasma oxidized LDL (A), erythrocyte glutathione peroxidase activity (B), and plasma MDA (C) excursions during the no vitamin arm (black square), low dose vitamin arm (black circle), medium dose vitamin arm (open triangle), and high dose vitamin arm (open diamond). The range for the standard error of the mean (SEM) for oxidized LDL at all data points was: no vitamin arm = 4.3–5.4, low dose vitamin arm = 4.1–5.1, medium dose vitamin arm = 4.5–5.9, and high dose vitamin arm = 3.2–3.6. The range for the SEM for erythrocyte glutathione peroxidase activity at all data points was: no vitamin arm = 10.4–12.6, low dose vitamin arm = 8.5–12.3, medium dose vitamin arm = 10.0–12.1, and high dose vitamin arm = 9.3–12.9. The range for the SEM for plasma MDA at all data points was: no vitamin arm = 0.026–0.112, low dose vitamin arm = 0.037–0.331, medium dose vitamin arm = 0.040–0.098, and high dose vitamin arm = 0.032–0.166. For each of the markers of oxidative stress, no significant difference was observed between the different dosages of vitamin E supplementation, p>0.05.
Surrogate Markers of Inflammation
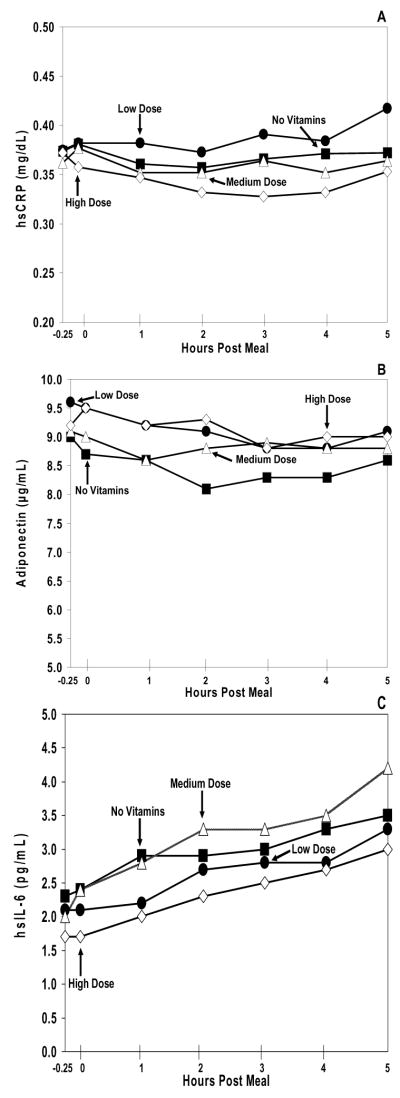
There were no significant differences seen in each of the baseline levels of CRP, adiponectin, and IL-6 (Figure 3) during the various study conditions (p>0.05 for all). Additionally, the study arm curves for each marker also failed to show any significant variation between each other (p>0.05 for all).
Figure 3. Inflammation Markers.
Plasma hsCRP (A), adiponectin (B), and hsIL-6 (C) excursions during the no vitamin arm (black square), low dose vitamin arm (black circle), medium dose vitamin arm (open triangle), and high dose vitamin arm (open diamond). The range for the standard error of the mean (SEM) for hsCRP at all data points was: no vitamin arm = 0.13–0.14, low dose vitamin arm = 0.11–0.13, medium dose vitamin arm = 0.10–0.11, and high dose vitamin arm = 0.14–0.17. The range for the SEM for adiponectin at all data points was: no vitamin arm = 1.4–1.5, low dose vitamin arm= 1.4–1.7, medium dose vitamin arm= 1.4–1.6, and high dose vitamin arm= 1.6–1.8. The range for the SEM for hsIL-6 at all data points was: no vitamin arm = 0.4–0.8, low dose vitamin arm = 0.3–0.4, medium dose vitamin arm = 0.2–0.6, and high dose vitamin arm = 0.2–0.5. For each of the markers of inflammation, no significant difference was observed between the different dosages of vitamin E supplementation, p>0.05.
Surrogate Markers of Hypercoagulation
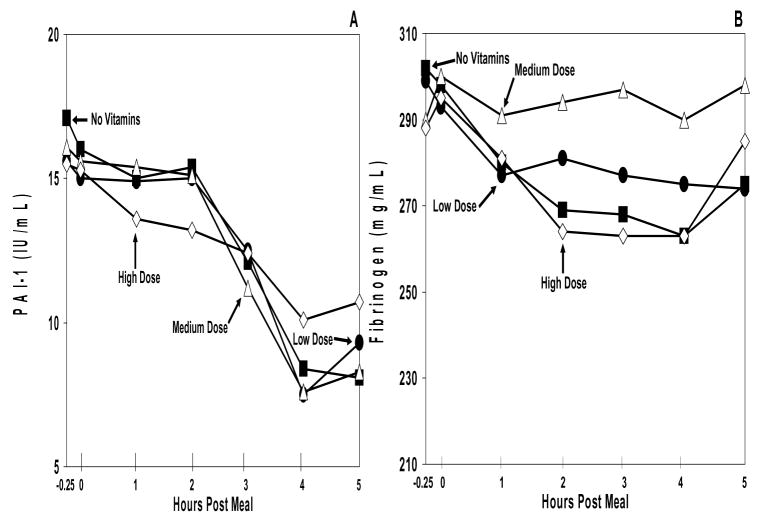
Baseline levels of PAI-1 and fibrinogen (Figure 4) were similar across all study arms (p>0.05). PAI-1 levels displayed a significant test meal-induced fall in all study arms (p=0.0004), representing a normal diurnal decline. For both PAI-1 and fibrinogen, the placebo and vitamin arms showed similar baselines (p>0.05) and similar curves across time (p>0.05).
Figure 4. Hypercoagulation Markers.
Plasma PAI-1 (A) and fibrinogen (B) excursions during the no vitamin arm (black square), low dose vitamin arm (black circle), medium dose vitamin arm (open triangle), and high dose vitamin arm (open diamond). The range for the standard error of the mean (SEM) for PAI-1 at all data points was: no vitamin arm = 2.2–5.3, low dose vitamin arm = 2.1–4.9, medium dose vitamin arm = 2.7–5.7, and high dose vitamin arm = 2.7–4.3. The range for the SEM for fibrinogen at all data points was: no vitamin arm= 19–24, low dose vitamin arm= 12–19, medium dose vitamin arm = 13–19, and high dose vitamin arm = 18–24. For each of the markers of hypercoagulation, no significant difference was observed between the different dosages of vitamin E supplementation, p>0.05.
Other Markers
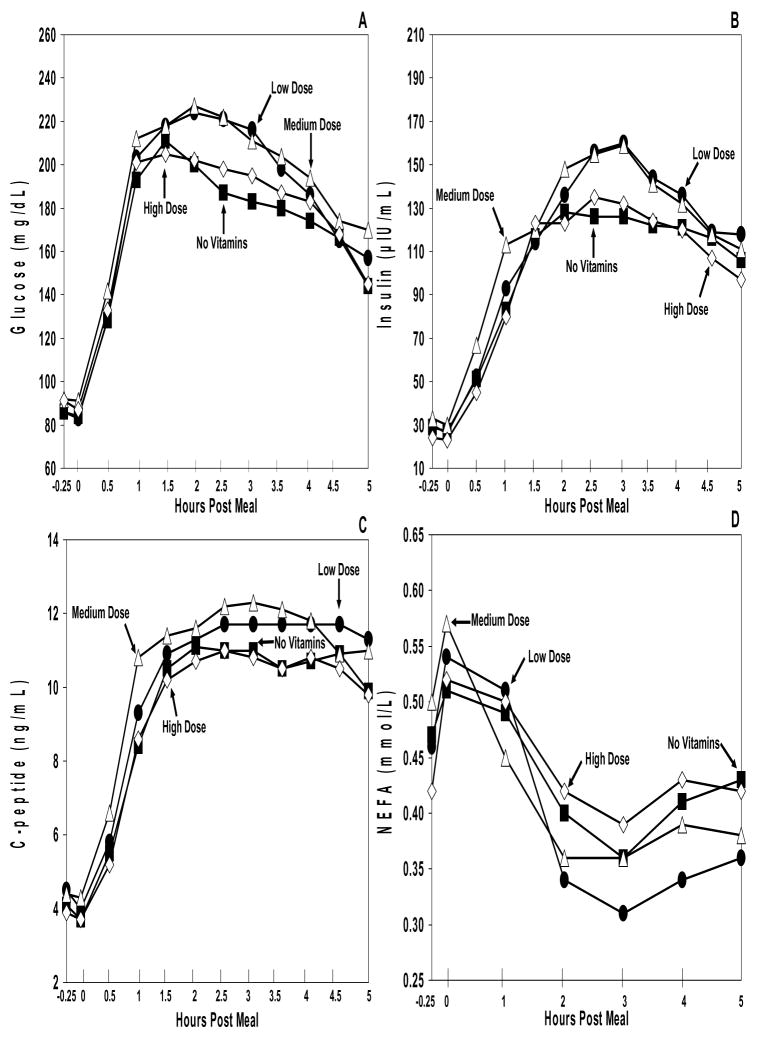
Mean baseline levels of glucose, C-peptide, and insulin (Figure 5) were similar for all study conditions (P>0.05 for all). Each marker showed a statistically significant post meal elevation (p<0.0001 for all). There were no statistically significant differences seen between the curves of each study arm for each marker (p>0.05 for all).
Figure 5. Metabolic Markers.
Plasma glucose (A), insulin (B), C-peptide (C), and NEFA (D) excursions during the no vitamin arm (black square), low dose vitamin arm (black circle), medium dose vitamin arm (open triangle), and high dose vitamin arm (open diamond). The range for the standard error of the mean (SEM) for glucose at all data points was: no vitamin arm = 4–17, low dose vitamin arm = 5–21, medium dose vitamin arm = 5–21, and high dose vitamin arm = 5–17. The range for the SEM for insulin at all data points was: no vitamin arm = 8–26, low dose vitamin arm = 7–40, medium dose vitamin arm = 6–43, and high dose vitamin arm = 5–40. The range for the SEM for C-peptide at all data points was: no vitamin arm= 0.5–1.1, low dose vitamin arm= 0.5–1.2, medium dose vitamin arm= 0.6–1.1, and high dose vitamin arm= 0.5–1.0. The range for the SEM for NEFA at all data points was: no vitamin arm= 0.02–0.07, low dose vitamin arm = 0.02–0.07, medium dose vitamin arm = 0.03–0.05, and high dose vitamin arm = 0.04–0.06. Although there was no significant difference between the different dosages of vitamin E supplementation and the metabolic markers (p>0.05), the metabolic effects of the high fat are evident.
For cholesterol and triglycerides, no significant differences were observed between mean baseline levels (p>0.05) and the study arm curves (p>0.05) (data not shown).
Discussion
This study demonstrated that daily supplementation of alpha tocopherol plus vitamin C significantly reduced the circulating concentration of gamma tocopherol. This suppression (by approximately 50%) was observed at all three dosages (low, medium, and high) of alpha tocopherol. These dosages included all of the dosages used in clinical trials that observed or failed to observe a significant reduction in cardiovascular events (6). In addition, at all dosages, alpha tocopherol supplementation failed to lower surrogate markers of atherosclerotic risk and oxidative stress, inflammation, and hypercoagulation, in a well-controlled type 2 diabetic population. This observation was made in spite of the fact that 1) the protocol used a high-risk population with increased potential benefit from reduction of oxidative stress, 2) inclusion of a high-fat meal which has been shown to enhance oxidative stress, 3) co-administration of vitamin C with vitamin E in order to replenish the vitamin E antioxidative radical, and 4) administration of both vitamins for 2 weeks prior to study. These vitamins were administered for two weeks and also several hours prior to the test meal to increase their efficacy in reducing inflammation and increasing fibrinolysis (38). The bioavailability of oral vitamin E is greatest when administered with food (39) – which is why the vitamins were given with a small breakfast (to ensure maximal absorption, and hence antioxidant capacity). We gave natural RRR-αlpha-tocopherol, because it is twice as potent as the racemic synthetic alpha tocopherol (40).
At high concentrations, vitamin C functions as an antioxidant which protects lipids from oxidative damage induced by aqueous peroxyl radicals (10,11). It also regenerates other antioxidants, such as flavonoids and glutathione. It may reverse endothelial dysfunction in peripheral arteries of individuals with high oxidative stress conditions, such as type 2 diabetes. Combined administration of vitamins E and C produces antioxidant effects primarily through the action of vitamin C elimination of the alpha-tocopherol radical. In vivo animal studies and in vitro studies show vitamin C accomplishes this by exporting the radical away from a lipid medium into an aqueous medium (41–42). The ability of vitamin C to preserve vitamin E in individuals who smoke has recently been demonstrated (43).
Type 2 diabetic individuals, when compared to healthy individuals, show greater oxidative stress in response to a standardized meal (44). Hyperglycemia may increase free radical production through mechanisms of lipoprotein glycation, glucose auto-oxidation, and polyol pathway activation (44). These individuals also have greater postprandial increments in triglyceride levels, when compared with a healthy population. This leads to a postprandial lipidemia which causes increased oxidative stress and vascular dysfunction (45). In general, populations with increased oxidative stress (e.g., smokers, patients with coronary heart disease, ESRD, transplant recipients, etc.) have responded maximally to antioxidant vitamins (6,36,46,47).
The high fat test meal, simulating a McDonald’s® Big Mac meal, was administered to enhance the level of oxidative stress. Previous studies have shown that a single high fat meal transiently impairs endothelial function for at least four hours in healthy subjects, and that oxidative stress is likely the mechanism, because the administration of antioxidant vitamins E and C was able improve flow-mediated vasodilation (48,49). Our high fat meal used reheated cooking oil identical to that used in commercial fast food restaurants (50), which has been shown to contribute to decreased arterial endothelial-dependent vasodilation (51).
Choices of surrogate markers for this study were based on their roles in the atherosclerotic process. Oxidized LDL was measured because the oxidative conversion of LDL to oxy-LDL is a key event in the development of the fatty streak, and oxy-LDL is more atherogenic than its native form (52). The antioxidant enzyme glutathione peroxidase-1 has a central role in the preventing the development of reactive oxygen species, and low levels of this enzyme are associated with increased risk of cardiovascular events (24). Our study measured glutathione peroxidase activity, but it should be noted that glutathione is affected not only by glutathione peroxidase activity but also glutathione peroxidase reductase activity as well as glutathione synthase. Even though glutathione peroxidase activity was unchanged in our study, it is possible that glutathione levels could have been reduced in patients with diabetes (53). Additional studies will be required to determine the actual effects of alpha vitamin E and vitamin C on glutathione. Malonyldialdehyde is a byproduct of lipid peroxidation in atherosclerosis (25). Non-esterified fatty acids (FFA) increase insulin resistance and serve as a substrate for hepatic triglyceride production (54). C reactive protein (CRP) has a pro-atherogenic role in vascular smooth muscle cells, monocyte-macrophages, and endothelial cells, and elevated levels of CRP predict a greater risk of cardiovascular disease (55). Adiponectin has been shown to increase fatty acid oxidation and glucose uptake, reduce fatty acid synthesis, and reduce molecules involved in gluconeogenesis (56). Low adiponectin levels are found in obese and diabetic individuals, and high levels are associated with lower atherosclerotic risk of MI in men (57). The pro-inflammatory response involves the secretion of the cytokine IL-6 from macrophages, which ultimately stimulates the secretion of CRP (55). PAI-1 is a suppressor of fibrinolysis through the inhibition of tissue plasminogen activator (tPA). Increased PAI-1 activity has been demonstrated in people with coronary artery disease, and elevated PAI-1 is a strong risk factor for cardiovascular disease (58). Fibrinogen, which is a product of the coagulation cascade, is also a risk factor for cardiovascular disease (31). Our study’s observation that alpha tocopherol supplementation had no effect on surrogate atherosclerotic markers is best explained by its most important finding: low, medium and high doses of alpha tocopherol plus vitamin C suppressed circulating levels of gamma tocopherol below those observed during the placebo arm of the study. Gamma tocopherol constitutes 70–80% of the vitamin E in an average American diet. It is especially abundant in nuts, vegetable oils, and plant seeds (59,60). Compared with alpha tocopherol, it has a much lower concentration in the human body but much greater bioactivity.
Recent studies have made strong arguments for the unique benefits of gamma tocopherol. Saldeen et al (61) showed that both alpha and gamma tocopherol decreased platelet aggregation and slowed arterial thrombus formation in rats. They also reduced LDL oxidation and lipid peroxidation, superoxide generation, and increased superoxide dismutase activity. However, in all of these effects, gamma tocopherol was significantly more potent than alpha tocopherol (61). Also, gamma tocopherol and its metabolite have unique anti-inflammatory properties (62) and detoxify reactive nitrogen oxide species in a matter superior to alpha tocopherol (63). In regards to human in vivo studies, two recent case-control trials showed lower concentrations of gamma tocopherol in subjects with coronary heart disease (CHD) when compared to a healthy control group (64,65). One of these studies noted that these changes were not seen with alpha tocopherol (64).
Large epidemiological studies have shown that vitamin E from food (which is mostly gamma tocopherol) is inversely associated with the risk of death from CHD and stroke, while vitamin E from supplements (mainly alpha tocopherol) showed no benefits (66,67). Both alpha and gamma tocopherol compete for the hepatic alpha tocopherol transfer protein, which preferentially binds to gamma tocopherol (68,69). The degree of binding to this protein determines the biological activity of the vitamin E molecule (69). This may explain why alpha tocopherol supplementation reduces gamma tocopherol levels (70). It is interesting that alpha vitamin E, even at the lowest replacement dose, significantly suppressed gamma vitamin E. This indicates that this suppression is very sensitive to low circulating levels of alpha vitamin E. The finding that vitamin E and vitamin C supplements failed to worsen the indices of oxidative stress and inflammation in spite of suppressing the circulating levels of gamma vitamin E may indicate that there may not be a major effect of a 50% decrease in gamma tocopherol.
A potential limitation of this study is that there was no washout scheduled between each change in vitamin E dosage. A washout period was omitted because the entire study for any individual required two months to complete, and we did not want to prolong the study for that individual because of the potential change in diet and negative adherence might occur. Since each of the treatment groups was randomized, this may have taken into account any problem with washout that may have existed. In addition, an alternative interpretation to our results is that the lack of effect on various biomarkers might result from the combined effects of increased alpha vitamin E and decreased gamma vitamin E or to a compensatory effect of alpha vitamin E in the presence of reduced concentrations of gamma vitamin E.
Our study demonstrates that low, medium, and high dose alpha tocopherol plus vitamin C had no effect on markers of atherosclerosis, when compared to placebo, in a high risk population. This is best explained by the suppression of the powerful antioxidant gamma tocopherol via alpha tocopherol supplementation. A recent study (71) demonstrates the superiority of gamma vitamin E over alpha vitamin E in improving oxidative stress in patients with the metabolic syndrome. No effect was observed when only alpha vitamin E was administered, whereas a partial response was observed when both alpha and gamma vitamin E were given to the patients. The maximal response was observed when only purified vitamin E was given. This is likely to be one mechanism to explain the failure of several recent large clinical trials demonstrating failure of vitamin E supplementation to improve cardiovascular outcomes (6–9). A recent publication has indicated that high doses of vitamin C increases the vitamin E content of LDL in type 2 diabetes (72). Our study does not directly address this possibility, although it may have contributed to the suppression of gamma vitamin E following the increasing doses of alpha vitamin E plus vitamin C in our protocol. Additional studies will be required to separate out the separate effects of alpha vitamin E and vitamin C on the suppression of gamma vitamin E. Because low dose alpha tocopherol is an ineffective antioxidant, and doses >400 IU/day may increase all-cause mortality (6), we do not recommend the use of these supplements on a daily basis.
Acknowledgments
This research was supported by the University of New Mexico General Clinical Research Center (NIH NCRR GCRC Grant # 5 M01-RR00997) and by the American Diabetes Association Grant # 7-04-CR-41
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Absalon D. Gutierrez, University of New Mexico Health Sciences Center.
Daniela Gonzalez de Serna, University of New Mexico Health Sciences Center.
Irina Robinson, University of New Mexico Health Sciences Center.
David S. Schade, University of New Mexico Health Sciences Center.
References
- 1.Neuzil J, Weber C, Kontush A. The role of Vitamin E in atherogenesis: linking the chemical, biological and clinical aspects of the disease. Atherosclerosis. 2001;157:257–83. doi: 10.1016/s0021-9150(00)00741-3. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Q, Christen S, Shigenaga, Ames BN. γ –Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–22. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 3.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328(20):1450–56. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 4.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328(20):1444–49. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 5.Pruthi S, Allison TG, Hensrud D. Vitamin E supplementation in the prevention of coronary heart disease. Mayo Clin Proc. 2001;76:1131–36. doi: 10.4065/76.11.1131. [DOI] [PubMed] [Google Scholar]
- 6.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 7.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer. The Women’s Health Study: A Randomized Controlled Trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. The HOPE and HOPE-TOO trial investigators. Effects of long-term Vitamin E supplementation on cardiovascular events and cancer. JAMA. 2005;293(11):1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 10.Frei B. Ascorbic acid protects lipids in human plasma and low-density lipoprotein against oxidative damage. Am J Clin Nutr. 1991;54:1113S–1118S. doi: 10.1093/ajcn/54.6.1113s. [DOI] [PubMed] [Google Scholar]
- 11.Niki E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr. 1991;54:1119S–1124S. doi: 10.1093/ajcn/54.6.1119s. [DOI] [PubMed] [Google Scholar]
- 12.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97(1):22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr A, Zhu B-Z, Frei B. Potential antiatherogenic mechanisms of ascorbate (vitamin C) and α-tocopherol (vitamin E) Circ Res. 2000;87:349–54. doi: 10.1161/01.res.87.5.349. [DOI] [PubMed] [Google Scholar]
- 14.Fang JC, Kinlay S, Beltrame J, Hikiti H, Wainstein M, Behrendt D, et al. Effects of vitamins C and E on the progression of transplant-associated arteriosclerosis: a randomised trial. Lancet. 2002;359:1108–13. doi: 10.1016/S0140-6736(02)08154-0. [DOI] [PubMed] [Google Scholar]
- 15.Salonen JT, Nyyssonen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala-Sarataho E, et al. Antioxidant supplementation in atherosclerosis prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. J Intern Med. 2000;248:377–86. doi: 10.1046/j.1365-2796.2000.00752.x. [DOI] [PubMed] [Google Scholar]
- 16.Salonen RM, Nyyssonen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Rissanen TH, et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic program: the antioxidant supplementation in atherosclerosis prevention study (ASAP) Circulation. 2003;107:947–53. doi: 10.1161/01.cir.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- 17.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, et al. Secondary Prevention with Antioxidants of Cardiovascular disease in Endstage renal disease (SPACE): randomized placebo-controlled trial. Lancet. 2000;356:1213–18. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Appel LJ. Supplementation of diets with alpha-tocopherol reduces serum concentrations of γ-and δ-tocopherol in humans. J Nutr. 2003;133:3137–40. doi: 10.1093/jn/133.10.3137. [DOI] [PubMed] [Google Scholar]
- 19.Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol. J Nutr. 1985;115(6):807–13. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- 20.Thurnham DI, Davies JA, Crump BJ, Situnayake RD, Davis M. The use of different lipids to express serum tocopherol: lipid ratios for the measurement of vitamin E status. Ann Clin Biochem. 1986;23:514–20. doi: 10.1177/000456328602300505. [DOI] [PubMed] [Google Scholar]
- 21.Hansen LG, Warwick WJ. An improved assay method for serum vitamins A and E using fluorometry. Am J Clin Pathol. 1978;70:922–23. doi: 10.1093/ajcp/70.6.922. [DOI] [PubMed] [Google Scholar]
- 22.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malonyldialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–94. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 23.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 24.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Eng J Med. 2003;349:1605–13. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 25.Yeo HC, Helbock HJ, Chyu DW, Ames BN. Assay of malonyldialdehyde in biological fluids by gas chromatography-mass spectrometry. Anal Biochem. 1994;220:391–6. doi: 10.1006/abio.1994.1355. [DOI] [PubMed] [Google Scholar]
- 26.Elphick MC. Modified colorimetric ultramicro method for estimating NEFA in serum. J Clin Pathol. 1968;21(5):567–70. doi: 10.1136/jcp.21.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2 Clin Chem. 2001;47(3):418–25. [PubMed] [Google Scholar]
- 28.Suominen P. Evaluation of an enzyme immunometric assay to measure serum adiponectin concentrations. Clin Chem. 2004;50(1):219–21. doi: 10.1373/clinchem.2003.025833. [DOI] [PubMed] [Google Scholar]
- 29.Moody MD, Van Arsdell SW, Murphy KP, Orencole SF, Burns C. Array-based ELISAs for high-throughput analysis of human cytokines. Biotechniques. 2001;31(1):186–90. 192–94. doi: 10.2144/01311dd03. [DOI] [PubMed] [Google Scholar]
- 30.Declerck PJ, Alessi MC, Verstreken M, Kruithof ED, Juhan-Vague I, Collen D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood. 1988;71(1):220–5. [PubMed] [Google Scholar]
- 31.Geffken DF, Keating FG, Cornell ES, Bovill EG, Tracy RP. The measurement of fibrinogen in population-based research. Studies on instrumentation and methodology. Arch Pathol Lab Med. 1994;118(11):1106–9. [PubMed] [Google Scholar]
- 32.Bondar RJ, Mead DC. Evaluation of glucose-6-phophate dehydogenase from leuconostoc mesenteroides in hexokinase method for determining glucose in serum. Clin Chem. 1974;20(5):586–90. [PubMed] [Google Scholar]
- 33.Hardy RW, Cohn M, Konrad RJ. Automated chemiluminescent assay for C-peptide. J Clin Lab Anal. 2000;14:17–19. doi: 10.1002/(SICI)1098-2825(2000)14:1<17::AID-JCLA4>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen L, Dinesen B, Jorgensen PN, Poulsen F, Roder ME. Enzyme immunoassay for intact insulin in serum or plasma. Clin Chem. 1993;39:578–82. [PubMed] [Google Scholar]
- 35.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28(10):2077–80. [PubMed] [Google Scholar]
- 36.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–5. [PubMed] [Google Scholar]
- 37.Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral alpha-tocopherol supplements decrease plasma gamma tocopherol levels in humans. J Nutr. 1985;348:241–51. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- 38.Carroll MF, Schade DS. Timing of antioxidant vitamin ingestion alters postprandial proatherogenic serum markers. Circulation. 2003;108:24–31. doi: 10.1161/01.CIR.0000074221.68903.77. [DOI] [PubMed] [Google Scholar]
- 39.Iuliano L, Micheletta F, Maranghi M, Frati G, Diczfalusy U, Violi F. Bioavailability of vitamin E as function of food intake in healthy subjects. Arterioscler Thromb Vasc Biol. 2001;21:e34–e37. doi: 10.1161/hq1001.098465. [DOI] [PubMed] [Google Scholar]
- 40.Brigelius-Flohe R, Traber M. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 41.Neuzil J, Thomas SR, Stocker R. Requirement for, promotion, or inhibition by α-tocopherol of radical-induced initiation of plasma lipoprotein lipid peroxidation. Free Radic Biol Med. 1997;22:57–71. doi: 10.1016/s0891-5849(96)00224-9. [DOI] [PubMed] [Google Scholar]
- 42.Suarna C, Dean RT, May J, Stocker R. Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of alpha-tocopherol and ascorbate. Arterioscler Thromb Vasc Biol. 1995;15(10):1616–24. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- 43.Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, et al. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Raadical Biology and Medicine. 2006;40:689–697. doi: 10.1016/j.freeradbiomed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 44.Ceriello A, Bortolotti N, Motz E, Crescentini A, Lizzio S, Russo A, et al. Meal-generated oxidative stress in type 2 diabetic patients. Diabetes Care. 1998;21:1529–33. doi: 10.2337/diacare.21.9.1529. [DOI] [PubMed] [Google Scholar]
- 45.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, et al. Postprandial endothelial activation in healthy subjects in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll of Cardiol. 2002;39(7):1145–50. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Meydani M, Mayer J. Combined vitamin C and E supplementation retards early progression of arteriosclerosis in heart transplant patients. Nutrition Reviews. 2002;60(11):368–71. doi: 10.1301/00296640260385810. [DOI] [PubMed] [Google Scholar]
- 47.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;1015:2107–11. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 48.Plotnick GD, Corretti MC, Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997;278:1682–86. [PubMed] [Google Scholar]
- 49.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–4. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 50.McSavage J, Trevisan S. The use and abuse of frying oil. Food Service Technology. 2001;1:85–92. [Google Scholar]
- 51.Williams MJ, Sutherland WH, McCormick MP, de Jong SA, Walker RJ, Wilkins GT. Impaired endothelial function following a meal rich in used cooking fat. Journal of the American College of Cardiology. 1999;33(4):1050–55. doi: 10.1016/s0735-1097(98)00681-0. [DOI] [PubMed] [Google Scholar]
- 52.Witzum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–92. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murakami K, Kondo T, Ohtsuka Y, Fujiwara Y, Shimada M, Kawakami Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism. 1989;38(8):753–758. doi: 10.1016/0026-0495(89)90061-9. [DOI] [PubMed] [Google Scholar]
- 54.McGarry JD. Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51(1):7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 55.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14,719 initially healthy American women. JAMA. 2005;294(1):56–65. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 56.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 57.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291(14):1730–6. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 58.Devaraj S, Chan AV, Jr, Jialal I. Alpha-tocopherol supplementation decreases plasminogen activator inhibitor-1 and p-selectin levels in type 2 diabetic patients. Diabetes Care. 2002;25(3):524–9. doi: 10.2337/diacare.25.3.524. [DOI] [PubMed] [Google Scholar]
- 59.Lehmann J, Martin HL, Lashley EL, Marshall MW, Judd JT. Vitamin E in foods from high and low linoleic acid diets. J Am Diet Assoc. 1986;86(9):1208–16. [PubMed] [Google Scholar]
- 60.Bieri JC, Evarts RP. Tocopherols and fatty acids in American diets. J Am Diet Assoc. 1973;62(2):147–51. [PubMed] [Google Scholar]
- 61.Saldeen T, Li D, Mehta JL. Differential effects of α- and γ-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation, and arterial thrombogenesis. J Am Coll Cardiol. 1999;34(4):1208–15. doi: 10.1016/s0735-1097(99)00333-2. [DOI] [PubMed] [Google Scholar]
- 62.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. γ –tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97(21):11494–99. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci USA. 1993;90:1771–75. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohrvall M, Sundlof G, Vessby B. Gamma, but not alpha, tocopherol levels in serum are reduced in coronary heart disease patients. J Intern Med. 1996;239:111–17. doi: 10.1046/j.1365-2796.1996.410753000.x. [DOI] [PubMed] [Google Scholar]
- 65.Kontush A, Spranger T, Reich A, Baum K, Beisiegel U. Lipophilic antioxidants in blood plasma as markers of atherosclerosis: the role of alpha-carotene and gamma-tocopherol. Atherosclerosis. 1999;144(1):117–22. doi: 10.1016/s0021-9150(99)00044-1. [DOI] [PubMed] [Google Scholar]
- 65.Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Eng J Med. 1996;334(18):1156–62. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 67.Yochum LA, Folsom AR, Kushi LH. Intake of antioxidant vitamins and risk of death from stroke in postmenopausal women. Am J Clin Nutr. 2000;72:476–83. doi: 10.1093/ajcn/72.2.476. [DOI] [PubMed] [Google Scholar]
- 68.Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, et al. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Letters. 1997;409:105–8. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 69.Catignani GL, Bieri JG. Rat liver alpha-tocopherol binding protein. Biochim Biophys Acta. 1977;497(2):349–57. doi: 10.1016/0304-4165(77)90192-1. [DOI] [PubMed] [Google Scholar]
- 70.Robinson I, Gonzalez de Serna D, Gutierrez A, Schade DS. Vitamin E in man – an explanation of clinical trial failure. Endocr Pract. 2006;12(5):576–82. doi: 10.4158/EP.12.5.576. [DOI] [PubMed] [Google Scholar]
- 71.Devaraj S, Leonard S, Trabet MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radical Biology and Medicine. 2008;44:1203–1208. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tessier D, Khalil A, Trottier L. Fülöp. Effects of vitamin C supplementation on antioxidants and lipid peroxidation markers in elderly subjects with type 2 diabetes. Arch Geront Geriatr. 2008 doi: 10.1016/i.archger.2007.10.005. [DOI] [PubMed] [Google Scholar]