Abstract
Divergence in acoustic signals between populations of animals can lead to species recognition failure, reproductive isolation, and speciation. Character displacement may facilitate coexistence of species in natural communities, yet evidence for character displacement in acoustic signals is scant. Here, we find evidence of character displacement in song as well as body size and bill size of 2 related African tinkerbirds. Playback experiments indicate that related species' songs are perceived differently in sympatry than in allopatry. We suggest character displacement occurs in phenotypic traits facilitating species recognition, which has important implications for understanding the processes that lead to speciation and diversification. Because many of the sites where the 2 species coexist are areas where pristine rainforest has been degraded, results also suggest that anthropogenic pressures resulting from deforestation may be a contributing cause of character displacement in these species.
Keywords: animal communication, environmental gradients, interspecific competition, species recognition, phenotypic evolution
Character displacement occurs where the ranges of 2 closely related species overlap, and morphological, ecological, or behavioral traits diverge in sympatry (1–6). Such divergence in sympatry is presumed to be adaptive—reducing niche overlap or heterospecific mating. Some of the best evidence for character displacement has come from work on Darwin's finches, where exaggerated divergence in bill morphology between sympatric species has been attributed to interspecific competition (2–4, 7). Yet, despite evidence that song frequencies are negatively correlated with body size and beak size (8, 9), character displacement in Darwin's finch songs has not been reported. Indeed, despite some evidence of call divergence in Hyla and Litoria tree frogs (10, 11) and Gryllus crickets (12), as well as plumage divergence in Ficedula flycatchers (13), there remains no convincing example of character displacement in bird song, and none that control for geographic and environmental variation (14). Bird songs of related species are predicted to diverge in sympatry to reduce species recognition errors when hybrids have lower fitness (14). Alternatively, natural selection may drive divergence in body size or beak shape because of interspecific competition for resources (1–6), leading to song divergence as a byproduct (8). Whether songs diverge directly by reinforcement to inhibit maladaptive hybridization or indirectly as a consequence of competition, song is fundamentally important in mate choice in many taxa, and divergence could lead to speciation (15).
We investigated a pattern of divergence in song in 2 African tinkerbirds where they coexist. Pogoniulus tinkerbirds are barbets (Family: Capitonidae) and emit vocalizations that are ideal for studying character displacement. Song structure is simple, differs in frequency and rate and, like simple vocalizations in other taxa (16, 17), songs may develop independently of cultural learning. Although the ability to learn songs has not been tested specifically in tinkerbirds, it has been demonstrated in just 3 Orders of birds: the Passeriformes, Psittaciformes, and Apodiformes (18, 19), with no evidence so far of song learning in Piciformes, the Order that includes Pogoniulus tinkerbirds. Morphologically, the yellow-rumped tinkerbird (Pogoniulus bilineatus) is typically larger than the yellow-throated tinkerbird (Pogoniulus subsulphureus), which has yellow facial markings. The 2 species have been shown to be closely related sister taxa based on phylogenetic analyses (20). Both species range across most of Central Africa, where they sing throughout the day from exposed perches in the forest canopy, with P. subsulphureus preferring dense forest and P. bilineatus more open habitats (Fig. 1) (21). Both species are omnivorous, foraging on fruits (including Loranthus mistletoe berries and the fruits of Macaranga, Allophyllus, and Trema) and insects (including ants, beetle larvae, and termites) (21).
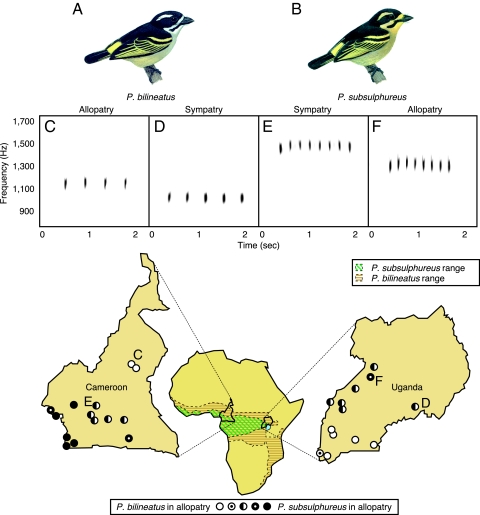
Fig. 1.
Songs in sympatry are more different from songs in allopatry. (A) P. bilineatus is typically larger than (B) P. subsulphureus. (C–F) Spectrograms are examples of the species' mean song frequencies for allopatric P. bilineatus at Wakwa (C), sympatric P. bilineatus at Mabira Forest (D), sympatric P. subsulphureus song at Obala (E), and allopatric P. subsulphureus song at Budongo (F). The Africa map illustrates the species' distributions. P. bilineatus completely encompasses the range of P. subsulphureus, but in lowland rainforests P. bilineatus is rare. Site locations are illustrated for P. bilineatus in allopatry (white circles); P. subsulphureus in allopatry (black circles); P. subsulphureus common, P. bilineatus rare, and thus effective allopatry for P. subsulphureus but sympatry for P. bilineatus (black circles with white dots); P. bilineatus common, P. subsulphureus rare, and thus effective allopatry for P. bilineatus (white circles with black dots); and both species common in sympatry (half-filled circles). [Tinkerbird illustrations are by Nik Borrow, from Birds of Western Africa (38), by Nik Borrow and Ron Demey, with permission from the publishers, Christopher Helm, London.]
We incorporated environmental data in our analyses to understand how phenotypic traits vary according to environmental and geographic factors, and thus to help eliminate alternative explanations for more exaggerated divergence in sympatry than in allopatry (5). In addition, incorporating environmental data could aid in detecting character displacement that has occurred but may have been overlooked because it was obscured by adaptation along environmental gradients, with differences appearing greater in allopatry than in sympatry (22). A recent theoretical study showed that the classic pattern of character displacement might only evolve in a trait under resource competition in the presence of an environmental gradient or in traits important in both ecology and mate choice (22).
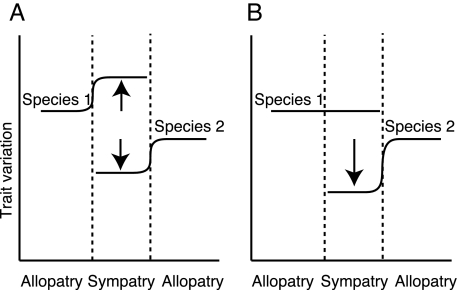
Frequency-dependent selection is centrally important in character displacement (23). All else being equal, extreme phenotypes will have higher fitness than phenotypes more similar to those of a commonly co-occurring congener, and thus divergence in sympatry is predicted to occur (24). However, because of positive frequency dependence, rare phenotypes are unlikely to have a selective impact on common phenotypes (25) but might lead to character displacement in only the rare phenotype (an example of asymmetric character displacement) (Fig. 2). Models support the prediction that divergence driven by resource competition or hybridization is unlikely to occur in a common species when its congener is rare but would be expected at higher congener abundances (22, 23). This effect has also been found empirically, with divergence in bill size in the Darwin's finch Geospiza fortis occurring only after the sympatric competing species Geospiza magnirostris increased in abundance (3).
Fig. 2.
Frequency-dependent selection determines symmetric or asymmetric character displacement. (A) When species 1 and 2 are both common in sympatry, both species' phenotypes diverge. (B) When species 1 is common but species 2 is rare, species 1 is not influenced by the rare presence of species 2 in sympatry and does not diverge (hereafter referred to as “effective allopatry”). Conversely, species 2 might still be affected by species 1's presence (sympatry) and might exhibit an asymmetric shift in phenotype. Arrows indicate direction of predicted divergence in sympatry.
Here, we report on a case of character displacement where the geographic range of P. subsulphureus is completely nested within the range of P. bilineatus. Although true allopatric populations exist for P. bilineatus, true allopatry does not exist for P. subsulphureus. Yet, populations exist within the sympatric range where one species is very common and the other rare. Under such circumstances, we would expect the rare species to have little or no effect on the common species. In such populations, we predict the common species would have a phenotype similar to that in true allopatry (Fig. 2). We call these populations where congeners are rare “effective allopatry.” We combined cases of effective allopatry with those of true allopatry for our analyses, thus allowing us to test for the prediction that divergence occurs when the coexisting related species is common.
We examined 27 populations of Pogoniulus at sympatric and allopatric sites in Uganda and Cameroon (Fig. 1). Sites were distributed across an environmental gradient from savanna with gallery forest and higher-elevation sites, where only P. bilineatus occurs, through transitional and disturbed forest, where both species coexist, to pristine lowland rainforest, where only P. subsulphureus is commonly found.
Results
Character Displacement in Song.
We used linear mixed models with maximum likelihood estimation (26) to determine the effect of congener presence on peak song frequency and song rate for each species. To control for the effects of environmental gradients, we incorporated remotely sensed data for percent tree cover [from the Moderate Resolution Imaging Spectroradiometer (MODIS)] and elevation. We included longitude as a measure of clinal variation and geographic isolation between populations (but not latitude, which was highly correlated with longitude for the sample locations). The data were nested by site/population, which was included as a random effect in the mixed model.
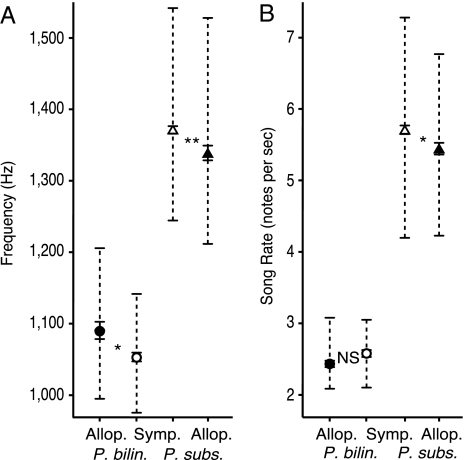
In sympatry, song frequencies were lower for P. bilineatus (df = 20, t = −2.39, P = 0.027) and higher for P. subsulphureus (df = 18, z = 3.31, P = 0.004) than in allopatry after controlling for percent tree cover, elevation, longitude, and site (Fig. 3A). Although both species sung faster songs further east (P. bilineatus: df = 203, t = 8.54, P < 0.001; P. subsulphureus: df = 209, t = 9.14, P < 0.001), P. subsulphureus songs were faster in sympatry than in allopatry (df = 18, t = 2.75, P = 0.013), but no difference was found in P. bilineatus song rate (t = 1.65, P = 0.114) (Fig. 3B). Elevation was found to influence P. subsulphureus song, with faster, higher-frequency songs at lower elevations (peak frequency: df = 209, t = −4.21, P < 0.001; rate: t = −1.98, P = 0.049), but elevation did not influence P. bilineatus song (peak frequency: df = 203, t = −0.74, P = 0.461; rate: t = 0.65, P = 0.517). Percent tree cover had no effect on song for either species (P. bilineatus, peak frequency: df = 203, t = −1.00, P = 0.320; rate: t = −1.06 P = 0.291; P. subsulphureus, peak frequency: df = 209, t = −0.80, P = 0.427, rate: t = −0.57, P = 0.568).
Fig. 3.
Tinkerbird songs diverge in sympatry in peak frequency and rate after controlling for percent tree cover, elevation, longitude, and site. Peak song frequencies (A) diverge in sympatry in both P. bilineatus and P. subsulphureus, whereas song rate (B) shifts in P. subsulphureus but not in P. bilineatus. Dashed lines show the range of mean peak song frequencies of individuals before controlling for environmental and geographic factors, illustrating that some song frequencies of each species are similar in allopatry. Error bars are SEM; *, P < 0.05; **, P < 0.01; NS, not significant.
Responses to Playback Experiments.
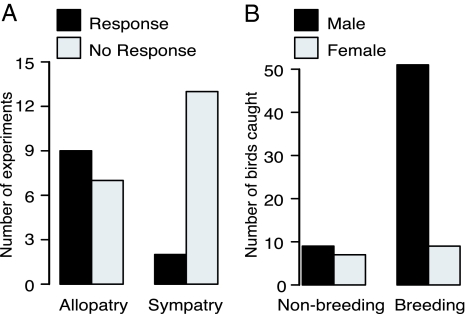
Character displacement may also arise in mate-recognition systems (27) via sexual imprinting to differentiate between conspecifics and heterospecifics in sympatry (28). To test the importance of song in species recognition and interspecific competition, we performed 31 playback experiments of synthetic mean heterospecific song to individuals in sympatry and allopatry. Significantly more individuals responded to mean heterospecific song in allopatry (multiway contingency table for survey data: df = 13, Pearson χ2 = 7.72, P = 0.016) than in sympatry (Fig. 4A), implying that songs become more distinct in sympatry to enable species recognition. A greater response in sympatry may have otherwise reflected maintenance of interspecific territoriality (28). Body size or plumage may also play a role in interspecific territoriality; however, both species were regularly found singing in close proximity to one another in sympatry (sometimes in the same tree), suggesting some territorial overlap. Vocalizations could also diverge because of acoustic interference (29) or background noise (30, 31); however, responses to heterospecific playback would not be expected if heterospecific song only represented background interference masking communication frequencies.
Fig. 4.
Allopatric tinkerbirds respond significantly more than sympatric tinkerbirds to heterospecific playback. (A) Bar charts indicating responses to playback of heterospecific song to both species, illustrating significantly greater responses in populations in allopatry (and effective allopatry). (B) Males respond significantly more than females to conspecific playback when they are in breeding condition, whereas there is no difference in responses between the sexes when they are not breeding.
Signal divergence could be driven by male–male competition or by female mate choice, with the latter potentially leading to reinforcement to avoid hybridization (15). We tested for the function of song in tinkerbirds by quantifying the strength of responses of males and females to conspecific playback. We found that at sites where breeding was evident (based on singing behavior and size of reproductive organs), the female-to-male response ratio was 1:5.8, whereas it was 1:1.3 at sites where breeding was not evident (multiway contingency table for survey data: n = 77, df = 14, Pearson χ2 = 15.84, P = 0.001) (Fig. 4B). We infer that males are more aggressive toward other males during the breeding season, competing with one another for mates, than during nonbreeding seasons, when both sexes may compete equally with conspecifics for resources. These results suggest that character displacement facilitates species recognition in sympatry, thus reducing costly interspecific aggression among males.
Morphological Divergence.
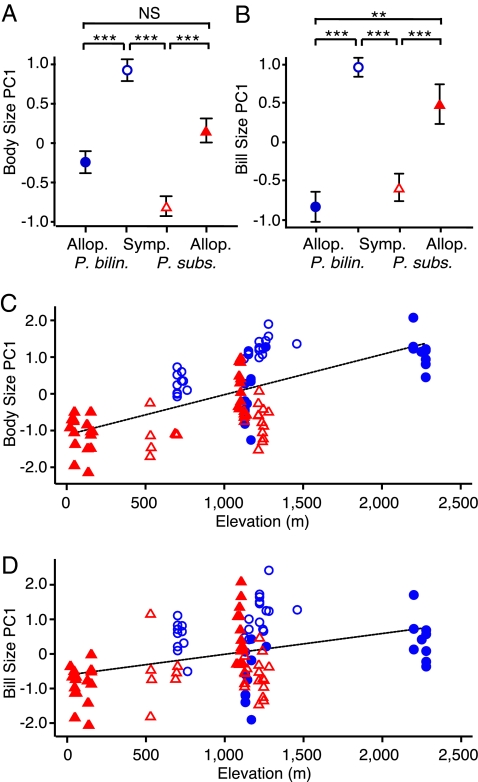
Previous research has reported a negative correlation between body size and song frequency in birds (30), as well as evidence that beak size may constrain song performance (8, 9). To investigate the extent to which songs might vary as a byproduct of morphological adaptation, we measured wing chord, tarsus, and tail length; bill length, depth, and width; and body mass from 37 P. bilineatus and 40 P. subsulphureus captured in mist nets in the field. We used linear regression with standard error adjusted for within-site correlation (32) to test for the effect of congener presence on body size (principal components analysis: PC1 explained 63% of variation, with factor loadings ranging from 61% for culmen to 90% for mass). We controlled for sex, elevation, and percent tree cover (but not longitude, which was highly correlated with elevation for the P. subsulphureus morphology data), nesting the data by site, and found that P. bilineatus are larger (df = 14, t = 6.76, P < 0.001), with larger bills (t = 9.86, P < 0.001), and P. subsulphureus are smaller (t = −5.17, P < 0.001), with smaller bills (t = −4.20, P = 0.001) in sympatry than in allopatry (Fig. 5 A and B). In fact, P. bilineatus and P. subsulphureus in allopatry were no different from one another in body size after controlling for sex, elevation, site, and percent tree cover (df = 14, t = 1.71, P = 0.109), but were different in sympatry (t = −8.85, P < 0.001) (Fig. 5A), consistent with the prediction of character displacement in body size. A pattern also existed of divergence in bill size (PC1 on bill length, depth, and width explained 62% of variation) in sympatry (df = 14, t = −6.92, P < 0.001) compared with allopatry (t = 3.46, P = 0.004) after controlling for sex, environmental gradients, and distance (Fig. 5B). Elevation also had a strong effect on both body size (t = 8.63, P < 0.001) and bill size (t = 5.30, P < 0.001), with size increasing with altitude (Fig. 5 C and D). Despite the correlation of size with elevation, body sizes in sympatry and allopatry were consistent with predictions of a negative correlation between body size and song frequency. Our results suggest there is selective pressure for the 2 species to diverge in morphology in sympatry, with selection occurring on either body size or bill size or both. Further research will be required to determine which trait is the possible target of selection (9, 33).
Fig. 5.
Character displacement in body size and bill size. (A) P. bilineatus are larger overall in body size and also in (B) bill size in sympatry, and P. subsulphureus are smaller after controlling for percent tree cover, elevation, site, and sex. No differences exist in allopatry in body size, and differences in bill size are smaller in allopatry than in sympatry. Error bars are SEM; *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant. Elevation has a strong effect on (C) body size and (D) bill size. P. bilineatus are larger in allopatry (solid blue circles) than P. subsulphureus (solid red triangles) in body size before controlling for the effects of elevation and tree cover, although not significantly (linear regression with standard error adjusted for within-site correlation, ref. 32; controlling for sex differences: df = 14, t = −1.56, P = 0.142), and are similar in bill size (df = 14, t = −0.07, P = 0.949). Controlling for the effects of elevation and tree cover in statistical models gives the impression seen in (A) and (B) of larger allopatric P. subsulphureus. P. bilineatus (open blue circles) are larger in sympatry than P. subsulphureus (open red triangles), even before accounting for the effects of elevation and tree cover (but still controlling for sex and site), in (C) body size (df = 14, t = −7.09, P < 0.001) and (D) bill size (df = 14, t = −6.81, P < 0.001).
Impacts of Anthropogenic Disturbance.
Many of the sites where we found the 2 species side by side were at edges and gaps of forest at which the open-habitat P. bilineatus has invaded the formerly pristine forest where only P. subsulphureus would typically exist. We performed a statistical analysis confirming that the 1-km2 pixels within which recordings were collected in those secondary forest sites where the 2 species coexist (thus excluding sympatric natural transition sites and secondary forest sites where P. subsulphureus occurred alone) had lower percent tree cover (X = 58.2% ± 1.9% SEM) than primary forest sites where only P. subsulphureus was found (X = 76.4% ± 1.0% SEM; linear regression with standard error adjusted for within-pixel correlation: df = 58, t = 3.71, P < 0.001). Secondary forest sites included several sites within the forest zone in Cameroon where human encroachment has fragmented the landscape and forest reserves and national parks in Uganda where extensive logging occurred historically. These patterns suggest that anthropogenic fragmentation of the landscape may result in the coexistence of the 2 species that would otherwise prefer different habitats (25), and it could play a role in driving divergence.
Discussion
Results represent the first compelling demonstration of character displacement in bird song, supported by parallel divergence in morphology. Song divergence is unambiguous, occurring in the pitch of very simple songs, which are presumed to develop independently of cultural learning. Both theoretical (25) and empirical studies (34, 35) have demonstrated that song-learning ability is more likely to lead to song convergence rather than divergence between closely related species in sympatry. In our study, divergence in body size mirrors patterns of song divergence, with larger P. bilineatus in sympatry singing lower-frequency songs and smaller P. subsulphureus singing higher-frequency songs, as predicted by the negative correlation typically found between body size and song frequency (30). Importantly, patterns of divergence are found after controlling for correlations between phenotype and environmental and geographic clines (22). Because song is important in mate choice (18) and reproductive isolation (11, 15), character displacement may also be important in speciation. Character displacement might also lead indirectly to speciation (11) when songs of species diverging in sympatry are not recognized by populations of the same species in allopatry. By examining divergence in phenotypic traits and controlling for variation along environmental gradients, further research can identify how character displacement in traits important in sexual selection might be a widespread phenomenon important in evolutionary diversification and adaptive radiation.
The mechanism driving character displacement in the present study could be reproductive interference, reinforcement against hybridization, or interspecific competition. Playback results suggest that reproductive interference may play a role because of the extent of male–male competition during the breeding season, but based on the lack of evidence of hybridization between the 2 species (36), we believe reinforcement is unlikely. The possibility that character displacement results from differential selection on body size and/or bill size in response to differences in resource use, with songs diverging as a byproduct, remains to be explored. In either case, divergence in traits important in resource competition (morphology) and/or reproductive isolation (song) is consistent with the theory on how character displacement can evolve along an environmental gradient (22).
The results also provide insight into how anthropogenic disturbance may influence species' phenotypes. Many of the sites where the 2 species commonly coexist are disturbed forest sites, where pristine rainforest has been fragmented and degraded. As deforestation advances in the Congo Basin, our results suggest that rapid divergence in songs and morphology of Pogoniulus tinkerbirds will likely ensue. Character displacement driven by anthropogenic change represents yet another class of examples of how evolution in human-altered environments may be taking place (37).
Materials and Methods
We recorded 228 P. bilineatus and 232 P. subsulphureus in Uganda and Cameroon between July 2004 and September 2007 by using a Sennheiser ME67/ME88 directional microphone and a Marantz PMD670 digital recorder/Sony TCD5M cassette recorder. Both regions were visited during dry and rainy seasons, thus controlling for variation in singing rates between species. Birds were target mist netted by using conspecific playback at 13 sites between July and September 2007, and 77 individuals were measured in the field. All birds caught were identified as adults based on bill and plumage characters (38), and 51 voucher specimens were collected (Natural History Museum of Los Angeles County catalog numbers 114786–114805; University of California, Los Angeles Donald R. Dickey Collection catalog numbers 43175–43205). We performed playback experiments to singing birds by using an iPod (Apple) with a PAL Tivoli Audio loudspeaker positioned ≈50 m linear distance from the subject and the following protocol: 2-min playback of synthetic mean heterospecific song, 1-min post-playback observation. Responses were scored 1 or 0 based on whether a visible approach occurred. Approaches, usually accompanied by visual searching and agonistic vocalizations, were deemed indicative of territorial behavior. If there was no response within the 3-min experiment, we performed a 2-min control playback of synthetic mean conspecific song followed by 1-min post-playback observation to establish that the singing bird was behaving territorially. If the subject did not respond to the conspecific control, the experiment was dropped. Twenty-nine playback experiments of synthetic mean conspecific song were also performed across sites (to different subjects) to confirm that approaches occur within the experimental framework. Male–female response rates were based on sex of individuals caught in mist nets in response to conspecific playback. Synthetic playback stimuli were based on each species' mean song frequency and rate from all of the recordings collected from 8 populations in Uganda [comprising 5 sympatric and 3 allopatric (2 of P. bilineatus and 1 of P. subsulphureus) populations] during 2004 and were produced by using SoundEdit 16 (Macromedia 1995). We used synthetic stimuli because they permit testing specifically for differences in frequency and rate between the 2 species, rather than motivational, signal-to-noise ratio, geographic population, background noise, or other differences inherent in field recordings (39). In this way, synthetic playbacks also avoid the problems of pseudoreplication associated with authentic recordings (39). All fieldwork was conducted under protocols approved by the University of California, Los Angeles Animal Research Committee (ARC no. 2004-094-11) and permits issued by the governments of Uganda and Cameroon.
Total DNA was extracted from samples of 40 individuals, including blood from birds released in the field and muscle tissue from voucher specimens for whom sex could not be determined, by using a Qiagen kit following the manufacturer's protocol. PCR was performed by using primers 2550F and 2718R to amplify the differently sized introns of Z- and W-linked CHD1 genes (40). PCRs were performed in 25-μL volumes by using 0.1 μL of Sigma Taq polymerase (Sigma-Aldrich), 2.5 μL of manufacturer-supplied buffer, 2.0 μL of 25 mM MgCl, 2.0 μL of 2.5 mM dNTPs, 1.0 μL of 10 μM of each primer, 14.4 μL of molecular-grade water, and 2.0 μL of DNA). PCR products were separated on a 1% agarose gel run in Tris-acetate ethylenediaminetetraacetic acid (TAE) buffer and visualized by ethidium bromide staining.
Tinkerbird songs were imported into Raven 1.3 (41), and spectrograms and power spectra were produced by using a Fast Fourier transformation size of 4,096, a 1,269 window size, a 3-dB filter bandwidth of 50 Hz, and 10.8-Hz frequency resolution. We measured the first 10 clean songs from recordings of each individual and calculated the mean peak frequency and rate. We noted the mean peak frequency from the power spectrum for each song was typically 1–3 sec long, including internote intervals. We calculated song rates by using the following equation:
where N = number of notes, Ds = song duration, and Dn = last note duration. The last note was deducted to avoid biasing rate values to faster rates for shorter songs; e.g., if an N/Ds equation is used, and N = 5, note duration = 1 sec, and the internote interval = 1 sec, Ds = 9 and Rate = 5/9. However, if N = 3, Ds = 5, Rate = 3/5; if N = 2, Ds = 3, Rate = 2/3; thus, rates are biased higher for shorter songs. With our equation, if N = 5, then Rate = 4/8; if N = 3, then Rate = 2/4; and if N = 2, then Rate = 1/2; and thus in each case the rate is the same.
Percent tree cover was extracted from the vegetation continuous field product, derived from the MODIS at a 1-km2 resolution. These data were produced from time-series composites of MODIS data from 2001 (42). We used elevation data provided by the Shuttle Radar Topography Mission, aggregated at a 1-km2 resolution. All environmental data were computed based on latitude–longitude coordinates obtained from handheld Global Positioning System devices from each recording and mist net location, except for 3 sites in Cameroon, where site coordinates were used.
“Effective allopatry” included sites where the congener was found (heard) in only one 1-km2 pixel for sites encompassing at least four 1-km2 pixels, implying congener presence was local compared with focal species presence. Each singing male's territory may be 200–400 m2 in size (21), and individuals are unlikely to be heard over more than 1 km2. We chose a threshold of 1 km2 in 4 km2 because it best reflects differences in levels of species interactions observed at sites. By using this method, 4 sites where 1 species was rare and local (P. subsulphureus rare: Bwindi Impenetrable National Park, Uganda; P. bilineatus rare: Budongo Forest Reserve, Uganda; and Etome and Zoebefam, Cameroon), were considered effective allopatry for the common species, and at these sites, where we typically sampled along a trail or road, the rare species was found locally in 1 pixel, usually at the edge of the site. The criterion needed to distinguish between such sites and those sites where both species were commonly found, but because only 2 or 3 pixels were sampled, 1 species may have only been recorded in one of the pixels. For example, in Simbok, Cameroon, we recorded 4 P. bilineatus and 3 P. subsulphureus, yet the former were concentrated in 1 of the 2 pixels sampled, whereas 1 individual of the latter extended slightly into a second pixel, yet clearly both species were present in similar numbers, and therefore were sympatric. We used the pixel presence method instead of calculating relative densities because densities could include cases where uncommon congeners were singing and interacting in virtually all pixels, such as Murchison Falls, where 36 P. subsulphureus and 12 P. bilineatus were recorded—both species are widespread, but the former is much more common. Because of tinkerbird vocal behavior, any widespread singing presence of even an uncommon congener is considered sympatry.
Uganda sites were at higher longitudes and lower latitudes than Cameroon sites, meaning longitude and latitude were highly correlated. We chose longitude as our measure of clinal variation and geographic isolation in the linear mixed models because it best reflected linear distances between sites. We elected to include elevation (rather than longitude) in the regression models because it better reflected the environmental gradient, with the cluster “site” controlling for geographic isolation. Linear mixed models were used for their flexibility in analyzing grouped data (26). We used linear regression with standard error adjusted for within-cluster correlation (32), which relaxes assumptions of independence between groups, for the morphological analyses to compare differences across species where morphological characters regularly overlap in allopatry. We tested residuals from all statistical models by using the Kolmogorov–Smirnov test; we could not reject normality (0.370 < P < 0.992). All tests were 2-tailed (α = 0.05). We used the χ2 test multiway contingency tables for survey data (43) because they allow computation of standard errors appropriate for complex survey data. By using this approach, the data are clustered by site by specifying site as the primary sampling unit when computing the variance, standard error, and confidence intervals.
We determined that tinkerbirds were not breeding at 2 sites (Wakwa, Cameroon; Bwindi Impenetrable National Park, Uganda) where individuals were caught in 2007. These 2 sites were the highest-elevation sites visited in each region during summer 2007, and previous work has found temporal variation in breeding seasons across an environmental gradient in Cameroon in another African forest bird (31). We based our assessment of whether tinkerbirds were breeding first qualitatively on the level of song performance during that time, and then quantitatively by inspection of reproductive organs: at Wakwa and Bwindi, 11 of 13 specimens collected had no evidence of enlargement in testes or ovaries, whereas 1 of 13 was described as having slightly enlarged testes, and 1 of 13 had enlarged testes. At the remaining sites where birds were caught, singing performance was widespread, and 21 of 38 birds had enlarged reproductive organs, 9 of 38 slightly enlarged, and only 8 of 38 had no evidence of enlargement of reproductive organs.
A comparison of percent tree cover was performed between secondary and primary forest sites, which largely mirror distributions of P. bilineatus in sympatry with P. subsulphureus in forested areas (present in secondary sites and absent from primary forest sites). The analysis excluded the Kanyawara site at Kibale National Park, which is a montane forest site where P. bilineatus typically would occur at much higher densities than P. subsulphureus, but here the latter is still common at the limit of its altitudinal range. Murchison Falls National Park is also omitted because this site encompasses a sharp natural ecotone from primary forest to savanna, and hence both species occur there naturally, rather than because of human influences. Finally, coastal sites are excluded because the higher elevation P. bilineatus is largely absent from them despite the extent of deforestation there. Values for percent tree cover for each recording location were regressed on binary classifications of primary and secondary forest and were nested per pixel by using linear regression with standard error adjusted for within-cluster correlation (32).
Acknowledgments.
We thank P. Grant, C. Gerhardt, H. Slabbekoorn, R. Moyle, G. Grether, N. Losin, G. Slater, C. Taylor, editor D. Schluter, and 3 anonymous reviewers for discussion and comments on the manuscript; and A. Freedman and X. Chen for assistance with analyses. We thank I. Onyutta, J. Ogubi, P. Adoch, J.-B. Dongmo, M. Wassaja, N. Francis, and H. Minick for assistance in the field; R. Jones and J. Chaves for assistance with laboratory work; and the Uganda Wildlife Authority and the Cameroon Biodiversity Conservation Society for assistance with research and collecting permits. We also thank K. Garrett and the Natural History Museum of Los Angeles County for training and supplies. A.N.G.K. was supported by a Lewis and Clark Fund grant from the American Philosophical Society, a Lida Scott Brown fellowship from the University of California, Los Angeles, and National Science Foundation Doctoral Dissertation Improvement Grant IBN-0808597. Partial support for this project came from the University of California, Los Angeles Dean's Recruitment and Retention Funds (D.T.B.), and National Science Foundation Grant IRCEB9977072 and National Aeronautics and Space Administration Grant IDS/03-0169-0347 (to T.B.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.S. is a guest editor invited by the Editorial Board.
References
- 1.Brown WL, Jr, Wilson EO. Character displacement. Syst Zool. 1956;5:49–64. [Google Scholar]
- 2.Grant PR. Convergent and divergent character displacement. Biol J Linn Soc. 1972;4:39–68. [Google Scholar]
- 3.Grant PR, Grant BR. Evolution of character displacement in Darwin's finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. [DOI] [PubMed] [Google Scholar]
- 4.Schluter D, Price TD, Grant PR. Ecological character displacement in Darwin's finches. Science. 1985;227:1056–1059. doi: 10.1126/science.227.4690.1056. [DOI] [PubMed] [Google Scholar]
- 5.Schluter D. Ecological character displacement in adaptive radiation. Am Nat. 2000;156:S14–S16. [Google Scholar]
- 6.Dayan T, Simberloff D. Ecological and community-wide character displacement: The next generation. Ecol Lett. 2005;8:875–894. [Google Scholar]
- 7.Lack D. Darwin's Finches. Cambridge, UK: Cambridge Univ. Press; 1947. [Google Scholar]
- 8.Podos J. Correlated evolution of morphology and vocal structure in Darwin's finches. Nature. 2001;409:185–188. doi: 10.1038/35051570. [DOI] [PubMed] [Google Scholar]
- 9.Huber SK, Podos J. Beak morphology and song production covary in a population of Darwin's finches. Biol J Linn Soc. 2006;88:489–498. [Google Scholar]
- 10.Höbel G, Gerhardt HC. Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea) Evolution. 2003;57:894–904. doi: 10.1111/j.0014-3820.2003.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoskin CJ, Higgie M, McDonald KR, Moritz C. Reinforcement drives allopatric speciation. Nature. 2005;437:1353–1356. doi: 10.1038/nature04004. [DOI] [PubMed] [Google Scholar]
- 12.Jang Y, Gerhardt HC. Divergence in the calling songs between sympatric and allopatric populations of a wood cricket Gryllus fultoni (Orthoptera: Gryllidae) J Evol Biol. 2006;19:459–472. doi: 10.1111/j.1420-9101.2005.01014.x. [DOI] [PubMed] [Google Scholar]
- 13.Sætre GP, et al. A sexually selected character displacement reinforces premating isolation. Nature. 1997;387:589–592. [Google Scholar]
- 14.Miller EH. In: Acoustic Communication in Birds. Kroodsma DE, Miller EH, editors. New York: Academic; 1982. pp. 213–252. [Google Scholar]
- 15.Price T. Speciation in Birds. Greenwood Village, CO: Roberts and Company; 2008. [Google Scholar]
- 16.Farnsworth A, Lovette IJ. Phylogenetic and ecological effects on interspecific variation in structurally simple vocalizations. Biol J Linn Soc. 2008;94:155–173. [Google Scholar]
- 17.Slabbekoorn H, Smith TB. Bird song, ecology and speciation. Philos Trans R Soc London SerB. 2002;357:493–503. doi: 10.1098/rstb.2001.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catchpole CK, Slater PJB. Bird Song: Biological Themes and Variations. 2nd Ed. Cambridge, UK: Cambridge Univ. Press; 2008. [Google Scholar]
- 19.Saranathan V, Hamilton D, Powell GN, Kroodsma DE, Prum RO. Genetic evidence supports song learning in the three-wattled bellbird Procnias tricarunculata (Cotingidae) Mol Ecol. 2007;16:3689–3702. doi: 10.1111/j.1365-294X.2007.03415.x. [DOI] [PubMed] [Google Scholar]
- 20.Moyle RG. Baton Rouge, LA: Louisiana State Univ; 2002. Molecular systematics of barbets and trogons: Pantropical biogeography, African speciation, and issues in phylogenetic inference. PhD dissertation. [Google Scholar]
- 21.Short LL, Horne JFM. Toucans, Barbets and Honeyguides. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 22.Goldberg EE, Lande R. Ecological and reproductive character displacement on an environmental gradient. Evolution. 2006;60:1344–1357. [PubMed] [Google Scholar]
- 23.Slatkin M. Ecological character displacement. Ecology. 1980;61:163–177. [Google Scholar]
- 24.Schluter D. Frequency dependent natural selection during character displacement in sticklebacks. Evolution. 2003;57:1142–1150. doi: 10.1111/j.0014-3820.2003.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 25.Olofsson H, Servedio MR. Sympatry affects the evolution of genetic versus cultural determination of song. Behav Ecol. 2008;19:596–604. [Google Scholar]
- 26.Cnaan A, Laird NM, Slasor P. Tutorial in biostatistics: Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Gerhardt HC. Reproductive character displacement of female mate choice in the grey treefrog, Hyla chrysoscelis. Anim Behav. 1994;47:959–969. [Google Scholar]
- 28.Irwin DE, Price TD. Sexual imprinting, learning and speciation. Heredity. 1999;82:347–354. doi: 10.1038/sj.hdy.6885270. [DOI] [PubMed] [Google Scholar]
- 29.Russo D, et al. Divergent echolocation call frequencies in insular rhinolophids (Chiroptera): A case of character displacement? J Biogeogr. 2007;34:2129–2138. [Google Scholar]
- 30.Ryan MJ, Brenowitz EA. The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am Nat. 1985;126:87–100. [Google Scholar]
- 31.Slabbekoorn H, Smith TB. Habitat-dependent song divergence in the little greenbul: An analysis of environmental selection pressures on acoustic signals. Evolution. 2002;56:1849–1858. doi: 10.1111/j.0014-3820.2002.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 32.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith TB. Bill size polymorphism and intraspecific niche utilization in an African finch. Nature. 1987;329:717–719. [Google Scholar]
- 34.Haavie J, et al. Flycatcher song in allopatry and sympatry - convergence, divergence and reinforcement. J Evol Biol. 2004;17:227–237. doi: 10.1111/j.1420-9101.2003.00682.x. [DOI] [PubMed] [Google Scholar]
- 35.Secondi J, Bretagnolle V, Compagnon C, Faivre B. Species-specific song convergence in a moving hybrid zone between two passerines. Biol J Linn Soc. 2003;80:507–517. [Google Scholar]
- 36.McCarthy EM. Handbook of Avian Hybrids of the World. Oxford: Oxford Univ Press; 2006. [Google Scholar]
- 37.Smith TB, Bernatchez L. Evolutionary change in human-altered environments. Mol Ecol. 2008;17:1–8. doi: 10.1111/j.1365-294X.2007.03607.x. [DOI] [PubMed] [Google Scholar]
- 38.Borrow N, Demey R. Birds of Western Africa. London: Christopher Helm; 2001. [Google Scholar]
- 39.McGregor PK, et al. In: Playback and Studies of Animal Communication. McGregor PK, editor. New York: Plenum Press; 1992. pp. 1–9. [Google Scholar]
- 40.Fridolfsson AK, Ellegren HA. A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol. 1999;30:116–121. [Google Scholar]
- 41.Charif RA, Clark CW, Fristrup KM. Raven 1.3 User's Manual. Ithaca, NY: Cornell Laboratory of Ornithology; 2006. [Google Scholar]
- 42.Hansen MC, et al. Towards an operational MODIS continuous field of percent tree cover algorithm: Examples using AVHRR and MODIS data. Remote Sens Environ. 2002;83:303–319. [Google Scholar]
- 43.Rao JNK, Scott AJ. On Chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat. 1984;12:46–60. [Google Scholar]