Abstract
The antifungal and amoebicidal drug clioquinol (CQ) was withdrawn from the market when it was linked to an epidemic of subacute myelo-optico-neuropathy (SMON). Clioquinol exerts its anti-parasitic actions by acting as a Cu/Zn chelator and ionophore. Here we show that local injections of CQ produce mechanical hyperalgesia and cold hypersensitivity through a mechanism involving TRPA1 in mice. We also show that CQ activates TRPA1 in a Zn2+-dependent manner. Using a different Zn2+-ionophore, zinc pyrithione (ZnPy), we demonstrate that low, nanomolar concentrations of intracellular Zn2+ ([Zn2+]i) stimulate TRPA1. Direct application of Zn2+ to the intracellular face of excised, inside-out patches activates TRPA1 with an EC50 value of 7.5 ± 1 nM. TRPA1 is expressed in a subpopulation of nociceptive dorsal root ganglion (DRG) neurons, where it acts as a sensory receptor for environmental irritants and oxidants. Using cultured DRG neurons from wild-type and TRPA1-deficient mice, we demonstrate that TRPA1 is the principal excitatory receptor for increased [Zn2+]i in DRG neurons. In conclusion, we have discovered that TRPA1 acts a sensor of intracellular Zn2+, and that Zn2+ ionophores, such as CQ and ZnPy, activate TRPA1 by increasing [Zn2+]i. We also demonstrate that CQ-evoked mechanical hyperalgesia and cold allodynia require TRPA1 in vivo.
Keywords: pain, sensory neurons, TRP channels, zinc
The antifungal and amoebicidal drug clioquinol (CQ) was once widely used to treat gastrointestinal disorders, but was withdrawn from oral preparations when CQ was linked to an epidemic of subacute myelo-optico-neuropathy (SMON) in Japanese patients. Patients with SMON suffer from sensory and motor disorders and visual impairment. Thirty years after the ban of oral CQ, 40% of patients with SMON are unable to walk independently and approximately 7% suffer from visual impairment. The most common complaints, however, have been different forms of sensory impairments, such as tactile hypo- and hypersensitivity (almost 90% of patients) and dysesthesias (97%) (1). Notably, almost 50% of patients experience pain and 40% have cold sensitivity. CQ also has acute sensory effects, and studies on isolated nociceptive fibers in dogs demonstrated that CQ recruited normally quiescent fibers to become sensitive to hyperosmotic stimuli and cold (2), suggesting a direct effect on sensory nerves.
CQ exerts its anti-parasitic actions by acting as a moderate affinity Cu/Zn chelator and ionophore. The ionophore activity of CQ contributes to its neurotoxicity (3, 4), but also makes it a useful drug for the treatment of acrodermatitis enteropathica, a rare genetic disorder characterized by insufficient Zn2+ uptake (5, 6). More recently CQ has been shown to reduce Cu/Zn deposits and beta-amyloid accumulation in transgenic mouse models of Alzheimer's disease (7, 8), findings that have led to clinical trials of CQ in Alzheimer's patients (9, 10).
TRPA1 is expressed in a subpopulation of dorsal root ganglion (DRG) neurons, where it acts as a sensory receptor for environmental irritants and both oxidation and thiol-reactive compounds, some of which are produced endogenously during oxidative stress (11–15). Furthermore, TRPA1 can be activated by increasing the osmolarity of the extracellular solution (16). Transgenic mice lacking TRPA1 have a reduced sensitivity to cold stimuli and punctate mechanical stimulation (17), in addition to a reduced chemical sensitivity to irritants and oxidants (11, 12, 17, 18). The similarity between the modalities affected by CQ in nociceptive fibers (increased sensitivity to cold stimulation and hyperosmotic solutions) and the behavioral deficits of mice lacking TRPA1 (reduced sensitivity to painful cold and mechanical stimuli) led us to examine whether CQ can induce pain behavior acutely in mice through the activation of TRPA1.
Results
Pronociceptive Effects of Clioquinol.
Tactile and cold hypersensitivity are very common symptoms in patients with CQ-induced SMON (1). Since CQ directly sensitizes peripheral nociceptive C fibers to painful stimuli (2), and TRPA1 is important for behavioral responses to mechanical and cold stimulation (17), we examined whether acute local administration of CQ could sensitize animals to mechanical and cold stimulation. These experiments were performed in wild-type (trpa1+/+) and TRPA1-deficient mice (trpa1−/−) to investigate a potential role of TRPA1 in the acute behavioral effects of CQ.
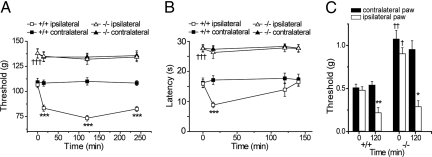
CQ (2.5 nmoles) was injected into the hind paws of trpa1+/+ and trpa1−/− mice and behavioral responses to cold (10 °C) and mechanical (paw pressure and von Frey hair) stimuli measured over a 4-h period (Fig. 1). Before administration of CQ, trpa1−/− mice had significantly higher mechanical thresholds to both paw pressure and von Frey hair stimulation and longer cold withdrawal latencies. These results confirm and extend the earlier findings of reduced responses to cold and von Frey hair stimulation in trpa1−/− mice (17). Following CQ injections, trpa1+/+ mice developed markedly reduced withdrawal thresholds in the injected limb in the paw pressure (Fig. 1A) and von Frey (Fig. 1C) tests and reduced withdrawal latencies in the cold plate assay (Fig. 1B). CQ did not affect the responses in the contralateral paw in any of the tests, indicating that CQ had a local effect in the injected paw. In trpa1−/− mice, CQ failed to affect either the paw pressure threshold or the cold withdrawal latency in the injected paw (Fig. 1 A and B). In contrast, CQ produced a large reduction in the von Frey withdrawal threshold in trpa1−/− mice (Fig. 1C), suggesting that although TRPA1 is important for the basal (predose) von Frey withdrawal threshold, CQ-evoked mechanical allodynia develops independently of TRPA1.
Fig. 1.
TRPA1 is required for the acute nociceptive effects of CQ. Mice (trpa1+/+ and trpa1−/−) received intraplantar injections of CQ (2.5 nmoles in 25 μl) and were examined for mechanical hyperalgesia (A, paw withdrawal threshold, paw pressure), cold hypersensitivity (B, paw withdrawal latency) and tactile allodynia (C, paw withdrawal threshold, von Frey). TRPA1-deficient mice had significantly higher thresholds and longer latencies than wildtype mice before administration of CQ (time = 0) in all three tests. CQ markedly reduced the withdrawal thresholds (A and C) and latency (B) in the injected paw of wild-type mice (+/+). In contrast, clioquinol only produced tactile allodynia (C) in TRPA1-deficient mice (-/-) and had no effect in the paw pressure and cold withdrawal tests. (n = 6; †, P < 0.05; ††, P < 0.01; †††, P < 0.001 compared to trpa1+/+ before administration of CQ; *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to the contralateral paw).
Clioquinol Is a TRPA1 Agonist.
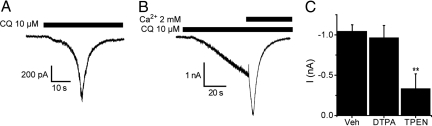
Our behavioral results from trpa1−/− mice demonstrated that TRPA1 is required for the nociceptive effects of CQ in vivo. Therefore, we examined whether CQ was able to activate TRPA1 directly. CQ (100 μM) failed to produce any significant membrane current (−3 ± 16 pA, n = 4) in untransfected CHO cells. In CHO cells expressing TRPA1, CQ (10 μM) readily evoked currents with the activation/inactivation current waveforms characteristic of TRPA1 (19). In Ca2+-containing solutions, the current increased to an apparent threshold amplitude and then rapidly increased before inactivating (Fig. 2A). CQ induced a non-inactivating current in the absence of external Ca2+, and subsequent addition of Ca2+ produced a sudden current surge followed by a rapid inactivation (Fig. 2B).
Fig. 2.
CQ is a TRPA1 agonist. CQ (10 μM) activates TRPA1 currents in CHO cells in Ca2+-containing (A) and Ca2+-free external solutions (B). (C) Pretreatment of the cells with a membrane permeable (TPEN), but not an impermeable (DTPA) Zn2+ chelator, significantly reduced the amplitudes of CQ-induced currents (**, P < 0.01).
CQ exerts its actions in other systems by acting as a moderate affinity Cu/Zn chelator and a Zn2+ ionophore (7, 8, 20). Therefore, we investigated whether CQ activates TRPA1 by increasing the concentration of intracellular zinc ([Zn2+]i). Inclusion of the membrane impermeable Zn2+ chelator DTPA (50 μM) in the external solution had no effect on CQ-induced currents, whereas the membrane permeable Zn2+ chelator TPEN (50 μM) significantly reduced the current amplitudes (Fig. 2C). The difference in the abilities of the two zinc chelators to inhibit CQ-evoked TRPA1 responses suggests that CQ can stimulate TRPA1 by mobilizing Zn2+ from intracellular compartments. The fact that CQ could evoke a current in some cells, even in the presence of TPEN (2 of 7 cells), raises the possibility that CQ can affect TRPA1 both through Zn2+-dependent and -independent mechanisms.
TRPA1 Is a Sensor of Intracellular Zn2+.
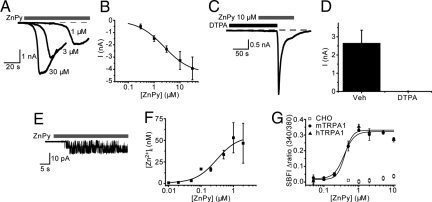
If CQ activates TRPA1 by increasing free [Zn2+]i, then TRPA1 should also be sensitive to other agents that raise the free [Zn2+]i, such as the commonly used Zn2+ ionophore zinc pyrithione (ZnPy). In whole-cell recordings, ZnPy acted as a time- and concentration-dependent TRPA1 agonist. Higher concentrations of ZnPy evoked currents that developed more rapidly and had larger amplitudes (Fig. 3 A and B). From these experiments, we calculated that ZnPy activated TRPA1 with an EC50 of 2.4 μM. No currents developed when ZnPy was applied in the presence of the zinc chelator DTPA. However, subsequent removal of DTPA with the continued presence of ZnPy evoked a large current, demonstrating that TRPA1 is sensitive to Zn2+ but not to pyrithione (Fig. 3 C and D).
Fig. 3.
Zn2+ is a potent TRPA1 activator. In the whole-cell configuration ZnPy stimulates TRPA1 with an EC50 of 2.4 μM (A and B; whole cell, −60 mV). DTPA, a membrane impermeable Zn2+ chelator completely prevents the ZnPy-induced activation of TRPA1 (C and D; n = 4–8). ZnPy (10 μM) rapidly evokes TRPA1 currents in cell attached patches (E). The patch pipette contained 5 mM BAPTA to remove any Zn2+ and Ca2+ from the extracellular side of the membrane. (F) FluoZin-3 measurements of [Zn2+]i in untransfected CHO cells exposed to different concentrations of ZnPy for 2 min. (G) Concentration dependence of ZnPy-evoked Na+ influx (measured with SBFI) in untransfected CHO cells and cells expressing mouse or human TRPA1.
In the cell-attached patch recording configuration the channels under study are protected from the extracellular solution by the tight seal between the electrode glass and the plasma membrane (in addition, the patch pipette contained 5 mM BAPTA to remove any Zn2+ and Ca2+ from the extracellular side of the membrane). In this configuration, addition of ZnPy (10 μM) to the cell rapidly induced TRPA1 single channel activity under the patch electrode (Fig. 3E) with a cord conductance of 83 ± 1 pS. This result strongly suggests that Zn2+ acts on TRPA1 from the intracellular side of the membrane.
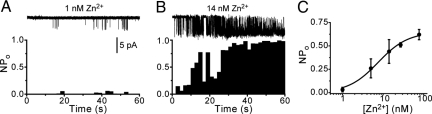
To obtain an estimate of the [Zn2+]i achieved with ZnPy, we treated untransfected CHO cells with different concentrations of ZnPy and measured the resulting [Zn2+]i using the zinc indicator dye FluoZin-3 (Fig. 3F). During a 2-min exposure to 1 μM ZnPy, the maximal [Zn2+]i reached was around 50 nM. This is inevitably an over-estimate of the intracellular concentrations achieved in our electrophysiological experiments, where the intracellular solutions contained 10 mM EGTA, which binds Zn2+ with a high affinity. Under this condition, we calculate that the equilibrium concentration of free [Zn2+]i is vanishingly low (in the tens of femtomolar range, calculated using WEBMAXC STANDARD, http://www.stanford.edu/∼cpatton/webmaxcS.htm). It is likely, however, that ZnPy produces higher local [Zn2+]i concentrations near the plasma membrane under our recording conditions. To obtain an estimate of the potency of Zn2+ without the complications of Zn2+ binding by intracellular EGTA, we took advantage of the fact that sodium ions are permeant through TRPA1 channels and used the Na+-sensitive dye SBFI to monitor TRPA1 activity. SBFI also has the advantage in that it is insensitive to changes in Zn2+ concentration unlike the available Ca2+-sensitive dyes, which are more sensitive to changes in the concentration of Zn2+ than Ca2+. Studies with SBFI-loaded cells showed that ZnPy evoked a Na+ influx through mouse and human TRPA1 channels with EC50 values of 460 ± 50 nM and 380 ± 30 nM, respectively (Fig. 3G). ZnPy failed to induce Na+-influx in untransfected cells. A comparison of the [Zn2+]i attained with a minimally effective concentration of ZnPy in the SBFI experiments (Fig. 3 F and G) indicates that TRPA1 is activated when the average [Zn2+]i reaches approximately 10–20 nM. Finally, to directly demonstrate that Zn2+ [not the ZnPy complex as has been shown for KCNQ channels (21)] activates TRPA1 by an intracellular, membrane-delimited mode of action and to obtain a direct measure of the potency of Zn2+, we examined the effect of Zn2+ applied directly to the intracellular face of excised patches from mTRPA1 CHO cells. In this configuration, we found that Zn2+ directly stimulates TRPA1-channel activity when the intracellular face of the membrane is exposed to concentrations of Zn2+ exceeding 1 nM (Fig. 4 A and B). We quantified the concentration dependence of the agonist activity of Zn2+ on inside-out patches by measuring the mean NPo during 60-s challenges with different Zn2+ concentrations to account for differences in both the rate of onset and the maximal NPo achieved. In this way, we found that Zn2+ activated TRPA1 in excised patches with an EC50 value of 7.5 ± 1 nM (Fig. 4C). We noted that the Zn2+-induced channel activation was characterized by a gradual increase in Po that often reached a plateau level where the channel has a Po close to 1, interrupted by frequent brief closures. The gradual increase in Po in the presence of a stable concentration of Zn suggests either that Zn2+ interacts with more than one site on the channel or that the interaction can produce a slow time-dependent conformational change in the channel.
Fig. 4.
TRPA1 is activated by intracellular Zn2+. Application of Zn2+ to the intracellular face of excised patches concentration-dependently stimulates TRPA1. (A) No or very little TRPA1 channel activity was seen in response to 1 nM Zn2+, whereas 14 nM Zn2+ (B) rapidly evoked a marked increase in channel activity (−60 mV). (C) Concentration dependence of the agonist activity of Zn2+ in inside-out patches (EC50 = 7.5 ± 1 nM). Each data point is the mean ± SEM. NPo during a 60-s local Zn2+-application of n = 4–6 patches. Only patches without significant channel activity before application of Zn2+ were analyzed.
TRPA1 Is Sensitive to Cu2+ and Cd2+ but Not to Fe2+.
Since the chelators and ionophores used in the present study (DTPA, TPEN, pyrithione, and CQ) bind Cu2+ and Fe2+ in addition to Zn2+, we examined whether TRPA1 is also sensitive to these ions. Additionally, we tested the toxic transition metal ion Cd2+. Using the SBFI assay, our experiments showed that TRPA1 is activated by Cu2+ (CuPy stimulated mTRPA1 and hTRPA1 with EC50 values of 0.6 ± 0.1 μM and 1.3 ± 0.1, respectively) and Cd2+ (CdPy stimulated a Na+-influx in mTRPA1 and hTRPA1 cells with EC50 values of 2.1 ± 0.1 μM and 1.4 ± 0.1 μM, respectively), whereas NaPy and FePy were inactive at concentrations up to 10 and 20 μM, respectively. The observation that Fe2+ does not stimulate TRPA1 confirms our earlier observation that Fe2+ can potentiate the agonist activity of H2O2 via the Fenton reaction, but does not stimulate the channel directly (11).
TRPA1 Is the Principal Sensor of Intracellular Zn2+ in Sensory Neurons.
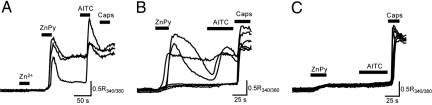
To assess whether elevated [Zn2+]i can stimulate TRPA1 in its native environment, we used cultured DRG neurons from trpa1+/+ and trpa1−/− mice. DRG neurons were exposed to Zn2+ [10 μM, a concentration that can occur during synaptic transmission (22)] and ZnPy (1 μM) for a relatively short period (20 s) to avoid occluding the Ca2+ signal measured with Fura-2 with an excessive Zn2+ entry. Subsequent exposures to AITC and capsaicin were used to identify TRPA1- and TRPV1-expressing neurons. We noticed a small uniform increase in the Fura-2 fluorescence ratio in all cells treated with ZnPy but not with Zn2+ (Fig. 5 A and B). This is consistent with a ZnPy-mediated increase in [Zn2+]i as Fura-2 binds Zn2+ with a KD of 3 nM and evokes changes in spectral properties similar to those seen with Ca2+ (23). A subpopulation of capsaicin-sensitive DRG neurons from trpa1+/+ mice (76 of 188 capsaicin-sensitive neurons) responded to stimulation with ZnPy with a rapid rise in [Ca2+]i, whereas treatment with Zn2+ did not evoke any [Ca2+]i response (Fig. 5 A and B). All of the ZnPy-sensitive neurons were sensitive to the TRPA1 agonist, AITC. In contrast, DRG neurons from trpa1−/− mice were unresponsive to stimulation with ZnPy with only 3 of 201 capsaicin-sensitive neurons responding with a small increase in [Ca2+]i (Fig. 5C). CQ had an almost identical effect on cultured DRG neurons compared to ZnPy. CQ (3 μM in the presence of 3 μM ZnSO4) selectively stimulated a Ca2+-influx in a subpopulation of capsaicin-sensitive DRG neurons from trpa1+/+ mice (91 of 210 capsaicin-sensitive neurons), whereas responses were almost absent in DRG neurons cultured from trpa1−/− mice (13 of 201 capsaicin-sensitive neurons). These results demonstrate that the agonist effects of ZnPy and CQ in DRG neurons are mediated by TRPA1.
Fig. 5.
TRPA1 is necessary for ZnPy evoked [Ca2+]i responses in DRG neurons. (A) ZnPy (1 μM) rapidly evokes a Ca2+-response in DRG neurons expressing TRPA1, whereas Zn2+ (10 μM) was unable to evoke responses. A brief pulse of ZnPy produced rapid elevations in [Ca2+]i in 40% (76 of 188) of capsaicin-sensitive DRG neurons cultured from TRPA1+/+ mice (B), but only evoked a small [Ca2+]i increase in 1% (3 of 201) of capsaicin-sensitive neurons from TRPA1−/− mice (C).
Discussion
In this study, we showed that TRPA1 is exquisitely sensitive to elevated [Zn2+]i and that Zn2+ ionophores, such as CQ and ZnPy, activate TRPA1 by increasing [Zn2+]i. Using cultured DRG neurons from TRPA1-deficient mice, we identified TRPA1 as the principal excitatory receptor for increased [Zn2+]i in DRG neurons. TRPA1 is also required for the mechanical hyperalgesia and cold hypersensitivity produced by local injections of CQ in vivo. TRPA1 is a sensory receptor for oxidation and reactive metabolites formed during oxidative conditions (11, 12, 24, 25). It is well-established that [Zn2+]i is increased as a consequence of ischemia and oxidative/nitrosative conditions, and that in such circumstances, Zn2+ exaggerates oxidative injuries and contributes to neuronal cell death (26–30). The Zn2+ sensitivity of TRPA1 described here underscores the channel's importance as a sensor of metabolic stress and insults. We have also demonstrated that TRPA1 is sensitive to Cd2+ and Cu2+ (but not Fe2+), putting TRPA1 forward as a potential mediator of heavy metal toxicity.
The intracellular concentration of Zn2+ in neurons is tightly regulated and normally below 1 nM [reviewed in (28)]. Our imaging experiments using Fura-2, which binds Zn2+ with a 100-fold higher affinity than Ca2+, revealed that ZnPy stimulates TRPA1-containing neurons as soon as there is a detectable but minimal Zn2+-mediated increase in the Fura-2 ratio in adjacent cells lacking TRPA1. This means that ZnPy activates TRPA1 in neurons at cellular concentrations of Zn2+ below the KD of Fura-2 for Zn2+ [3 nM; (23)]. Our results from experiments with FluoZin-3, used to measure ZnPy-mediated elevations of [Zn2+]i, and SBFI, used to measure ZnPy-induced Na+ influx through TRPA1, demonstrate that Zn2+ activates the channel in intact CHO cells at low nanomolar concentrations (≈10 nM). These estimates were confirmed by our results from experiments on excised, inside-out patches (free from metallothionein and other intracellular ligands for Zn2+), where [Zn2+]i readily activated TRPA1 with an EC50 of 7.5 nM.
A clinical analysis of over 1,000 patients with CQ-induced SMON found that among the most common symptoms of this syndrome were sensory disturbances, including hypersensitivities as well as loss of sensitivities and dysesthesias (1). The neurological basis for these chronic symptoms is thought to involve damage and alterations to various types of neurons in the sensory pathways including those located within the dorsal column of the spinal cord. The neurotoxic effects of CQ are not restricted to sensory neurons, and some of the most pronounced signs of neurotoxicity of CQ are observed in the optic nerve (1). Nevertheless, there is strong evidence that sensory fiber dysfunction is a significant feature of CQ intoxication. Repeated administration of CQ has been shown to lead to selective damage of small-diameter peripheral sensory nerve fibers, with few observable effects on larger diameter sensory or motor nerve fibers (31). Such damage is consistent with an action on TRPA1 expressed in small-diameter sensory nerve fibers.
CQ administration has acute as well as long-term sensory effects. Hypersensitivities to cold and osmotic stimuli were noted electrophysiologically in a preparation of polymodal sensory neurons after brief (up to 30 min) exposure to CQ (2). This agrees well with our findings that local intraplantar injection of CQ in mice produces hypersensitivities to noxious cold and mechanical stimuli. The concentrations of CQ that activate TRPA1 in vitro are in the range found in the plasma of CQ-treated patients in clinical trials (9), and it is therefore likely that activation of TRPA1 by CQ contributes to the acute sensory side effects associated with systemic administration of this drug.
Our studies on wild-type and TRPA1-deficient mice revealed that the increased cold and paw pressure sensitivities seen after CQ treatment are dependent on the presence of TRPA1. A TRPA1-mediated effect on cold responses is concordant with previous studies that revealed a role for TRPA1 in the increase in cold sensitivity observed after surgical injury to a peripheral nerve (17). Local administration of CQ also increased the sensitivity of mice to punctate mechanical stimuli delivered by calibrated von Frey hairs but, unlike the increases in cold and paw pressure sensitivities, this was seen in both wild-type and TRPA1-deficient mice. Different sensory fiber types are responsible for low-threshold and high-threshold mechanosensation, and the effect of locally administered CQ on low-threshold (von Frey) stimuli is likely to be due to an action on fibers that do not express TRPA1. A similar TRPA1-independent increase in sensitivity to von Frey hair mechanical thresholds was noted in a surgical model of neuropathic pain (17).
It is unclear how the expression of TRPA1 influences the transduction or transmission of cold or mechanical stimuli. The baseline cold, paw pressure, and von Frey hair thresholds are all elevated in TRPA1-deficient mice. TRPA1 has been proposed as a sensory transduction molecule for both cold and mechanical stimuli (17, 32–40), but a significant number of investigations have failed to demonstrate that TRPA1 is the primary transducer for these stimuli (34, 41, 42). Further studies are required to determine why the lack of TRPA1 expression reduces the sensitivity to cold and mechanical (paw pressure, Von Frey) stimuli in untreated mice, while the hypersensitivities seen after CQ treatment or neuropathy are differentially dependent on TRPA1.
CQ and other CQ-like compounds that are Zn2+/Cu2+ chelators have been proposed as therapies for the treatment of Alzheimer's disease. Although chelation will reduce the concentration of metals in some areas, these compounds can transport Zn2+/Cu2+ to other regions where the divalent cations can be released to exert biological effects. Drugs that act like CQ to increase [Zn2+]i may elicit sensory side effects via activation of TRPA1. As TRPA1 is expressed in sensory neurons innervating the viscera, the effects of Zn2+ activation may not be perceived as pain but as discomfort. Other sensations and reflexes associated with activation of these sensory nerves may also be elicited. CQ administration is known to induce gastrointestinal symptoms, such as severe abdominal pain, abdominal distension, and constipation in both humans and dogs (43). The sensory and other neurological activities of CQ-like drugs may impose limitations on their therapeutic utility.
The identification of Zn2+ as a potent intracellular TRPA1 activator makes it likely that cellular stressors that increase [Zn2+]i (44, 45) will activate TRPA1 and affect the excitability of neurons. Our results also raise the possibility that TRPA1 can be activated indirectly in response to Zn2+ entry through other neuronal ion channels, such as L-type Ca2+ and AMPA/kainate channels (23, 46), which are expressed by DRG neurons (47–49). This would be consistent with the conclusion of a recent report, which appeared during the revision of this manuscript, that Zn2+ passing through TRPA1 can stimulate the channel (50).
Materials and Methods
Behavioral Experiments.
All animal studies were carried out according to U.K. Home Office Animal Procedures (1986) Act. Data shown are from male and female homozygote trpa1−/− and trpa1+/+ littermates. CQ (2.5 nmol in 25 μL saline, 1% DMSO), was injected s.c. into the plantar surface of one of the hind paws using a 50-μL luer syringe (Hamilton Co.) fitted with a 26-gauge × 3/8-inch intradermal needle.
Mechanical hyperalgesia was measured following clioquinol injections using an Analgesymeter (Ugo-Basile). Briefly, the mice were kept in their holding cages to acclimatize (10–15 min) to the experimental room. The experimenter then lightly restrained the mouse and applied a constant increasing pressure stimulus to the dorsal surface of the hind paw using a blunt conical probe. The nociceptive threshold was defined as the force in grams at which the mouse withdrew its paw. To avoid tissue injury, a force cut-off value was set to 150 g.
Tactile allodynia was assessed by measuring withdrawal thresholds to calibrated von Frey hairs (0.008–2 g). Animals were placed into a Perspex chamber with a metal grid floor, giving access to the underside of their paws and allowed to acclimatize before the start of the experiment. Von Frey hairs were applied perpendicular to the mid plantar surface of the hind paw with sufficient force to cause slight bending against the paw and held for a few seconds. This was repeated 5 times at intervals of 1–2 s. A positive response was noted if the paw was sharply withdrawn or there was flinching upon removal of the hair. If no response was noted a higher force hair was tested and the filament producing a positive response recorded as the threshold.
Cold sensitivity was examined by lightly restraining the animal and placing one of the hind paws onto a cold plate (10 °C). The withdrawal latency was measured in both the ipsilateral and contralateral paws, before and 2 h after CQ injections (using 30 s as a cut-off).
Cell Culture.
Untransfected CHO cells and CHO cells expressing mouse TRPA1 [tetracycline inducible expression (39)] were grown in MEM-α medium supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), L-glutamine (2 mM), and FCS (10%). DRG neurons were prepared from adult male or female mice using methods described previously (51).
Intracellular [Ca2+] Measurements.
DRG neurons were loaded with 2 μM Fura-2 AM (Molecular Probes) in the presence of 1 mM probenecid for approximately 1 h. The dye loading and subsequent experiments were performed in a physiological saline solution containing (in mM) 140 NaCl, 5 KCl, 10 glucose, 10 Hepes, 2 CaCl2, and 1 MgCl2, buffered to pH 7.4 (NaOH). Compounds were applied to cells by local continuous microperfusion of solution through a fine tube placed very close to the cells being studied. Experiments were conducted at 30 °C. Images of a group of cells were captured every 2 sec using 340 and 380 nm excitation wavelengths with emission measured at 520 nm with a microscope-based imaging system (PTI). Analyses of emission-intensity ratios at 340 nm/380 nm excitation (R, in individual cells) were performed using the ImageMaster suite of software.
Measurements of Intracellular Zn2+ and Na+ Levels.
Intracellular Zn2+ concentrations [Zn2+]i were measured in untransfected CHO cells grown in 96-well black walled plates (Costar) using a Flexstation 3 (Molecular Devices). Cells were loaded with 5 μM FluoZin-3 AM (Molecular Probes) for 30 min. The dye loading experiments were performed in the physiological salt solution mentioned above. Fluorescence emission was measured at 515 nm after excitation at 496 nm. The increased emission following zinc pyrithione was used to determine the [Zn2+]i using the Grynkiewicz equation (52). A maximal signal was obtained using 20 μM ZnPy and the minimal signal using 50 μM TPEN for each well. Intracellular Na+ concentrations were measured in CHO cells (untransfected or expressing mouse or human TRPA1) using a Flexstation 3 as detailed above. Cells were loaded with 5 μM SBFI AM for 1 h and fluorescence emission ratios (505 nm) were collected after excitation at 340/380 nm.
Electrophysiology.
CHO cells (untransfected or expressing TRPA1) were studied under voltage-clamp conditions using an Axopatch 200B amplifier and pClamp 10.0 software (Molecular Devices). Whole-cell recordings from CHO cells were performed at a holding potential of −60 mV. Borosilicate glass pipettes (2–5 MΩ, 75–80% series resistance compensation) were filled with (in mM) 140 KCl, 1 CaCl2, 2 MgATP, 10 EGTA, and 10 Hepes buffered to pH 7.4 (KOH). Drugs were applied by local microperfusion with a rapid solution changer (RSC-200, Biologic) in the physiological saline mentioned above for [Ca2+]i measurements. When the effects of TPEN or DTPA were studied, the cells were superfused with the chelators for 90 s before the application of ZnPy or CQ. Cell-attached patches were recorded using a nominally Ca2+-free saline solution (containing in mM; 140 NaCl, 5 KCl, 1 MgCl2, 10 glucose, and 10 Hepes, pH 7.4) extracellularly, the recording pipette in addition contained 5 mM BAPTA to remove any Ca2+ and Zn2+ from the extracellular face of the patch. Inside-out patches were recorded with a Ca2+-free saline solution in the recording pipette (in mM) 140 NaCl, 5KCl, 1 MgCl2, 10 Hepes, and 1 EGTA, and the cytoplasmic side was superfused with a solution consisting of (in mM) 140 KCl, 2 MgATP [to maintain channel activity in excised patches (53)], 5 citric acid, 10 Hepes, 10 μM CaCl2, 1 ZnSO4, and 1–2.25 EGTA depending on the desired free Zn2+ (calculated using WEBMAXC Standard, http://www.stanford.edu/∼cpatton/webmaxcS.htm).
Acknowledgments.
The trpa1−/− mice were kindly provided by Drs. Kelwin Kwan and David Corey (Harvard Medical School, Boston). This work was supported by the Medical Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Konagaya M, et al. Clinical analysis of longstanding subacute myelo-optico-neuropathy: Sequelae of clioquinol at 32 years after its ban. J Neurol Sci. 2004;218:85–90. doi: 10.1016/j.jns.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Kumazawa T, Mizumura K. Abnormal activity of polymodal receptors induced by clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) Brain Res. 1984;310:185–188. doi: 10.1016/0006-8993(84)90026-x. [DOI] [PubMed] [Google Scholar]
- 3.Arbiser JL, et al. Clioquinol-zinc chelate: A candidate causative agent of subacute myelo-optic neuropathy. Mol Med. 1998;4:665–670. [PMC free article] [PubMed] [Google Scholar]
- 4.venisti-Zarom L, Chen J, Regan RF. The oxidative neurotoxicity of clioquinol. Neuropharmacology. 2005;49:687–694. doi: 10.1016/j.neuropharm.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Dillaha CJ, Lorincz AL, Avik OR. Acrodermatitis enteropathica; review of the literature and report of a case successfully treated with diodoquin. J Am Med Assoc. 1953;152:509–512. doi: 10.1001/jama.1953.03690060025009. [DOI] [PubMed] [Google Scholar]
- 6.Kury S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 7.Adlard PA, et al. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Cherny RA, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie CW, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 10.Sampson E, Jenagaratnam L, McShane R. Metal protein attenuating compounds for the treatment of Alzheimer's disease Cochrane. Database Syst Rev. 2008 doi: 10.1002/14651858.CD005380.pub3. CD005380. [DOI] [PubMed] [Google Scholar]
- 11.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bessac BF, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 15.Streng T, et al. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XF, Chen J, Faltynek CR, Moreland RB, Neelands TR. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci. 2008;27:605–611. doi: 10.1111/j.1460-9568.2008.06030.x. [DOI] [PubMed] [Google Scholar]
- 17.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colvin RA, et al. Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am J Physiol Cell Physiol. 2008;294:C726–C742. doi: 10.1152/ajpcell.00541.2007. [DOI] [PubMed] [Google Scholar]
- 21.Xiong Q, Sun H, Li M. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat Chem Biol. 2007;3:287–296. doi: 10.1038/nchembio874. [DOI] [PubMed] [Google Scholar]
- 22.Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158:126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 23.Atar D, Backx PH, Appel MM, Gao WD, Marban E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J Biol Chem. 1995;270:2473–2477. doi: 10.1074/jbc.270.6.2473. [DOI] [PubMed] [Google Scholar]
- 24.Macpherson LJ, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trevisani M, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley SN, Power JM, Coulson EJ, Bartlett PF. Zinc-mediated neuronal death is dependent on Trk activation. Exp Neurol. 2007;205:360–366. doi: 10.1016/j.expneurol.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Aizenman E, et al. Induction of neuronal apoptosis by thiol oxidation: Putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 28.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 29.Frederickson CJ, Hernandez MD, McGinty JF. Translocation of zinc may contribute to seizure-induced death of neurons. Brain Res. 1989;480:317–321. doi: 10.1016/0006-8993(89)90199-6. [DOI] [PubMed] [Google Scholar]
- 30.Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J Neurosci. 2009;29:1105–1114. doi: 10.1523/JNEUROSCI.4604-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozawa K, Saida K, Saida T. Experimental clioquinol intoxication in rats: Abnormalities in optic nerves and small nerve cells of dorsal root ganglia. Acta Neuropathol. 1986;69:272–277. doi: 10.1007/BF00688304. [DOI] [PubMed] [Google Scholar]
- 32.Karashima Y, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsura H, et al. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–123. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 34.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol. Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima T, et al. Noxious cold stimulation induces mitogen-activated protein kinase activation in transient receptor potential (TRP) channels TRPA1- and TRPM8-containing small sensory neurons. Neuroscience. 2006;140:1337–1348. doi: 10.1016/j.neuroscience.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Obata K, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrus M, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 40.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 41.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 42.Munns C, AlQatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Tateishi J. Subacute myelo-optico-neuropathy: Clioquinol intoxication in humans and animals. Neuropathology. 2000;20:S20–S24. doi: 10.1046/j.1440-1789.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- 44.Hwang JJ, Lee SJ, Kim TY, Cho JH, Koh JY. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J Neurosci. 2008;28:3114–3122. doi: 10.1523/JNEUROSCI.0199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiseman DA, et al. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol. 2006;291:C555–C568. doi: 10.1152/ajpcell.00509.2005. [DOI] [PubMed] [Google Scholar]
- 46.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci USA. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huettner JE. Glutamate receptor channels in rat DRG neurons: Activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- 48.Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. Presynaptic kainate receptors regulate spinal sensory transmission. J Neurosci. 2001;21:59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee CJ, Labrakakis C, Joseph DJ, Macdermott AB. Functional similarities and differences of AMPA and kainate receptors expressed by cultured rat sensory neurons. Neuroscience. 2004;129:35–48. doi: 10.1016/j.neuroscience.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 50.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol. 2009;5:183–190. doi: 10.1038/nchembio.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 53.Karashima Y, et al. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch. 2008;457:77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]