Abstract
Auditory thalamocortical connections are organized as parallel pathways originating in various nuclei of the medial geniculate body (MGB). The development of these pathways has not been studied. Therefore it remains unclear if thalamocortical connections segregate before the onset of hearing or if refinement of exuberant thalamocortical connections occurs following hearing onset. We studied this issue in the pallid bat. In adult pallid bats, parallel thalamocortical pathways represent two different sounds used in two different behaviors. The suprageniculate (SG) nucleus of the dorsal division of the MGB (MGBd) projects to a high-frequency cortical region selective for the echolocation calls, but not to a low-frequency cortical region sensitive to noise transients used in the localization of prey. Conversely, the ventral division (MGBv) projects to the low-frequency, but not the high-frequency, cortical region. Here we studied the development of these parallel pathways. Based on retrograde tracer injections in electrophysiologically characterized cortical loci, we show that there is an asymmetrical overlap in projection patterns from postnatal (P) day 15-60. The low-frequency region receives extensive input from both the SG and the MGBv. In contrast, the high-frequency region receives the great majority of its input from the SG, as in adults, while projections from MGBv appear to make only a minor contribution, if any. By P150, these pathways are segregated and adult-like. These data suggest that these anatomically segregated pathways arise through postnatal refinement of initially overlapping connections.
Keywords: auditory cortex, medial geniculate body, auditory development, plasticity, parallel pathways, echolocation
INTRODUCTION
Most sensory information reaches cortex via the thalamus through highly specific thalamocortical connections. In the auditory system, the medial geniculate body (MGB) provides the thalamic input to the auditory cortex (Raczkowski et al., 1976; Andersen et al., 1980; Morel and Imig, 1987; Winer et al., 1999; reviewed in Imig and Morel, 1983 and Rouiller, 1997). Parallel thalamocortical pathways arise from three distinct divisions of the MGB (reviewed in Winer et al., 2005). Thalamocortical connections include the tonotopically organized primary auditory pathway through the MGBv (ventral division), a multisensory pathway through the MGBm (medial division) that may be involved in multimodal integration and learning (Wepsic, 1966; Bordi and LeDoux, 1994; Edeline and Weinberger, 1992; reviewed in Hu, 2003), and a non-tonotopic pathway through the MGBd (dorsal division) that may be involved in representing complex sounds (Aitkin and Dunlop, 1968; Olsen and Suga, 1991; Schuller et al., 1991; Radtke-Schuller, 2004; Radtke-Schuller et al., 2004; Wenstrup and Grose, 1995).
The development of these parallel pathways between the MGB and the auditory cortex has been the subject of only one study. Gurung and Fritzsch (2004) found that the MGB innervates the auditory cortex before onset of patterned sensory input. However, whether segregation of MGB-auditory cortex inputs into parallel pathways depends on sensory experience remains unknown, Considerably more is known of the development of connectivity at lower levels of the auditory system. Studies of connections of the midbrain (Friauf and Kandler, 1990; Gabriele et al., 2000, 2007) and lower brainstem (Leake et al., 2002) suggest that the nucleotopic connections are mostly adult-like before hearing onset. Postnatal refinement or maintenance of connections, perhaps in an activity-dependent manner, is restricted to sharpening tonotopic organization (Kim and Kandler, 2003; Franklin et al., 2006; Leake et al., 2006). The main goal of the present study is to extend these developmental studies to the thalamocortical level.
The subject of this study is the pallid bat, a species well suited for the analysis of thalamocortical development. This species is a gleaning bat that uses passive hearing to detect and locate prey, and reserves echolocation largely for obstacle avoidance (Bell 1982; Fuzessery et al., 1993). In response to the need for simultaneous performance of two auditory functions while hunting, the pallid bat has, in effect, evolved two parallel auditory systems. Physiological and anatomical studies suggest that its inferior colliculus (IC)-MGB-auditory cortex connections are organized as two parallel, segregated pathways representing the FM (frequency modulated) sweeps used in echolocation and noise transients used in prey detection (Fuzessery, 1994; Fuzessery, 1997; Razak and Fuzessery, 2002; Razak et al., 2006). The tonotopic region in its auditory cortex is divided roughly into two halves; a high-frequency region (HFR) with neurons selective for FM sweeps used in echolocation, and a low-frequency region (LFR) sensitive to noise transients used in passive sound localization. The HFR receives its input largely from the suprageniculate (SG) nucleus of the MGBd, but not the MGBv (Razak et al., 2006). In contrast, the LFR receives tonotopic input from the MGBv, but not the SG. This present study focuses on the development of these parallel pathways.
Another advantage of the pallid bat is that the ontogeny of both echolocation behavior (Brown et al., 1978) and functional organization of auditory cortex (Razak and Fuzessery, 2007) has been studied. This provides a behavioral framework during development to compare anatomical and physiological data. The overall functional organization of auditory cortex, including frequency representation, is adult-like early in development. An exception is the prevalence and anatomical distribution of functionally bimodal neurons (Razak et al., 1999). These neurons appear to receive input from both pathways, and have two discrete tuning curves tuned to frequencies used in echolocation and passive sound localization. In adults, these bimodal neurons are found near the interface of the LFR and HFR. In pups, however, they are more widely distributed (Razak and Fuzessery, 2007), suggesting that projections from the MGBv and SG may show greater overlap in their cortical targets. We tested this hypothesis by retrograde tracing of MGB inputs from electrophysiologically identified injection sites in the LFR and HFR of auditory cortex. The results indicate that projections from the ventral and dorsal divisions of the MGB show overlap early in postnatal development, and become segregated as the system matures.
MATERIALS AND METHODS
Electrophysiology and retrograde tracing data were obtained from pallid bat pups that were born and raised in the University of Wyoming animal facility. Pregnant pallid bats were netted in New Mexico and housed in cages. The bats were fed mealworms raised on ground Purina rat chow (St. Louis, MO). The room was heated and maintained on a reversed 12:12 hour light/dark cycle. As pups were born, they were moved, with their mothers, to a nursery cage that housed pups born within 2-3 days of each other. Therefore, the ages reported in this study are accurate to within 2-3 days. Pups and moms were maintained in these cages until the day of recording from the pups. All procedures followed animal welfare guidelines required by the National Institutes of Health and the Institutional Animal Care and Use Committee.
Surgical procedures
The methodology used in this study was identical to that reported previously in adults (Razak et al., 2006). Briefly, recordings were obtained from bats that were anesthetized with Metofane (methoxyflurane) inhalation, followed by an intraperitoneal injection of pentobarbital sodium (30 μg/g body wt) and acepromazine (2 μg/g body wt). The level of anesthesia was evaluated by the toe-pinch reflex. Surgery commenced only when this reflex was absent. In addition, this reflex was tested every two hours during the recording session, and an additional dose of pentobarbital sodium (one-third of presurgical dose) was injected if required. To expose the auditory cortex, the head was held in a bite bar, a midline incision was made in the scalp, and the muscles over the dorsal surface of the skull were reflected to the sides. The bat was then placed in a Plexiglas restraining device. A cylindrical aluminum head pin was inserted through a cross bar over the bat’s head and cemented in place. This pin held the bat’s head secure during recording and injection. The location of the auditory cortex was determined relative to the rostrocaudal extent of the midsagittal sinus, the distance laterally from the midsagittal sinus, and the location of a prominent lateral blood vessel that travels parallel to the midsagittal sinus (Razak and Fuzessery, 2002). The size of the exposure was usually ∼2 mm2.
Recording procedures
Experiments were conducted in a heated (85-90°F), soundproofed chamber lined with anechoic foam. Bats were kept anesthetized throughout the course of the experiments. Stimuli were generated by using Modular Instruments, Inc. and Tucker Davis Technologies digital hardware and custom-written software (Fuzessery et al., 1991). The waveforms were amplified with a stereo amplifier and presented as closed-field stimuli through Infinity emit-K ribbon tweeters (Harman International Industries, Inc.) fitted with funnels that were inserted into the bat’s pinnae and sealed there with petroleum jelly. The speaker-funnel frequency response curve showed a gradual increase of 20 dB from 6 to 70 kHz, as measured with a Bruel and Kjaer 1/8 in. microphone placed at the tip of the funnel.
Recordings were obtained with glass microelectrodes (1 M NaCl, 2-7 MΩ impedance) at cortical depths between 200 and 600 μm. Penetrations were made orthogonal to the surface of the cortex. Response magnitudes and poststimulus time histograms were acquired and stored with a Modular Instruments high-speed clock controlled by custom-written software. Responses were quantified as the total number of spikes elicited over 20 stimulus repetitions.
Data acquisition
The functional organization of the cortex was mapped to determine the LFR and HFR, with an emphasis on best frequencies (BFs) and response selectivity. The BF was defined as the frequency of the tone burst (5 msec duration, including 1/1 msec rise/fall time) that elicited response at the lowest tested intensity to at least five successive presentations. Details on determining response selectivity are presented elsewhere (Razak and Fuzessery, 2002). Briefly, by using BF tone, broadband and narrowband noise, upward and downward FM sweeps as probes, the neuron was classified as selective for the stimulus that elicited at least 30% more response than the other stimuli. In both pups (Razak and Fuzessery, 2007) and adults (Razak and Fuzessery, 2002), the LFR is characterized by frequency tuning of <35 kHz and preference for noise transients over FM sweeps, while neurons of the HFR are tuned above 30 kHz and have a preference for downward FM sweeps over upward sweeps (Razak and Fuzessery, 2006) and noise.
Injection of tracers
Electrophysiological mapping of auditory cortex followed by retrograde tracer injections was successful in 14 pallid bats (12 pups with ages between P15-60 and two 5 month old bats). For more details on the functional organization of auditory cortex during development, see Razak and Fuzessery (2007). In 5 bats, either flurogold (FG) or fluroruby (FR) was injected in a site with known BF and response selectivity. In the remaining 9 bats, two different tracers (FG and FR) were injected in loci with different BF. All tracer injections were made using iontophoresis. The tip of a glass micropipette (10-20 μm tip diameter) was filled with FG or FR through capillary action. The micropipette was back-filled with NaCl (1 M) to permit recording and injecting with the same electrode. A Stoelting Precision Current Source was used to inject FG or FR (1 to 5 μA current, 7 seconds on, 7 seconds off, 5-30 minutes) at cortical depths of 200-600 μm.
Identification of retrogradely labeled neurons in the thalamus
After a survival period of ∼6 days, the bat was lethally anesthetized with pentobarbital sodium and perfused intracardially with phosphate-buffered saline (PBS, pH 7.4; 30-50 ml, ice-cold). The brain was fixed with 0.1 M phosphate-buffered 4% paraformaldehyde (80-100 ml, ice-cold). The perfusion rate was between 3 and 4 ml/minute. The brain was removed after measurements of the cortical exposure were made and refrigerated overnight in 30% sucrose (in 0.1 M phosphate buffer). Forty μm thick frozen coronal sections were collected in 0.1 M phosphate buffer.
Alternate sections were mounted onto slides from PBS. The remaining series of sections were stained with cresyl violet. After coverslipping with permount (for cresyl violet) or DPX (for FG/FR), the slides were viewed under either brightfield (cresyl violet) or fluorescent (FG/FR) illumination. Sections were observed under a Nikon Eclipse E800 brightfield microscope. Fluorescently labeled cells were observed by switching from a UV-2E/C filter to detect FG (excitation wavelength 340-380 nm) to a Y-2E/C filter for detection of FR (excitation wavelength 540-580 nm). Images were adjusted for contrast and brightness in either Canvas X (ACD systems) or Photoshop 6.0 (Adobe). The location of retrogradely labeled cells within the various divisions of the MGB was noted across the rostrocaudal extent of the MGB.
RESULTS
Organization of the MGB in pups
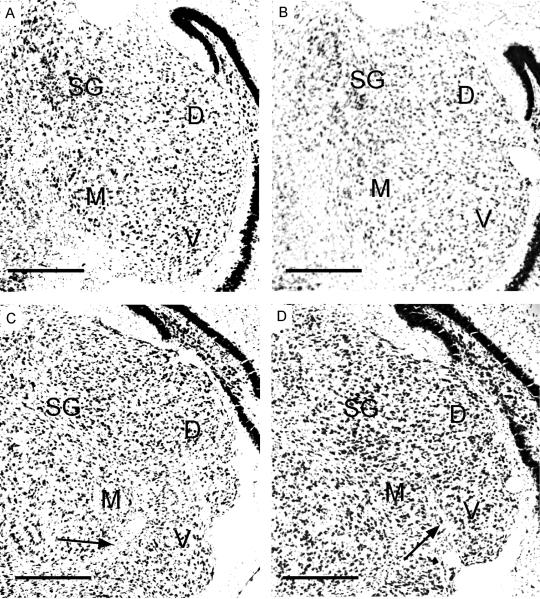
Cell size, darkness of staining and packing density in Nissl-stained sections were used to determine the boundaries of various divisions of the MGB in pups. Figure 1 shows photomicrographs of four Nissl-stained sections obtained from a P15 pup, the youngest age tested. The sections are shown in the caudal to rostral direction. The three major divisions of the MGB described in other species (Morest, 1964) including adult pallid bats (Razak et al., 2006) can be seen in the P15 pup: ventral (MGBv), dorsal (MGBd), and medial (MGBm). The MGBv contains a high packing density of cells with similar sizes. The MGBm has a wider range of cell sizes and a lower packing density. The lower cell density in the MGBm can be seen in Figure 1B. MGBm and MGBv are also separated by fiber tracts in the more rostral sections through the MGB (arrows in Fig. 1C, D). The SG, considered a part of the MGBd, is prominent throughout the rostrocaudal extent of the MGB. Its large darkly stained cells have a distinctive appearance in Nissl-stained sections facilitating its demarcation from the dorsolateral part of the MGBd and other divisions of the MGB. The darker staining in SG compared to the other regions can be clearly seen in all four sections shown in Figure 1. Because the distinctive Nissl-based features of the SG and the MGBv are present from at least P15 in the pallid bat, it was possible to determine the connections of these two MGB regions to the auditory cortex from the onset of adult-like high frequency hearing (∼P14, Brown et al., 1978).
Figure 1.
Nissl stained coronal sections through the MGB from a P15 pallid bat pup. Arrows point to fiber tracts that separate MGBv and MGBm. Scale bars = 250 μm. A, B, C and D show sections cut at 15%, 25%, 50% and 75% from the caudal end of the MGB, respectively. D - dorsal division, M - medial division, V - ventral division, SG - suprageniculate nucleus.
Thalamic sources of inputs to cortical LFR in pups
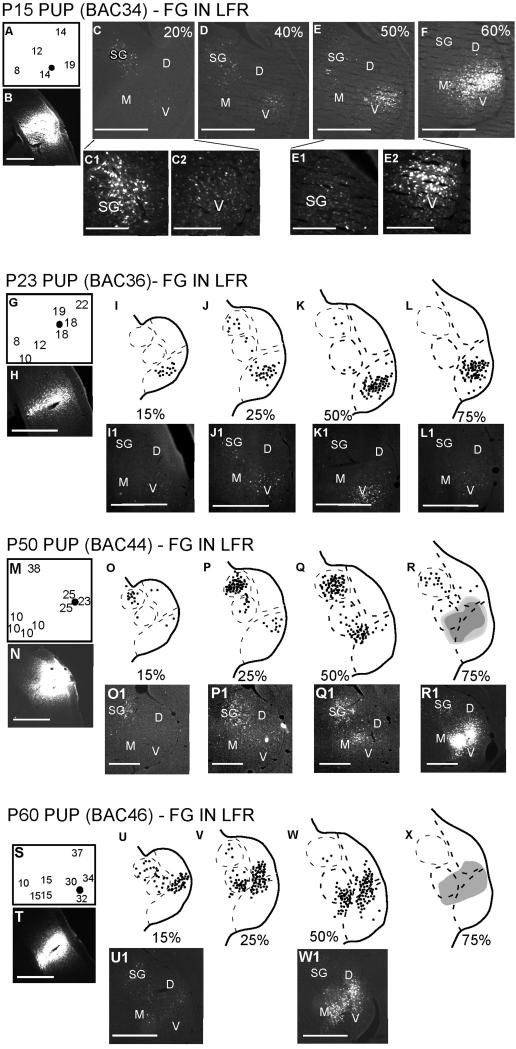
In adults, the LFR receives input from the MGBv, but not the SG. In contrast, pup LFR receives input from both the MGBv and the SG. Figure 2 shows data from four different pups at ages ranging between P15 and P60. In each experiment, FG was injected in the LFR. Both frequency tuning and response selectivity were used as physiological markers to target injections. In the case of LFR injections, the injection sites responded better to noise than to FM sweeps and typically had BF between 8-35 kHz (Fig. 2).
Figure 2.
Both SG and MGBv are labeled following injections in the LFR of the cortex. Four different experiments are shown. (A, G, M, S) Best frequency map around the cortical injection site (black dot). Note that all injections were made near neurons with BF<35 kHz. These neurons were selective for noise used in prey localization. (B, H, N, T) Cortical injection sites. For each experiment, the locations of labeled neurons in four different sections of the MGB are shown in a caudal to rostral direction. In the schematic representation of the MGB, each dot represents one or two neurons. A solid shape is used if the labeling is dense (e.g., R, X). Scale bars = 500 μm in all figures, except in C1, C2, E1, E2, where the scale bar = 200 μm.
The first two panels of each experiment in Figure 2 show the BF map around the injection site and a photomicrograph of the cortical injection site, respectively. The remaining panels for each experiment in Figure 2 show the MGB locations in which FG labeled neurons were observed. The MGB sections are shown in the caudal to rostral direction. In every case, FG labeled neurons were seen in the SG and the MGBv, indicating that both of these thalamic regions project to the LFR. More SG neurons appear to project to the LFR from the caudal half of the MGB compared to the rostral half. The number of FG labeled MGBv neurons increased in the rostral direction. The data are not clear on whether there is a decrease between P15-P60 in the absolute number of SG neurons projecting to the LFR. This is primarily because the injection sizes and BF of the injection sites were not similar across experiments. As in adults, both MGBm and MGBd project to the LFR.
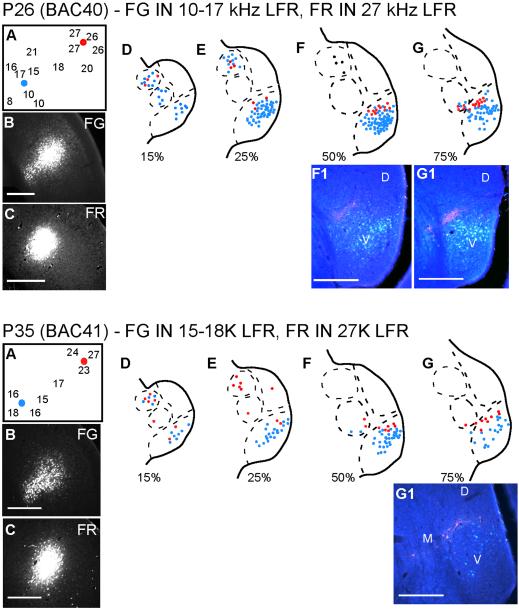
In the adult pallid bat the MGBv-LFR connections are organized tonotopically, as reported for the MGBv-primary auditory cortex connections in other species. Injections in LFR sites with BF<20 kHz label lateral parts of MGBv while injections in LFR sites with BF>20 kHz label more medial loci of the MGBv (Razak et al., 2006). In 2 pups, we tested if such tonotopically organized connections were present (ages P26 and P35). In both cases, FG was injected in low-BF sites, while FR was injected in higher-BF sites within the LFR (Fig. 3). The caudal SG was labeled with FG and FR in both experiments, consistent with the conclusion that the SG projects to the LFR in pups. In the MGBv, while there was some overlap, FR labeled neurons occupied more medial loci than FG labeled neurons suggesting that the MGBv-LFR connections in pups show at least a gross tonotopic organization.
Figure 3.
MGBv input to the LFR is organized tonotopically in young bats. In each experiment, two different tracers were injected in the LFR. Each tracer targeted a different frequency band within the LFR. Scale bars = 250 μm.
Thalamic sources of inputs to cortical HFR in pups
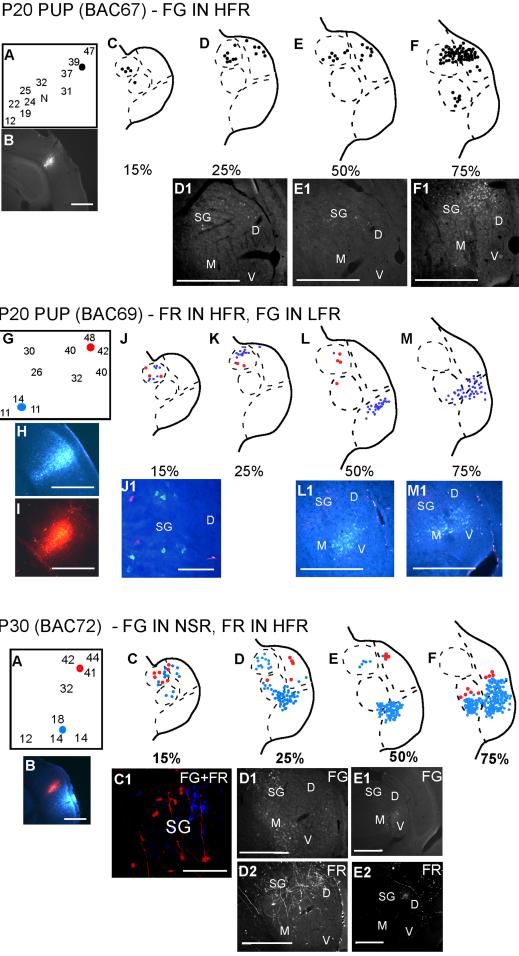
In adults, the HFR receives inputs from the SG, but not the MGBv. A similar pattern was observed in pups. Figure 4 shows three different experiments addressing thalamic inputs to the HFR. HFR injections were targeted based on both frequency tuning (35-60 kHz) and selectivity for downward FM sweeps over upward FM sweeps and noise. In the first case (P20) in Figure 4, FG was injected in the HFR near neurons with BF ∼40 kHz. The SG was labeled throughout the caudorostral extent, with increasing cell numbers in the rostral direction. No FG label was found in the MGBv. In the second experiment (P20) shown in Figure 4, FR was injected in the HFR, while FG was injected in the LFR. In this experiment, the caudal SG was labeled with FG, providing additional support to the conclusion that the SG projects to the LFR in pups. The SG was also labeled with FR, while the MGBv was not, supporting the conclusion that the HFR receives input from the SG, but not the MGBv.
Figure 4.
SG, but not MGBv, is labeled following injections in the HFR of the cortex. The second and third experiments shown had injections in both the LFR and the HFR. Scale bars 500 μm in all panels except J1, P1. In J1 and P1, scale bar = 100 μm.
The third experiment (P30) shown in Figure 4, followed the trend seen in the previous experiments with the SG showing connections to both the LFR and the HFR. In this experiment, neurons labeled with FR (from the HFR) were seen more ventrally (see section at 75%) than in any other experiment with HFR injections. These neurons are immediately adjacent, but not intermingled with, neurons labeled following LFR injections. The location of the label is consistent with the tonotopic organization of the MGBv, with the injection into the region of higher frequency tuning being more dorsal and medial (see Fig. 3). We therefore cannot rule out that these neurons are within the MGBv. If this is the case, it is the only evidence found that MGBv neurons may provide a minor input to the HRF in pups.
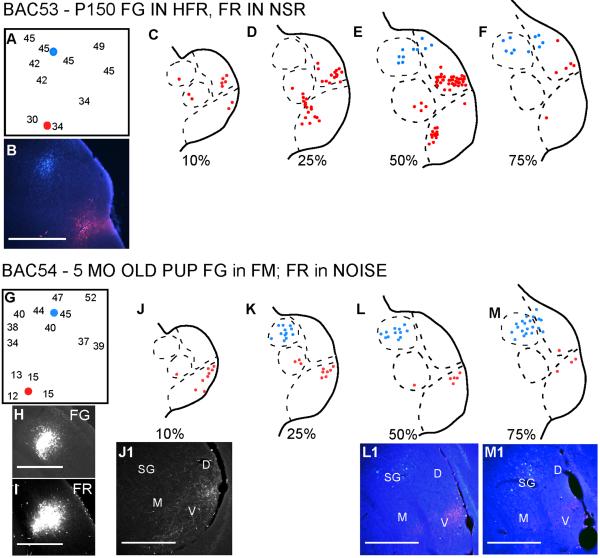
Thalamocortical connections in P150 bats
Figure 5 shows two experiments conducted in P150 bats, an age at which the bats can be considered adults. In both experiments, FG was injected in the HFR and FR was injected in the LFR. Except for one FR labeled neuron in the SG in the first example (Fig. 5C), the connections from the MGBv and the SG were to the LFR and HFR, respectively. This suggests that the pathways are mostly segregated by P150.
Figure 5.
Pathways are segregated in 5 month old bats. In both experiments tracers were injected in the LFR and HFR. LFR tracer labeled neurons in the MGBv, but not the SG. HFR injections labeled neurons in the SG, but not the MGBv. The non-SG areas of the MGBd project to both LFR and HFR. Scale bars = 500 μm.
Summary of results
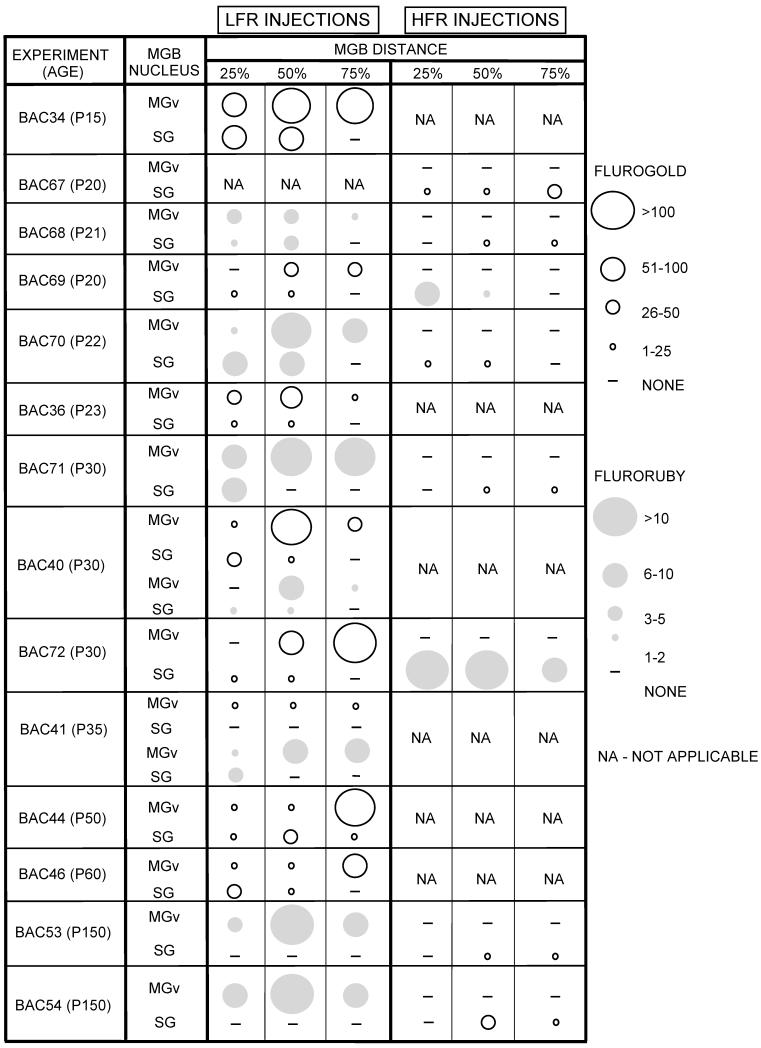
Table 1 presents a summary of all the experiments. The table schematically shows the number of labeled neurons in the SG and MGBv at three MGB locations (arranged caudal to rostral). In every pup younger than P60, the LFR receives input from both the SG and the MGBv. Caudal SG sends more projections to the LFR compared to the rostral SG. In case BAC41, only the caudal 15% of the SG projected to the LFR (Fig. 3 K-N). Therefore the FG data for this case appears as no SG label in Table 1. The HFR receives input from the SG, but not the MGBv. Taken together, these data show that during early development the LFR differs from adults in that it receives input from both the SG and the MGBv, while the HFR is similar to adults as it receives input from the SG, but not the MGBv.
Table 1.
Summary of 14 experiments performed in this study. The number of labeled SG and MGBv neurons in three different caudorostral sections is shown schematically by the size of the circle. Light circles - FG. Gray circles - FR. Note that FG labeled more cells than FR. NA - not applicable (injection not made)
|
DISCUSSION
This study sought to determine the time course of development of the parallel thalamocortical pathways for prey localization and echolocation observed in the adult pallid bat. The main result is that between the time of acquisition of adult-like hearing (2 weeks postnatal) and at least until the time of weaning (8 weeks postnatal), there is overlap in the thalamocortical connections. The overlap was due primarily to the SG projecting to both the LFR and the HFR in pups. The pup MGBv projects mainly to the LFR as seen in adults. SG projections to the LFR were seen in every experiment regardless of the frequency range of the LFR injection site (8-35 kHz). Because the distance from the HFR, which is the target of SG projections in adults, increases as best frequencies within the LFR decrease, this suggests that retrograde SG labeling is not due simply to the proximity of LFR injection sites to the HFR. This suggests that the observed overlap of the pathways is not likely to be a result of LFR injections spreading into the HFR in pups. In addition, the similarity of results for different injection sizes and the asymmetry in projection patterns (SG projects to both LFR and HFR, but MGBv projects mainly to LFR) also indicate that the results were not due to spread of tracers across cortical regions.
The SG neurons projecting to the LFR were located predominantly in the caudal half of the MGB. The SG neurons projecting to the HFR were distributed throughout the rostrocaudal extent of the MGB, as is the case in adults. In contrast, projections from the MGBv were much more adult-like at the earliest ages tested, and were restricted largely to the LFR. We found little evidence that the MGBv also projects to the HFR; this nucleus thus appears more faithful to its adult target, even early in development. Therefore it appears that the thalamocortical pathways underlying representation of two different sounds used in two behaviors become segregated postnatally, and may be subject to experience-dependent refinement.
This asymmetry in the degree of overlap in these two thalamocortical projections is not what would have been predicted based upon the anatomical distribution of functionally bimodal neurons early in development (Razak and Fuzessery, 2007). These neurons are widely distributed in both the LFR and HFR. If both regions are receiving converging low- and high-frequency input, we would have anticipated the degree of overlap in projections from the SG and MBGv would be more symmetrical, but this is not the case. Projections from the MGBv to the HFR of auditory cortex are minor, leaving in question the origin of bimodal neurons in this region. It is possible that the SG is receiving both low- and high-frequency input from the IC or other lower level auditory nuclei (Casseday et al., 1989; Gordon and O’Neill, 2000). If this is the case, then it must also be explained why it is only the caudal SG that projects to the LFR. Since no physiological evidence of high-frequency tuning was recorded at the injection sites in the LFR, this would suggest that the caudal SG selectively receives low-frequency input. These issues need to be resolved through physiological examination of the frequency tuning of SG neurons, and anatomical examination of the inputs to SG, over the course of development.
It is perhaps not surprising that the parallel pathways arise through developmental plasticity of connections originating in the SG. The SG appears to be more susceptible to plasticity during both evolutionary and developmental time courses. The SG in rodents and carnivores is a multisensory nucleus dominated by visual inputs from the superior colliculus (Calford and Aitkin, 1983; Katoh and Benedek, 1995; Tanaka et al., 1985). The auditory input comes primarily from extralemniscal sources such as the nucleus of the central acoustic tract and the external nucleus of the IC (Casseday et al., 1989; Gordon and O’Neill, 2000). Only in chiroptera does the SG receive input from the central nucleus of the IC (ICc, Wenstrup et al., 1994). In the mustached bat, the SG, as well as the rest of the MGBd, receives input from all frequency bands of the ICc (Wenstrup et al., 1994, Wenstrup and Grose, 1995). In both horseshoe (Radtke-Schuller, 2004) and mustached (Pearson et al., 2007) bats, the MGBd projects to both primary and non-primary auditory cortices. Thus in bats, the increase in thalamic participation in auditory processing appears primarily through enhancing the contribution of MGBd, specifically the SG, in auditory processing.
The developmental plasticity of SG connections is illustrated by cross-modal plasticity in ferrets (Pallas et al., 1990). By appropriate lesions of retinal axon targets, visual input can be guided to the MGB. This rewiring leaves the MGBv-primary auditory connections similar to controls. However, an anomalous input to the primary auditory cortex arises from dorsal thalamus, including the SG, in the cross-modal animals. This suggests that the SG typically receives and sends more exuberant connections during early development. These connections may either be stabilized or pruned by activity-dependent mechanisms.
The present data on the pallid bat thalamocortical development support the hypothesis that the SG sends exuberant projections during early development. Between P15 and P60, the SG projects to both HFR and LFR. In adults, the SG projects only to the HFR, suggesting developmental pruning of exuberant connections from the SG. The pruning may be a result of activity-dependent competition. As the bat matures and begins to hunt, competition for cortical tissue by pathways serving echolocation and passive sound localization may eliminate SG projections serving echolocation from the LFR, which serves passive sound localization in adult.
An unusual aspect of thalamocortical connections in the adult pallid bat is the lack of projections from the MGBv to the HFR. The cortical LFR and HFR represent the audible range of the pallid bat (5-70 kHz). The lack of projections from the MGBv to the HFR suggests that the HFR may not be part of the primary auditory cortex. If only the LFR is the primary auditory cortex, then it is unusual in that nearly half the audible range (BF >40 kHz) is not represented in the primary auditory cortex. If the tonotopic map formed by LFR and HFR is primary auditory cortex, then it is unusual in that it receives half the input from the MGBv and the other half from the SG. We have previously suggested (Razak et al., 2006) that this is an adaptation specific to gleaning behavior wherein parallel pathways provide a substrate for improved segregation of prey-generated noise and echoes if they arrive within a short time interval. Studies of other gleaning bat species’ thalamocortical organization are likely to shed more light on this hypothesis. Given the paucity of MGBv projections to the HFR in pups, it may be that this aspect of thalamocortical connectivity develops in an experience-independent manner, in contrast to the refinement of SG projections over the course of development.
In summary, too few studies have addressed the issue of thalamocortical development in the mammalian auditory system. The present study has examined this topic in the pallid bat, a species whose auditory system has an unusual and specialized functional organization. We found considerable differences in the development of thalamocortical projections from the dorsal and ventral divisions of the MGB. Whether a similar developmental pattern occurs in nonecholocating mammals remains to be determined.
Acknowledgements
We thank Donal Skinner for his generosity in sharing his microscope facilities, and Jeff Winer for authoritative discussions about the auditory thalamus. Research was funded by NIDCD Grant DC05202 to ZMF and INBRE P20 RR0 16474-04.
REFERENCES
- Aitkin LM, Dunlop CW. Interplay of excitation and inhibition in the cat medial geniculate body. J Neurophysiol. 1968;31:44–61. doi: 10.1152/jn.1968.31.1.44. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections AI, AII, and the anterior auditory field (AAF) in the cat: evidence for two largely segregated systems of connections. J Comp Neurol. 1980;194:663–701. doi: 10.1002/cne.901940312. [DOI] [PubMed] [Google Scholar]
- Bell GP. Behavioral and ecological aspects of gleaning by the desert insectivorous bat, Antrozous pallidus (Chiroptera: Vespertilionidae) Behav Ecol Sociobiol. 1982;10:217–223. [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res. 1994;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- Brown P, Grinnell AD, Harrison J. The development of hearing in the pallid bat, Antrozous pallidus. J Comp Physiol A. 1978;126:169–182. [Google Scholar]
- Calford MB, Aitkin LM. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983;3:2365–2380. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casseday JH, Kobler JB, Isbey SF, Covey E. Central acoustic tract in an echolocating bat: an extralemniscal auditory pathway to the thalamus. J Comp Neurol. 1989;287:247–259. doi: 10.1002/cne.902870208. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Franklin SR, Brunso-Bechtold JK, Henkel CK. Unilateral cochlear ablation before hearing onset disrupts the maintenance of dorsal nucleus of the lateral lemniscus projection patterns in the rat inferior colliculus. Neuroscience. 2006;143:105–115. doi: 10.1016/j.neuroscience.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, Kandler K. Auditory projections to the inferior colliculus of the rat are present by birth. Neurosci Lett. 1990;120:58–61. doi: 10.1016/0304-3940(90)90167-8. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM, Gumtow RG, Lane R. A microcomputer-controlled system for use in auditory physiology. J Neurosci Methods. 1991;36:45–52. doi: 10.1016/0165-0270(91)90136-n. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM, Buttenhoff P, Andrews B, Kennedy JM. Passive sound localization of prey by the pallid bat (Antrozous p. pallidus) J Comp Physiol [A] 1993;171:767–777. doi: 10.1007/BF00213073. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM. Response selectivity for multiple dimensions of frequency sweeps in the pallid bat inferior colliculus. J Neurophysiol. 1994;72:1061–1079. doi: 10.1152/jn.1994.72.3.1061. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM. Acute sensitivity to interaural time differences in the inferior colliculus of a bat that relies on passive sound localization. Hear Res. 1997;109:46–62. doi: 10.1016/s0378-5955(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Development of afferent patterns in the inferior colliculus of the rat: projection from the dorsal nucleus of the lateral lemniscus. J Comp Neurol. 2000;416:368–382. doi: 10.1002/(sici)1096-9861(20000117)416:3<368::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Shahmoradian SH, French CC, Henkel CK, McHaffie JG. Early segregation of layered projections from the lateral superior olivary nucleus to the central nucleus of the inferior colliculus in the neonatal cat. Brain Res. 2007;1173:66–77. doi: 10.1016/j.brainres.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M, O’Neill WE. An extralemniscal component of the mustached bat inferior colliculus selective for direction and rate of linear frequency modulations. J Comp Neurol. 2000;426:165–181. doi: 10.1002/1096-9861(20001016)426:2<165::aid-cne1>3.0.co;2-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung B, Fritzsch B. Time course of embryonic midbrain and thalamic auditory connection development in mice as revealed by carbocyanine dye tracing. J Comp Neurol. 2004;479:309–327. doi: 10.1002/cne.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B. Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res. 2003;153:543–549. doi: 10.1007/s00221-003-1611-5. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Organization of the thalamocortical auditory system in the cat. Annu Rev Neurosci. 1983;6:95–120. doi: 10.1146/annurev.ne.06.030183.000523. [DOI] [PubMed] [Google Scholar]
- Katoh YY, Benedek G. Organization of the colliculosuprageniculate pathway in the cat: a wheat germ agglutinin-horseradish peroxidase study. J Comp Neurol. 1995;352:381–397. doi: 10.1002/cne.903520306. [DOI] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–90. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Chair L, Snyder RL. Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2006;497:13–31. doi: 10.1002/cne.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, Imig TJ. Thalamic projections to fields A, A1, P and VP in cat auditory cortex. J Comp Neurol. 1987;265:119–144. doi: 10.1002/cne.902650109. [DOI] [PubMed] [Google Scholar]
- Morest DK. The neuronal architecture of the medial geniculate body of the cat. J Anat. 1964;98:611–630. [PMC free article] [PubMed] [Google Scholar]
- Olsen JF, Suga N. Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of target range information. J Neurophysiol. 1991;65:1275–1296. doi: 10.1152/jn.1991.65.6.1275. [DOI] [PubMed] [Google Scholar]
- Pallas SL, Roe AW, Sur M. Visual projections induced into the auditory pathway of ferrets. I. Novel inputs to primary auditory cortex (AI) from the LP/pulvinar complex and the topography of the MGN-AI projection. J Comp Neurol. 1990;298:50–68. doi: 10.1002/cne.902980105. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Crocker WD, Fitzpatrick DC. Connections of functional areas in the mustached bat’s auditory cortex with the auditory thalamus. J Comp Neurol. 2007;500:401–418. doi: 10.1002/cne.21175. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Diamond IT, Winer J. Organization of thalamocortical auditory system in the cat studied with horseradish peroxidase. Brain Res. 1976;101:345–354. doi: 10.1016/0006-8993(76)90275-4. [DOI] [PubMed] [Google Scholar]
- Radtke-Schuller S. Cytoarchitecture of the medial geniculate body and thalamic projections to the auditory cortex in the rufous horseshoe bat (Rhinolophys rouxi). I. Temporal fields. Anat Embryol. 2004;209:59–76. doi: 10.1007/s00429-004-0424-z. [DOI] [PubMed] [Google Scholar]
- Radtke-Schuller S, Schuller G, O’Neill WE. Thalamic projections to the auditory cortex in the rufous horseshoe bat (Rhinolophys rouxi). II. Dorsal fields. Anat Embryol. 2004;209:77–91. doi: 10.1007/s00429-004-0425-y. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM, Lohuis TD. Single cortical neurons serve both echolocation and passive sound localization. J Neurophysiol. 1999;81:1438–1442. doi: 10.1152/jn.1999.81.3.1438. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Functional organization of the pallid bat auditory cortex: Emphasis on binural organization. J Neurophysiol. 2002;87:72–86. doi: 10.1152/jn.00226.2001. [DOI] [PubMed] [Google Scholar]
- Razak KA, Shen W, Zumsteg T, Fuzessery ZM. Parallel thalamocortical pathways for echolocation and passive sound localization in a gleaning bat, Antrozous pallidus. J Comp Neurol. 2006;500:322–338. doi: 10.1002/cne.21178. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Neural mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex of the pallid bat. J Neurophysiol. 2006;96:1303–1319. doi: 10.1152/jn.00020.2006. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Development of functional organization of the pallid bat auditory cortex. Hearing Res. 2007;228:69–81. doi: 10.1016/j.heares.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM. Functional organization of the auditory pathways. In: Ehret G, Romand R, editors. The central auditory system. Oxford University Press; New York: 1997. pp. 3–96. [Google Scholar]
- Schuller G, O’Neill WE, Radtke-Schuller S. Facilitation and Delay Sensitivity of Auditory Cortex Neurons in CF - FM Bats, Rhinolophus rouxi and Pteronotus p. parnellii. Eur J Neurosci. 1991;3:1165–1181. doi: 10.1111/j.1460-9568.1991.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Otani K, Tokunaga A, Sugita S. The reciprocal connections of the suprageniculate nucleus and the superior colliculus in the rat. Neurosci Res. 1985;3:79–85. doi: 10.1016/0168-0102(85)90040-9. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, Grose CD. Inputs to combination-sensitive neurons in the medial geniculate body of the mustached bat: the missing fundamental. J Neurosci. 1995;15:4693–711. doi: 10.1523/JNEUROSCI.15-06-04693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup JJ, Larue DT, Winer JA. Projections of physiologically defined subdivisions of the inferior colliculus in the mustached bat: targets in the medial geniculate body and extrathalamic nuclei. J Comp Neurol. 1994;346:207–236. doi: 10.1002/cne.903460204. [DOI] [PubMed] [Google Scholar]
- Wepsic JG. Multimodal sensory activation of cells in the magnocellular medial geniculate nucleus. Exp Neurol. 1966;15:299–318. doi: 10.1016/0014-4886(66)90053-7. [DOI] [PubMed] [Google Scholar]
- Winer JA, Sally SL, Larue DT, Kelly JB. Origins of medial geniculate projections to physiologically defined zones of rat primary auditory cortex. Hear Res. 1999;130:42–61. doi: 10.1016/s0378-5955(98)00217-2. [DOI] [PubMed] [Google Scholar]
- Winer JA, Miller LM, Lee CC, Schreiner CE. Auditory thalamocortical transformation: structure and function. Trends Neurosci. 2005;28:255–263. doi: 10.1016/j.tins.2005.03.009. [DOI] [PubMed] [Google Scholar]