Abstract
Several nonclassical major histocompatibilty antigens (class Ib molecules) have emerged as key players in the early immune response to pathogens or stress. Class Ib molecules activate subsets of T cells that mount effector responses before the adaptive immune system, and thus are called innate T cells. MR1 is a novel class Ib molecule with properties highly suggestive of its regulation of mucosal immunity. The Mr1 gene is evolutionarily conserved, is non-Mhc linked, and controls the development of mucosal-associated invariant T (MAIT) cells. MAIT cells preferentially reside in the gut, and their development is dependent on commensal microbiota. Although these properties suggest that MAIT cells function as innate T cells in the mucosa, this has been difficult to test, due to the (i) paucity of MAIT cells that display MR1-specific activation in vitro and (ii) lack of knowledge of whether or not MR1 presents antigen. Here we show that both mouse and human MAIT cells display a high level of cross-reactivity on mammalian MR1 orthologs, but with differences consistent with limited ligand discrimination. Furthermore, acid eluates from recombinant or cellular MR1 proteins enhance MAIT cell activation in an MR1-specific and cross-species manner. Our findings demonstrate that the presentation pathway of MR1 to MAIT cells is highly evolutionarily conserved.
Recent studies of plants, insects, and mammals have shown that the innate immune system displays a remarkable ability to discriminate self from nonself (1) and discriminate dangerous from harmless (2). At the molecular level, much of this discrimination is accomplished by innate immune cells expressing surprisingly extensive families of receptors capable of pattern recognition. These pattern recognition receptors (PRRs) bind the evolutionarily conserved ligands expressed by pathogens, commensal microbiota, or stress-induced cells (3). Innate cells with PRR expression also must be capable of mounting rapid effector responses without dependency on clonal expansion. This strategy of early pathogen detection contrasts with that of the acquired immune system using conventional T cells restricted by classical MHC molecules. Classical MHC molecules on infected cells are bound by a heterogeneous pool of self and nonself peptides that conventional T cells discriminate as a result of thymic education during ontogeny. This peptide-based discrimination is dependent on clonal expansion of pathogen-specific T cells. Alternatively, certain class Ib molecule–restricted T cells, such as CD1d-restricted iNKT cells (4) and H2-M3– restricted CD8+ T cells (5), appear to be less ligand-discriminating than classical T cells, making them less dependent on clonal expansion. These more pattern-like recognition systems allow class Ib–restricted T cells to mount quick and appropriate immune responses to pathogens or stress.
It has been speculated that mucosal-associated invariant T (MAIT) cells, which are restricted by the novel class Ib molecule MR1, function as innate T cells similar to CD1d-restricted iNKT cells (6, 7). Indeed, MAIT cells and iNKT cells have some striking similarities, including the fact that both populations have a memory or activated phenotype, at least in humans (6–8). Like iNKT cells, MAIT cells also express an invariant mVα19 TCRα in mouse and the homologous hVα7.2 in human, and use very limited Vβ chains in both species (9). Also like iNKT cells, MAIT cells expand poorly in vitro on TCR ligation and rapidly release IFN-γ and IL-10 (refs. 6, 10; O.L. et al., unpublished work). However, unlike iNKT cells, the peripheral expansion/activation of MAIT cells is dependent on B cells and gut commensal flora (8, 11). Furthermore, MAIT cells preferentially reside in the gut lamina propria, whereas iNKT cells preferentially reside in the peripheral lymphoid organs. Based on these similarities and differences, an attractive model is that MAIT cells and iNKT cells have similar regulatory functions but service different immune compartments and/or recognize a different class of ligands. Definitive support for this model requires better characterization of the nature of the MR1 ligand, if indeed it binds one.
MR1 is encoded by a single gene that is not linked to Mhc in mouse and human but is linked to Cd1d in human. The Mr1 gene is the most highly conserved Mhc gene between human and mouse, particularly in coding sequences for the α1 and α2 domains that form the ligand-binding platform for classical MHC class I molecules. The Mr1 message and encoded protein have been found to be expressed ubiquitously in all cell types and tissues tested thus far (12); however, surface expression of endogenous MR1 has not been detected despite considerable effort. In contrast, surface MR1 is detected on cells overexpressing transduced/transfected MR1, but mouse MAIT cell hybridomas are only rarely activated by these cells. Thus, whether or not ligand availability is the limiting factor controlling MR1 expression and whether or not MAIT cell activation requires a diverse set of ligands remain open questions. We report here that clonal mouse and polyclonal human MAIT cells are highly cross-reactive on mammalian MR1 orthologs, but with interesting differences. We also present evidence showing that an acid eluate of MR1 enhances MAIT cell activation. These findings imply that MAIT cells likely recognize discrete ligands and that the MR1 ligand presentation to MAIT cells is highly conserved in mammals.
Results
Sequence Comparisons of Mr1 Gene Orthologs Indicate Evolution Under Purifying Selection.
Insight into the selective pressure reflective of a gene's evolution can be provided by comparing genetic sequences and functional interactions of orthologs from disparate species. The Mr1 gene sequence is known for human, rat, mouse, and nonhuman primates chimpanzee, orangutan, and monkey (13). To better compare the sequence homology and functional conservation of MR1 molecules, we cloned an Mr1 cDNA from bovine, an even-toed ungulate (Rumania), which has relatively high MAIT cell expression (9). The cDNA was reverse-transcribed from bovine (Bos taurus) total RNA, cloned using the TA cloning vector, and expressed on the cell surface for MAIT cell activation [supporting information (SI) Fig. S1]. Interestingly, the bovine MR1 sequence was quite disparate, with the lowest sequence identity to the other known MR1 sequences (Fig. S2), and thus made a significant addition to our study. Comparisons of the 7 MR1 orthologs showed that synonymous substitution predominated, indicating purifying selection. However, class Ia alleles showed the expected excess of nonsynonymous mutations, indicating that polymorphism is a selective advantage, particularly in the α1/α2 domains (Fig. S3). Purifying selection also has been implicated among murid species in the evolution of H2-M3 orthologs that bind peptide ligands containing a conserved N-formylated methionine as presented to CD8+ T cells, as well as among primate HLA-E orthologs that present conserved class Ia signal peptides to NK cells (5, 14, 15). Thus, there are clear precedents implicating purifying selection resulting in the presentation of molecular patterns. MR1 conservation also is likely mandated by its need to interact with the invariant Vα and limited Vβ chains of the MAIT cell TCR repertoire. If the purifying selection determines the interaction of MR1 with TCR, then MR1 orthologs could potentially activate species-disparate MAIT cells.
Mouse MAIT Cell Hybridoma 6C2 Displays Extensive Cross-Species Activation by MR1 Orthologs.
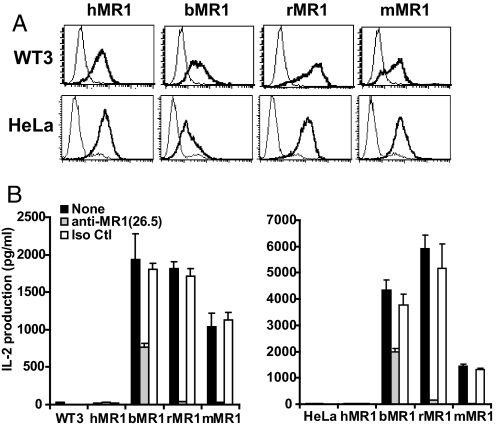
To test for physiological conservation of MR1 functions, we next determined whether cells expressing various MR1 orthologous proteins could activate MAIT cells. Toward this end, we transduced bovine, rat, human, and mouse MR1 molecules into human HeLa cells and mouse WT3 cells (Fig. 1). The surface expression of these species-disparate MR1 proteins was monitored by MR1−/− mouse anti-human MR1 mAb 26.5 (16). Based on the known cross-reactivity of mAb 26.5 with mouse MR1 as well as epitope mapping (16), we believed that mAb 26.5 also would likely detect bovine and rat MR1, but not necessarily with the same avidity. Cell lines expressing significant levels of surface mouse, rat, or human MR1 were established (Fig. 1A). Interestingly, surface expression of bovine MR1 required concomitant expression of bovine β2m. This finding is consistent with bovine MR1 being a more sequence-disparate molecule than human or mouse MR1 (Fig. S2), as well as the fact that genes encoding class Ia and β2m are known to have coevolved in different species. In any case, mouse and human cell lines expressing similar levels of the various MR1 orthologous proteins were obtained, allowing us to test for functional conservation of MAIT cell activation. Importantly, cells expressing either bovine or rat MR1 stimulated a strong response of the MAIT cell hybridoma (Fig. 1B); indeed, the level of activation by bovine and rat MR1 was higher than that of mouse MR1 on both WT3 and HeLa cells. The greater activity of bovine and rat MR1 compared with mouse MR1 might be explained by an underestimation of the surface MR1 on these cells due to their weaker binding by mAb 26.5. Indeed, this seems likely with bovine MR1, because its activation of the MAIT cell hybridoma was only partially blocked. Alternatively, rat and bovine MR1 could more favorably bind a putative ligand compared with mouse MR1. In any case, this cross-species activation of MAIT cells was clearly MR1-dependent, because the nontransduced cell lines failed to activate MAIT cells, and because mAb to MR1 blocked most, if not all, of the activation of the MAIT cell hybridoma (Fig. 1B).
Fig. 1.
Activation of mouse MAIT cells by bovine, rat, and mouse MR1 proteins. (A) Human, bovine, rat, and mouse MR1 were expressed on both mouse (WT3) and human (HeLa) cell lines using a retroviral transduction system. The surface expression was evaluated by FACS using anti-MR1 antibody 26.5 (coarse lines) compared with negative controls (thin lines) using the secondary antibody only (WT3) or the nontransduced HeLa cells. Similar results were seen with human 293T and mouse CH27 cells. (B) The mouse MAIT cell hybridoma 6C2 could be activated by bovine, rat, or mouse MR1 proteins in a manner blocked or inhibited by anti-mouse MR1 antibody 26.5.
Identification of a Single Human MR1 Residue Preventing Activation of Mouse MAIT Cells.
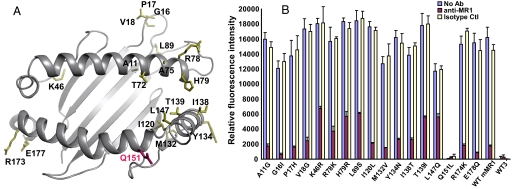
In contrast to rat and bovine MR1, human MR1 failed to activate the mouse MAIT cell hybridoma (Fig. 1B). To determine whether this failure resulted from impaired interaction with the MAIT cell TCR or a putative MR1 ligand, a mutagenesis approach was taken. Such an approach is validated by the high probability that MR1 adopts a canonical MHC fold topology, as has been suggested by analysis of the MR1 α1/α2 domain sequences (17, 18). An MR1 threading model constructed using the templates of class Ia molecules with known 3-dimensional structures has 19 variable residues in the α1 and α2 domains between human and mouse MR1 (Fig. 2A). To investigate which residues of human MR1 contribute to its failure to activate mouse MAIT cells, we singly mutated each of the 19 residues in mouse MR1 to its human MR1 counterpart. Two of these 19 disparate residues (T72M and A75V) were tested previously and found to not ablate activation of the MAIT cell hybridoma (16). Thus, each of the remaining 17 mutant MR1 constructs was expressed on mouse WT3 fibroblasts at levels comparable to wild-type hMR1 or mMR1 molecules. Surprisingly, only 1 out of these 17 mutants (Q151L) completely abrogated activation of the mouse MAIT cell hybridoma (Fig. 2B), suggesting functional conservation in all of the other residues. Furthermore, human is the only species tested that does not share this residue with MR1 proteins from other species.
Fig. 2.
The inability of human MR1 to activate mouse MAIT cell activation is mapped to the residue L151. (A) A mouse MR1 threading model was generated with the template of H-2Kb (1VAC) using the PHYRE Web server (32). The variable residues in the murine MR1 α1/α2 domains that were mutated to their human MR1 counterparts are shown with colored side chains using the PyMOL program. (B) The mMR1 mutants expressed on WT3 cells were tested for their ability to activate mouse MAIT cell hybridoma. Of the 17 mutants tested, only the mutation of Q151L [shown in magenta in (A)] resulted in decreased mouse MAIT cell activation.
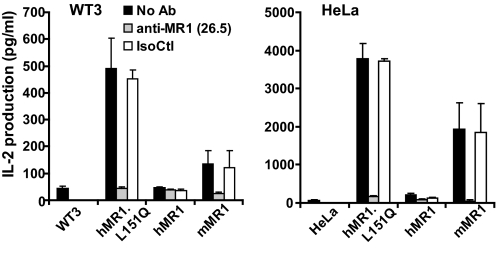
To confirm the critical importance of MR1 residue Q151 for mouse MAIT cell activation, we generated the reciprocal mutation (L151Q) by replacing the residue in hMR1 with its counterpart from mMR1. Again, this reciprocal mutation was similarly expressed on the cell surface. Strikingly, mouse or human cells expressing the hMR1.L151Q mutant strongly stimulated the MAIT cell hybridoma (Fig. 3). This indicates that residue Q151 is not only required for MR1 activation of mouse MAIT cells, but is the sole reason that human MR1 fails to activate mouse MAIT cells. Interestingly, if MR1 has a canonical MHC fold, as predicted, then residue 151 would be expected to interact only with the TCR and not with a putative ligand. Of note, class I residue 151 and the homologous position in class II are frequent TCR contacts (19).
Fig. 3.
Human-to-mouse reversal mutation at residues L151 restores the ability of hMR1 to activate mouse MAIT cells. The mutation of Leu-151 in hMR1 to Gln of mMR1 rescued the activation of mouse MAIT cells by hMR1 and was blocked by anti-MR1 antibody 26.5. Irrelevant isotype-matched antibody 34–2-12 was used as an isotype control (IsoCtl).
Additional Mouse MAIT Cell Clones and Polyclonal Human MAIT Cells Also Display Significant Reactivity on MR1 Orthologs.
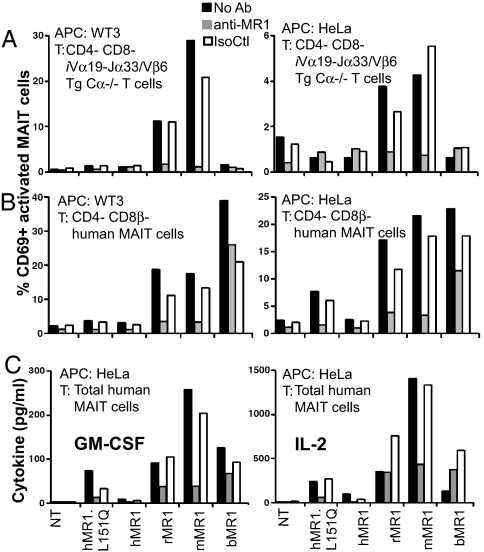
To examine whether cross-species reactivity is a general feature of MAIT cells, we tested an additional MAIT cell hybridoma and T cells from an iVα19 TCRα/Vβ6 TCRβ double-transgenic (Tg) mouse (8). The 8D12 mouse hybridoma displayed a pattern of reactivity on the species orthologs similar to that of the 6C2 hybridoma (data not shown). Both 6C2 and 8D12 are Vβ8, whereas the TCRβ used in the Tg mouse was obtained from a Vβ6 mouse hybridoma (18G7) reactive to mouse MR1 expressed by transfected cells (data not shown). These quasi-monoclonal T cells from Vα19/Vβ6Tg Cα−/− mice had more limited reactivity toward the MR1 homologs, such that rat and mouse MR1 stimulated them, but human and bovine MR1 did not (Fig. 4A). Interestingly, T cells from iVα19 single Tg Cα−/− mice displayed very weak reactivity toward rat MR1 and no reactivity against any other MR1 homolog (data not shown). It is noteworthy that <10% of the 40 iVα19 hybridomas that we have made exhibited reactivity against cells overexpressing mouse MR1. These results suggest that negative selection may effectively delete the autoreactive MR1 repertoire and eliminate MAIT cells reactive with self-MR1/ligand(s).
Fig. 4.
Primary quasi-monoclonal murine and polyclonal human MAIT cell are differentially activated on coculture with xenogeneic MR1 transfectants. TCRβ+/Vβ6+/CD4−/CD8α− (double-negative) transgenic iVα19-Jα33/Vβ6/Cα−/− mouse (A), TCRγδ−/3C10+/CD3+/CD4−/CD8β−/CD161+ (double-negative and CD8αα) (B), and unseparated human MAIT T cells (C) were cultured with the indicated transduced cell lines for 16 h (A and B) or 48 h (C). Both mouse and human MAIT cells were activated when cocultured on mouse and rat MR1 overexpressed on WT3 and HeLa. Human MAIT cells also were activated by hMR1.L151Q and bovine MR1. Interactions were specifically blocked by the anti-MR1 26.5 antibody. NT, nontransduced cells; IsoCtl, isotype control antibody.
To study the diversity of the MAIT cell repertoire, we tested polyclonal human MAIT cell reactivity toward the different species of MR1. Primary human MAIT cells, but not conventional T cells, were strongly activated, with enhanced surface CD69 expression, by the different species of MR1 proteins but not by human MR1 (Fig. 4B). Importantly, primary human MAIT cells were able to secrete IL-2, GM-CSF (Fig. 4C), IFN-γ, and TNF-α (data not shown) after brief stimulation with cross-species MR1 molecules. But only limited rescue of hMR1 reactivity was observed with the L151Q mutation, raising the possibility that human MAIT cells are self-tolerant. Consistent with this hypothesis, a very small number of cells were activated by native human MR1 in most, but not all, experiments (data not shown), suggesting that a very small repertoire reacts to the human MR1/ligand(s) presented on the transfected cells. Confirming these results, a small but reproducible MR1-dependent (blocked by the anti-MR1 26.5 mAb) IL-2 and GM-CSF secretion was observed after stimulation by HeLa cells expressing the native human MR1 (Fig. 4C). This weak reactivity toward native human MR1 suggests that most of the self-MR1 reactive repertoire was deleted. Alternatively, human MR1 uses Leu-151 instead of the glutamine used by the other 3 species to enhance the threshold of self MAIT cell activation. Thus, the very high frequency of human MAIT cells reacting toward xenogeneic MR1 could be related either to a monomorphic ligand presented by MR1 or to the recognition of a common epitope generated in part by MR1 residue Leu-151 in human or Gln-151 in other species. Indeed, mutation of the residue 151 (L151Q) rescued the ability of hMR1 to partially activate polyclonal self MAIT cells (Fig. 4 B and C) and activate xenogeneic MAIT cell hybridomas 6C2 (Fig. 3) and 8D12 (data not shown). The much lower reactivity toward MR1 orthologs of polyclonal mouse MAIT cells as studied in iVα19 single Tg Cα−/− mice (data not shown) could be related to stronger structural constraints imposed by the monomorphic mouse iVα19 in comparison with human Vα7.2, in which the 2 central codons of the CDR3α loop are polymorphic (8, 20). Together, these findings demonstrate for the first time that primary human and mouse MAIT cells can be activated ex vivo by MR1-expressing cells. Moreover, if a conserved ligand is involved in MAIT cell activation, then MAIT cells are capable of at least limited ligand discrimination. In the case of mouse MAIT cells, this ligand discrimination was demonstrated by the disparate cross-reactivities of Vβ8 versus Vβ6 hybridomas, and in the case of polyclonal human MAIT cells, ligand discrimination could explain in part why they appear to be mostly tolerant to human MR1, but highly cross-reactive with the MR1 orthologs.
Acid Eluates of MR1 Ligand Enhance MAIT Cell Activation in an MR1-Dependent Manner.
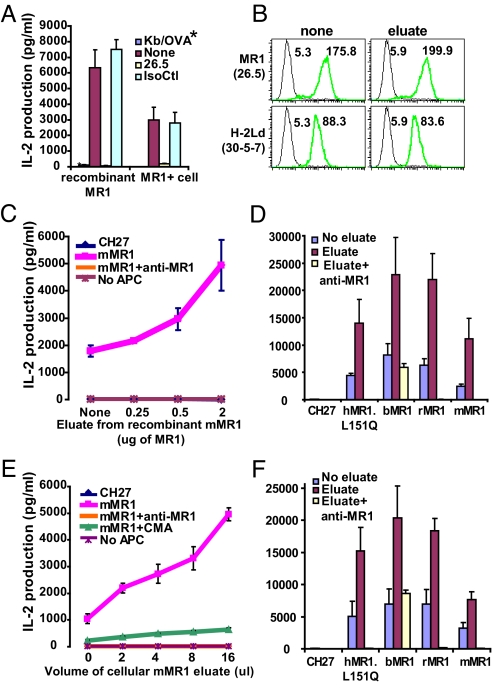
Although circumstantial evidence supports the existence of an endogenous MR1 ligand, to date there is no direct evidence of this. The circumstantial evidence for ligand binding is that (i) MR1 undergoes a conformational change from open to folded conformers analogous to that of classical MHC molecules after binding ligand, (ii) only antibodies to folded MR1 block MAIT cell activation, (iii) extensive mutagenesis of MR1 predicts that it interacts with ligand in a manner similar to classical MHC molecules, and (iv) all αβ T cells described thus far detect MHC or MHC-like molecules bound by ligand (16, 21). To obtain direct evidence for the MR1-binding ligand, we used established approaches for ligand extraction from classical MHC molecules (22). For these studies, recombinant MR1 was produced by insect cells and purified by affinity and size-exclusion chromatography. MR1-dependent MAIT cell activation with plate-bound recombinant MR1 protein (Fig. 5A) and Western blot analysis with anti-MR1 antibody 4E3 were used to confirm its specificity and functionality. Our results also show that MAIT cell (6C2) activation by MR1-overexpressing B cells was substantially enhanced by the acid eluate from recombinant MR1 in a dose-dependent manner (Fig. 5C). Furthermore, the MAIT cell activation up-regulated by the acid eluate from recombinant MR1 was not seen when antigen-presenting cells (APCs) lacking surface MR1 were added to the culture or in the presence of mAb to MR1. This indicates that the acid eluates from recombinant MR1 did not have a mitogenic effect on MAIT cell activation, but more likely provided an MR1 ligand required for MAIT cell activation.
Fig. 5.
MR1 ligand isolated from recombinant MR1 enhances mouse MAIT cell activation by MR1 orthologous proteins. (A) The MAIT cell hybridoma (6C2) was activated by plate-bound recombinant MR1 protein compared with activation by the MR1-expressing cell. The plate-bound recombinant H-2Kb/OVA (*) complex was used as a negative control. (B) Surface staining of MR1 and H-2Ld molecules on mMR1–overexpressing CH27 cells after incubation with the acid eluate from recombinant MR1. The numbers indicate the mean fluorescent intensity. The green lines indicate the staining with anti-MR1 or Ld antibodies; the black lines indicate isotype controls. (C) Acid-eluted components (<10 kd) from recombinant mMR1 activated mouse MAIT cells in an MR1-dependent manner. (D) MAIT cell activation by MR1 protein from different species with addition of the acid eluate from recombinant MR1. (E) Acid-eluted components (<10 kd) from immunoprecipitated cellular mMR1 expressed in/on CH27 cells activated mouse MAIT cells in an MR1-dependent manner, and this activation was blocked by proton (H+) ATPase inhibitor CMA. (F) MAIT cell activation by MR1 protein from different species with addition of the acid eluate from cellular mMR1 protein. IsoCtl, isotype control antibody.
Interestingly, the enhanced MAIT cell activation in these assays occurred with minimally changed MR1 surface expression (Fig. 5B), supporting the existence of a specific ligand in the acid extract favorable for activation of the MAIT cell hybridoma. Similarly, the low–molecular weight components isolated from MR1-expressing B cells using affinity purification enhanced MAIT cell activation in an MR1-specific and dose-dependent manner (Fig. 5E). This finding demonstrates that the small molecules dissociated from cellular MR1 provided an enriched source of ligands for MAIT cell activation. Furthermore, concanamycin A (CMA) was found to block enhancement of activation of the MAIT cell hybridoma by the acid eluate from the B-cell line (Fig. 5E). These findings support our previous report demonstrating the importance of endosomal ligand loading by MR1 (12). Furthermore, the MR1 ligand was found to be not transporter of antigen presentation– or proteasome-dependent, making it unlikely that this ligand is a simple unmodified peptide. The finding of a putative MR1 ligand in eluates of MR1 expressed by mouse and insect cells strongly suggests that this ligand is evolutionarily conserved. To examine this prediction, MR1 from bovine, rat, and human (with the L151Q substitution) were tested for enhanced MAIT cell activation in the presence of the acid eluate. As shown in Fig. 5 D and F, the acid eluates gave an approximately 2- to 3-fold enhancement of all 3 MR1 orthologous proteins. This directly demonstrates that small molecules bound to mouse recombinant or cellular MR1 can be presented by MR1 proteins from disparate mammalian species, strongly supporting ligand conservation. It is important to note that the extensive cross-species presentation of a conserved ligand was observed on MR1 expressed by a B-cell line (Fig. 5F), because B cells appear to be the physiological APCs required for maintenance of MAIT cells in the periphery (8, 11).
The finding of enhancement when using APCs expressing hMR1-L151Q indicates that residue 151 is a TCR contact site that has no effect on ligand binding. Thus, of the 19 aa differences between mouse and human MR1, only 1 prevents activation of mouse MAIT cell hybridomas, and it would appear to prevent TCR engagement, not ligand binding. This implies a remarkable functional conservation in MR1 activation of MAIT cells. Indeed, the striking conservation of TCR engaging and antigen-binding sites in MR1 orthologs (Fig. S4) further supports MR1's comprehensive cross-species activation of MAIT cells and indicates a different interactive mechanism from that of CD1d or MHC class Ia molecules. More specifically, most of the species-disparate residues in MR1 are located distal from the platform groove, with the exception of a small cluster located around sites where the C terminus of peptide ligands are anchored to classical MHC proteins. This observation supports the model of MR1 binding an evolutionarily conserved ligand and also predicts an orientation for TCR engagement (Fig. S4).
Discussion
It is attractive to speculate that class Ib presentation to innate T-cell populations is reflective of primordial pathways that preceded classical MHC molecules and the acquired immune system. Demonstration of cross-species activation lends credence to the physiological conservation of the MR1 and other class Ib presentation pathways. For example, polyclonal mouse iNKT cells can develop in vivo in the presence of the human CD1d molecule, and polyclonal mouse iNKT cells can be activated in vitro by either human or mouse CD1 presentation of the α-GalCer (23–25). In addition, peptides presented by human HLA-E bind to the mouse Qa-1b molecule, and Qa-1b is able to bind class I leader peptides of class I from several mammalian species (26). In addition, as we report here, both mouse and human MAIT cells display considerable cross-species reactivity of MR1 orthologs, suggesting that they too define a functionally conserved presentation pathway.
Consistent with the antiquity of class Ib presentation pathways, it has been noted that ligand binding by class Ib molecules has similarities to PRRs. Like PRRs, class Ib molecules present ligands with conserved chemical features to immune effector cells that have limited ligand discrimination. Similar to PRRs that evolved to bind pathogen-derived or stress-induced specific molecular patterns (1, 2), ligand-binding class Ib molecules have been found to bind both self and nonself ligands. Importantly, this dual presentation of self and nonself ligands has been critically implicated in the function of several class Ib molecules; for example, CD1d-mediated presentation of both self and nonself ligands has been implicated in iNKT cell responses to bacteria (27). In addition, Qa1 binds signal peptides derived from self class I molecules as NK sensors for viral infection, as well as GroEL peptides from Salmonella typhimurium or Mycobacterium tuberculosis to generate a cytotoxic T-lymphocyte response to infection (28, 29). Interestingly, these GroEL-specific cytotoxic T-lymphocytes cross-react with the stress-induced HSP60 peptide bound to Qa1 as a potential danger signal. This is another example of presentation of an endogenous ligand by class Ib molecules to T cells (28, 29). Based on these findings, we consider it likely that the presentation of the self ligand by MR1 to MAIT cells reported here is of physiological importance, although by no means does this obviate the additional presentation of bacterial antigens.
The presentation of conserved ligands coupled with the relatively limited ligand discrimination of class Ib–restricted T cells has additional functional implications; for example, these properties could explain why class Ib–reactive T cells have an activated/memory phenotype. More specifically, H2-M3–restricted T cells may remain partially activated in the periphery due to the presentation of self M3 ligands derived from mitochondrial proteins. Alternatively, cross-reactive conserved antigens from commensal bacteria have been proposed to be “priming” elements for the memory state of H2-M3–restricted T cells (30). In addition, it was recently reported that the development and/or peripheral expansion of H2-M3–restricted T cells is dependent on commensal bacteria (5, 29). Indeed, the reactivity to self antigen or the cross-reaction between a self ligand and a microbial antigen from commensal flora also could explain the activated/memory phenotype of MAIT cells. Given that the gut flora is required for expansion of MAIT cells in the periphery, it also is attractive to speculate that differences in the gut flora may be responsible for the greater abundance of MAIT cells in human and bovine compared with mouse (9). In fact, in contrast to NKT cells, MAIT cells exit the thymus as naïve cells in both human and mouse before becoming memory cells and accumulate in high numbers after birth in human, whereas they stay naïve and in low numbers in the periphery of mouse (8). The reason for the differences in MAIT cell number and phenotype between human and mouse is not clear; it could be related to the cleanness of specific pathogen-free mouse facilities or to the genetic makeup of the mouse, which might be related to the genetic “bottleneck” through which laboratory mice have passed. Whatever the reason, the intrathymic MR1-dependent selection and the naïve phenotype of mouse MAIT cells contrast with these cells' reactivity to syngeneic and xenogeneic MR1 as expressed in transduced cells in culture. This difference may be related to the high expression of MR1 on the transduced cells or to the costimulation context. More interestingly, this contrasting reactivity could indicate that the ligand bound to MR1 in culture differs from that selecting the MAIT cells in vivo. Again, this finding suggests that MAIT cells display some form of ligand discrimination. Favoring this hypothesis is the very low but reproducible reactivity of human MAIT cells toward native human MR1 as expressed in cultured cells. In any case, the activation/memory phenotype of class Ib–restricted T cells allows them to mediate rapid, polyclonal antimicrobial responses to narrow sets of self or exogenous antigens with conserved motifs (5, 29, 31). Furthermore, the ability to mount quick responses to self and/or microbial ligands is relevant to the effector or regulatory function of innate T cells restricted by CD1d or H2-M3 in antimicrobial responses.
In summary, the findings reported here represent the best evidence thus far that MR1 presents ligands to MAIT cells and that this pathway is highly conserved in mammals. Given that MAIT cell activation is ligand-dependent, we also present the first evidence that both mouse and human MAIT cells are capable of ligand discrimination at least at some level. In mouse MAIT cells, this ligand discrimination was demonstrated by the disparate cross-reactivities of Vβ8 versus Vβ6 hybridomas, and in polyclonal human MAIT cells, ligand discrimination may partially explain why these cells appear to be mostly tolerant to human MR1 but are highly cross-reactive with the MR1 orthologs. Thus, 3 highly conserved elements in the antigen-presenting complex of MR1/ligand/iVα19TCR shape the unique, functionally conserved antigen-presentation pathway. Furthermore, this pathway is likely a key determinant of the activated/memory phenotype and the gut flora developmental dependency of MAIT cells, adding credence to their importance in mucosal immune regulation.
Materials and Methods
Cell Lines, mAbs, and Flow Cytometry.
The mouse embryonic fibroblast WT3 (H-2b), mouse B cell line CH27 (H-2a), human cervical cancer cell HeLa, and human kidney cell 293T were used for retroviral transduction (16, 21). The MAIT T-T hybridoma cells 6C2 and 8D12 have been described (9, 16). All cells were maintained as described (16). Anti-MR1 mAb 26.5 was obtained from an immunization of MR1-deficient mice with soluble recombinant human MR1 made in insect cells and used to detect surface MR1 expression by flow cytometry. mAb 4E3 was used for Western blot analysis as described (12, 16).
Site-Directed Mutagenesis (SDM) and Retroviral Transduction.
The variable residues between mouse and human shown in Fig. 2A were directionally mutated from the mouse to human using SDM, as described previously (16).
MAIT Hybridoma Activation.
Here, 105/mL of mMR-expressing cells or plate-bound 10 μg recombinant MR1 proteins were cultured with 106/mL of MAIT hybridoma cells for 24 h, after which the secreted IL-2 was tested as described (12, 16). Anti-MR1 mAb 26.5 was used to block MAIT cell activation, and mAb 34–2-12 was used as an isotype control (16). Blockade of MAIT cell activation with protein (H+) ATPase inhibitor CMA was performed as described (12).
Primary Transgenic Murine and Polyclonal Human MAIT Cell Activation.
Mesenteric lymph nodes from iVα19-Jα33/Vβ6 transgenic mice on a Cα−/− background were harvested, and T cells were sorted by flow cytometry as CD5+/CD19− cells. Human MAIT cells were isolated from peripheral blood mononuclear cells of healthy donors by FACS sorting as CD5+/CD19−/3C10+/CD161+ cells. The 3C10 mouse monoclonal antibody recognizes the human Vα7 segment of human MAIT cells (8). Sorted mouse and human MAIT cells (5 × 105/mL) were cultured with irradiated MR1 transfectants (5 × 104/mL) in the presence or absence of 10 μg/mL of blocking 26.5 or isotype control antibodies. Activation (CD69 expression) of MAIT cells was analyzed after overnight incubation. Murine and human MAIT cells were labeled with TCRβ/Vβ6/CD4/CD8α/CD69 and TCRγδ/3C10/CD3/CD4/CD8β/CD161/CD69 antibodies, respectively. Supernatants were harvested after 48 h and assayed with a Luminex-based assay (Bio-Rad).
Isolating mMR1 Ligand From Recombinant and Cellular mMR1 Protein.
The recombinant mMR1 protein was generated using baculovirus coexpressing GFP and a bicistronic construct (see SI Materials and Methods). The cellular MR1 protein from overexpressed CH27 cells (12) were immunoprecipitated with protein A–conjugated 26.5 antibody. The purified MR1 proteins were treated with 0.1% TFA (Pierce), and the component <10 kd was collected using a YM-10 Centriprep filter (Millipore) as reported previously (22). The concentrated eluate was added to the coculture of MR1-expressing cells and MAIT cell hybridoma to test its effect on enhancing MAIT cell activity.
Supplementary Material
Acknowledgments.
This study was supported by the National Institutes of Health (Grant AI046553, to T.H.) and INSERM and the Ligue Contre le Cancer (Grant ANR MIME 2006, to O.L.) .We thank Dr. Lonnie Lybarger for providing the rat Mr1 cDNA construct, Drs. Emil Unanue and Anish Suri for advice concerning affinity purification of MR1 and ligand extraction, and Dr. Janet Connolly for critiquing the manuscript.
Footnotes
The authors declare no conflict of interest.
Data deposition: Bovine (Bos taurus) Mr1 cDNA sequences were deposited in the GenBank database (accession nos. EU792881 and EU841914).
This article contains supporting information online at www.pnas.org/cgi/content/full/0903196106/DCSupplemental.
References
- 1.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 2.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: Innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 5.Colmone A, Wang CR. H2–M3–restricted T cell response to infection. Microbes Infect. 2006;8:2277–2283. doi: 10.1016/j.micinf.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Kawachi I, Maldonado J, Strader C, Gilfillan S. MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol. 2006;176:1618–1627. doi: 10.4049/jimmunol.176.3.1618. [DOI] [PubMed] [Google Scholar]
- 7.Treiner E, et al. Mucosal-associated invariant T (MAIT) cells: An evolutionarily conserved T cell subset. Microbes Infect. 2005;7:552–559. doi: 10.1016/j.micinf.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Martin E, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilloy F, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib–restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croxford JL, et al. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol. 2006;7:987–994. doi: 10.1038/ni1370. [DOI] [PubMed] [Google Scholar]
- 11.Treiner E, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 12.Huang S, et al. MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J Exp Med. 2008;205:1201–1211. doi: 10.1084/jem.20072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I–related MR1 gene. J Immunol. 1998;161:4066–4077. [PubMed] [Google Scholar]
- 14.Doyle CK, et al. Hyperconservation of the N-formyl peptide-binding site of M3: Evidence that M3 is an old eutherian molecule with conserved recognition of a pathogen-associated molecular pattern. J Immunol. 2003;171:836–844. doi: 10.4049/jimmunol.171.2.836. [DOI] [PubMed] [Google Scholar]
- 15.Knapp LA, Cadavid LF, Watkins DI. The MHC-E locus is the most well conserved of all known primate class I histocompatibility genes. J Immunol. 1998;160:189–196. [PubMed] [Google Scholar]
- 16.Huang S, et al. Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J Biol Chem. 2005;280:21183–21193. doi: 10.1074/jbc.M501087200. [DOI] [PubMed] [Google Scholar]
- 17.Hansen TH, Huang S, Arnold PL, Fremont DH. Patterns of nonclassical MHC antigen presentation. Nat Immunol. 2007;8:563–568. doi: 10.1038/ni1475. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 19.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 20.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miley MJ, et al. Biochemical features of the MHC-related protein 1 consistent with an immunological function. J Immunol. 2003;170:6090–6098. doi: 10.4049/jimmunol.170.12.6090. [DOI] [PubMed] [Google Scholar]
- 22.Suri A, et al. Specificity of peptide selection by antigen-presenting cells homozygous or heterozygous for expression of class II MHC molecules: The lack of competition. Proc Natl Acad Sci USA. 2003;100:5330–5335. doi: 10.1073/pnas.0330859100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumann J, et al. Targeted expression of human CD1d in transgenic mice reveals independent roles for thymocytes and thymic APCs in positive and negative selection of Valpha14i NKT cells. J Immunol. 2005;175:7303–7310. doi: 10.4049/jimmunol.175.11.7303. [DOI] [PubMed] [Google Scholar]
- 24.Kjer-Nielsen L, et al. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J Exp Med. 2006;203:661–673. doi: 10.1084/jem.20051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brossay L, et al. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurepa Z, Hasemann CA, Forman J. Qa-1b binds conserved class I leader peptides derived from several mammalian species. J Exp Med. 1998;188:973–978. doi: 10.1084/jem.188.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 28.Soloski MJ, Metcalf ES. The involvement of class Ib molecules in the host response to infection with Salmonella and its relevance to autoimmunity. Microbes Infect. 2001;3:1249–1259. doi: 10.1016/s1286-4579(01)01485-x. [DOI] [PubMed] [Google Scholar]
- 29.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 30.Lenz LL, Bevan MJ. CTL responses to H2–M3–restricted Listeria epitopes. Immunol Rev. 1997;158:115–121. doi: 10.1111/j.1600-065x.1997.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 31.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 32.Bennett-Lovsey RM, Herbert AD, Sternberg MJ, Kelley LA. Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins. 2008;70:611–625. doi: 10.1002/prot.21688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.