Abstract
Gray matter pathology is increasingly recognized as an important feature of multiple sclerosis (MS), but the nature of the immune response that targets the gray matter is poorly understood. Starting with a proteomics approach, we identified contactin-2/transiently expressed axonal glycoprotein 1 (TAG-1) as a candidate autoantigen recognized by both autoantibodies and T helper (Th) 1/Th17 T cells in MS patients. Contactin-2 and its rat homologue, TAG-1, are expressed by various neuronal populations and sequestered in the juxtaparanodal domain of myelinated axons both at the axonal and myelin sides. The pathogenic significance of these autoimmune responses was then explored in experimental autoimmune encephalitis models in the rat. Adoptive transfer of TAG-1–specific T cells induced encephalitis characterized by a preferential inflammation of gray matter of the spinal cord and cortex. Cotransfer of TAG-1–specific T cells with a myelin oligodendrocyte glycoprotein-specific mAb generated focal perivascular demyelinating lesions in the cortex and extensive demyelination in spinal cord gray and white matter. This study identifies contactin-2 as an autoantigen targeted by T cells and autoantibodies in MS. Our findings suggest that a contactin-2–specific T-cell response contributes to the development of gray matter pathology.
Keywords: cortical pathology, experimental autoimmune encephalitis, Th1/Th17 T cells, node of Ranvier, myelin glycoproteins
Multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) are usually considered as immune-mediated inflammatory disorders of the CNS (1–3). Over the years, several myelin antigens, including myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated oligodendrocytic basic protein, and myelin oligodendrocyte glycoprotein (MOG), and nonmyelin antigens such as S100b and neurofilament have been shown to be capable of inducing EAE (1, 2). Antigen microarrays comprising protein and lipid autoantigens have been used to analyze immune responses in MS and other autoimmune diseases (4, 5) and recently provided potential biomarkers for subtypes of MS patients (6). In analogy to EAE, the striking clinical heterogeneity of human MS is often explained by the assumption that different myelin and nonmyelin antigens are targeted in different patients. In human MS, however, the precise role of these candidate antigens remains to be defined.
Recently, attention has been redirected toward neuronal and axonal damage in MS (7, 8), based on classical findings in neuropathology (9–12), together with advances in clinical imaging techniques (13–15). Gray matter is affected at many sites throughout the CNS, including the basal ganglia, hippocampus (16), spinal cord (17), and cortex (10, 11, 18–20). Recent imaging studies showed that damage in the gray matter reflects disability to a greater extent than lesions in white matter (14, 15).
In this regard, the following key questions have remained unresolved:
Are neurons targeted directly by an adaptive immune response against neuronal antigen(s), or is neuronal injury merely a consequence of myelin loss?
If there exists an antineural response, is it similarly heterogeneous as the antimyelin response? Is it directed against multiple neuronal antigens?
If yes, what is the role of different neuronal target antigens in human MS?
We started out with an unbiased proteomics approach based on affinity-purified glycoproteins from human brain tissue to probe the antibody repertoire of MS patients for reactivities. We thereby identified the axoglial protein contactin-2/transiently expressed axonal glycoprotein 1 (TAG-1) as a candidate autoantigen, which was recognized by both autoantibodies and T helper (Th) 1/Th17 T cells.
Contactin-2/TAG-1 was originally discovered to be transiently expressed on axons during development, and was therefore named TAG-1, a term still used for the rodent orthologue. Contactin-2/TAG-1 is expressed during adulthood in the juxtaparanodal region of myelinated fibers by oligodendrocytes, Schwann cells, and axons, where it interacts with itself (21, 22). Contactin-2/TAG-1 is also expressed by neurons in the gray matter of the hippocampus (23) and the spinal cord (24). This distribution led us to speculate that autoimmune responses to contactin-2 might be involved in the development of gray matter lesions in MS. Indeed, our functional experiments in EAE showed that contactin-2 (TAG-1)–specific T cells induce gray matter inflammation in experimental animals, paving the way for antibody-mediated cortical demyelination.
Results
Identification of Contactin-2 as a Candidate Autoantigen in Multiple Sclerosis.
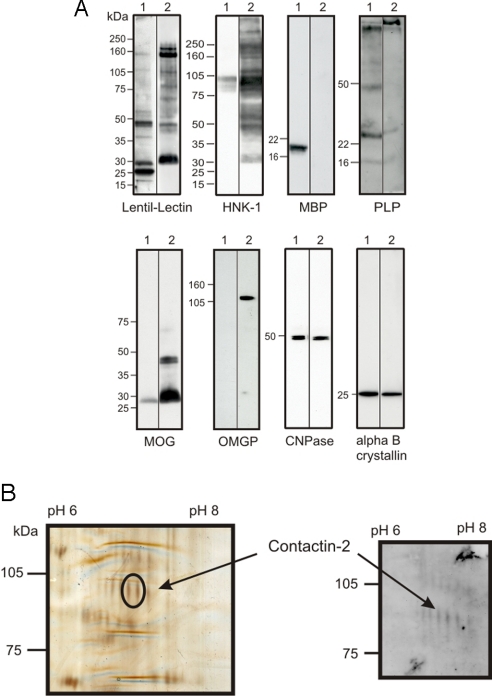
An autoimmune response to contactin-2 was detected in patients with MS using a proteomics-based analysis of the disease-associated autoantibody repertoire. As the target in this screen, we used a preparation of lentil lectin-binding glycoproteins isolated from human myelin. This protein preparation contains purely myelin proteins and proteins shared by myelin and axons (25). This approach allowed us to investigate autoreactivity to a subset of quantitatively minor glycoproteins that may be exposed at the membrane surface. This glycoprotein preparation was highly enriched in several well-characterized myelin glycoproteins, including MOG, oligodendrocyte myelin glycoprotein (OMGP), and myelin-associated glycoprotein (MAG), whereas the major structural proteins MBP and PLP were largely depleted (Fig. 1A). Screening this glycoprotein preparation after resolution by 2D gel electrophoresis by Western blotting using Ig preparations from patients undergoing immunoadsorption therapy (26) identified a series of spots migrating with an apparent molecular weight of ≈100 kDa (Fig. 1B). This pattern was seen in 3 of 5 MS samples but in neither of the 2 controls (cardiomyopathy and peripheral neuropathy) used in this initial screen. Mass spectrometry analysis of 2 spots excised from silver-stained gels identified the parent protein as contactin-2 with sequence coverage of 19% and a probability for alpha error of 0.005. The identification of these spots at about 100 kDa as contactin-2 indicates that they represent the low molecular weight form of this glycoprotein (24).
Fig. 1.
Identification of contactin-2 as a potential autoantigen by a proteomics approach. (A) Using lentil lectin affinity chromatography, glycoproteins were purified from human myelin. Both fractions (myelin, 1; myelin glycoproteins, 2) were analyzed by Western blot analysis with antibodies to known myelin components. Staining with lentil-lectin and HNK-1 (anti-CD57), which both recognize specific carbohydrates, shows enrichment of certain proteins in the glycoprotein fraction. In particular, MAG at around 100 kDa can be detected in the HNK-1 stain. Anti-MBP and anti-PLP recognize the major unglycosylated myelin proteins that are largely depleted in the myelin glycoprotein fraction. Anti-MOG and anti-OMGP recognize glycosylated myelin proteins that are enriched in the purified myelin glycoprotein fraction. Anti-cyclic nucleotide phosphatase and anti-alpha B crystalline recognize proteins that are present in both fractions. (B) Myelin glycoproteins were separated in parallel on two 2D gels. One gel was used for silver staining (Left), and the other one was used for Western blotting (Right) with immunoadsorption eluates from an MS patient. This allowed the identification of glycoproteins recognized by IgG of MS patients. Two spots with a similar molecular weight (encircled at a molecular weight of about 100 kDa) were cut, and both spots were identified as contactin-2.
Autoreactivity to Contactin-2 in MS Patients and Controls.
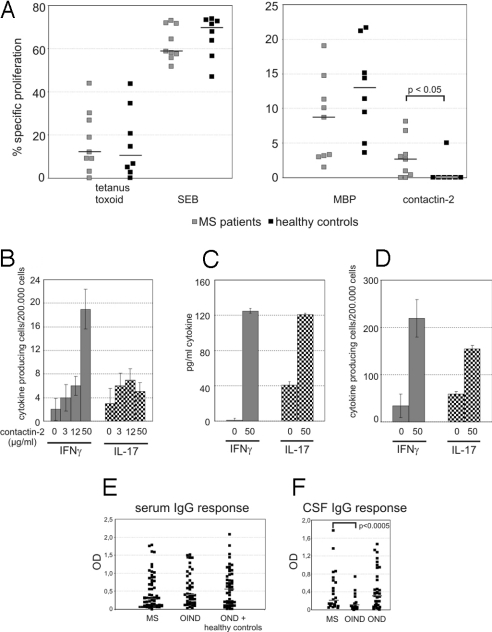
Following the identification of contactin-2 as a potential candidate autoantigen in MS, we investigated whether patients developed a disease-associated T-cell response to this autoantigen. We compared the proliferative response of peripheral blood mononuclear cells (PBMCs) of MS patients (n = 9) and healthy controls (n = 8) with contactin-2, MBP, tetanus toxoid, and staphylococcal enterotoxin B (SEB). The response to contactin-2 was significantly increased in MS patients compared with healthy controls (P < 0.05), whereas the proliferative response to the other antigens was similar in both groups (Fig. 2A).
Fig. 2.
T-cell reactivity and autoantibody response against contactin-2 in MS and control patients. (A) PBMCs from 9 untreated MS patients and 8 healthy controls were stimulated with contactin-2 and for comparison with MBP, tetanus toxoid, and SEB. The proliferation is indicated as the percentage of cells dividing in the presence of antigen minus the percentage of cells dividing in the absence of antigen using 5-chloromethylfluorescein diacetate. Contactin-2 but not tetanus toxoid, MBP, or SEB induced a stronger proliferation of PBMCs from MS patients than from controls (P < 0.05 using the U test). Gray squares represent MS patients, and black squares represent healthy controls. The bars represent the median. (B) PBMCs from an MS patient were tested for contactin-2–specific production of IFN-γ and IL-17 using a direct ex vivo ELISPOT assay. The cells were stimulated with the indicated concentrations of contactin-2 and left for 21 h in culture. The number of cytokine-producing cells per 2 × 105 cells is shown (filled, IFN-γ; dotted, IL-17). For a list of all tested patients, see Table S1. (C and D) PBMCs from 2 MS patients are shown. After restimulation, a contactin-2–specific IFN-γ response could be detected in patient 14 and an IL-17 response could be detected in patient 12 using an ELISPOT assay (C) and an ELISA (D). For a list of all results, see Table S2. (E) IgG reactivity against recombinant contactin-2 was determined by ELISA using sera diluted 1:400. The ODs corrected for the individual background are shown for each tested patient. There was no significant difference between the different groups. (F) The CSF samples were diluted 1:1 and tested by ELISA for recognition of contactin-2. The CSF-IgG response against contactin-2 was significantly stronger in MS patients than in OIND control patients (P < 0.0005, t test). There was no significant difference between the anti-contactin-2 reactivity in MS patients and OND control patients. The ODs corrected for the individual background are shown for each tested patient.
We then investigated the cytokine profile associated with this antigen-specific proliferative response focusing on IFN-γ and IL-17, both of which are implicated in the pathogenesis of MS (2, 27, 28). At a concentration of 50 μg/mL contactin-2, an IFN-γ response was detected in 9 of 12 MS patients after direct ex vivo analysis by enzyme-linked immunospot (ELISPOT) assay, with a median frequency of 7.5 cells/2 × 105 (range: 0–24 cells). In contrast, the number of cells secreting IL-17 in response to contactin-2 was lower, with a median value of only 1 cell/2 × 105 (range: 0–2 cells) [Fig. 2B and supporting information (SI) Table S1]. These contactin-2–specific IL-17 and IFN-γ responses were markedly increased following antigen-specific restimulation in vitro, as shown by both ELISA and ELISPOT assay (Fig. 2 C and D). Contactin-2–induced IL-17 production was magnified by the addition of IL-15 and IL-23 to the cultures after the primary stimulation (29) (Fig. S1 and Table S2). As anticipated from studies of T-cell responses to other CNS autoantigens (2), we also observed antigen-specific responses to contactin-2 in our control donors [IFN-γ: 3.1 cells/2 × 105 cells (range: 0–17 cells); IL-17: 1 cell/2 × 105 cells (range: 0–5 cells); Table S1].
In addition to investigating the antigen-specific T-cell response, we characterized the autoantibody response to contactin-2 by ELISA. Sera from 153 subjects were analyzed [56 MS patients, 45 other inflammatory neurological disease (OIND) patients, 12 noninflammatory neurological disease (OND) patients, and 40 healthy controls] as well as 84 cerebrospinal fluid (CSF)/serum pairs obtained from 24 MS patients (16 patients with clinical definite MS and 8 patients with probable MS), 35 OND patients, and 25 OIND patients. A serum antibody response to contactin-2 was detected in the majority of donors, irrespective of their clinical status (Fig. 2E). However, there was a significantly higher IgG response against contactin-2 (median = 0.2) in CSF from MS patients than in that from OIND patients (median = 0.054; P < 0.0005), although there was no difference between that from MS and OND patients (median = 0.3; P = 0.67) (Fig. 2F). Analysis of the corrected antibody index (30) revealed that 17% (4 of 24) of MS patients compared with only 4% (1 of 25) of OIND patients and 9% (3 of 35) of OND patients showed an intrathecal IgG response to contactin-2. Isotype usage was investigated in 24 subjects (11 MS patients, 5 OIND patients, 3 OND patients, and 5 healthy controls) selected on the basis of having high serum titers to contactin-2. IgG2 was the dominant IgG isotype in both MS and control cohorts, whereas some patients also showed a considerable IgM response (Fig. S2A). Deglycosylation experiments using 2 of these high-titer sera demonstrated that the autoantibody response to contactin-2 is directed against both glycosylated and peptide epitopes (Fig. S2B). A prerequisite for this autoantibody response to contactin-2 to mediate any pathological effect is recognition of the native protein at the membrane surface (31). We therefore investigated whether either IgG or IgM antibodies present in the sera of patients with a high antibody titer as determined by ELISA bound to the surface of contactin-2–transfected cell lines by flow cytometry. Analysis of 11 sera and 7 CSF samples as well as 7 immunoadsorption eluates identified 2 samples with a low level of Ig binding to the contactin-2–transfected cells (Fig. S2C).
Contactin-2/TAG-1–Specific T Cells Target Cortical and Spinal Cord Gray Matter.
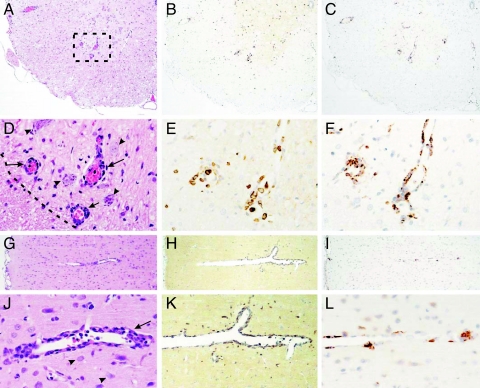
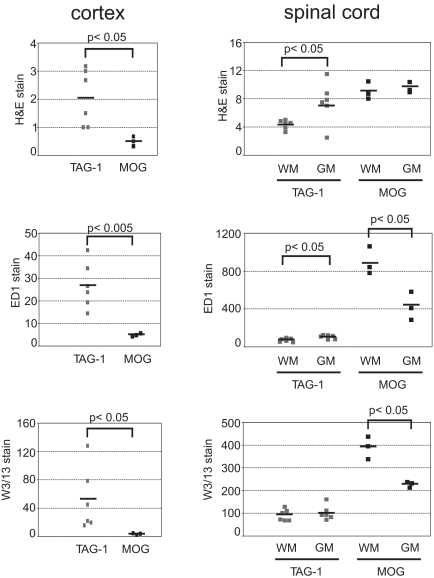
Our observation that the proliferative response to contactin-2 is associated with IFN-γ and IL-17 secretion in patients with MS led us to speculate that this response could actively participate in disease pathogenesis. We therefore investigated the pathogenic potential of this antigen-specific response in an adoptive transfer model of EAE. Adoptive transfer of 107 CD4+ T cells specific for aa 31–240 of rat TAG-1 induced EAE characterized by loss of weight, transient loss of tail tone, and occasional hind limb paraparesis; clinical EAE was seen in 3 of 9 animals (Table 1). Histopathological studies revealed that an inflammatory response was present in all animals. This immune response targeted the cerebral cortex as well as the spinal cord white and gray matter (Fig. 3). A quantitative analysis of the histopathology comprising H&E staining and immunostaining for W3/13 T cells and ED1 macrophages revealed that the pattern of inflammation induced by TAG-1–specific T cells differs from that induced by MOG-specific T cells (Fig. 4). Whereas in MOG transfer EAE (tEAE) cortical involvement is minimal and the inflammatory response preferentially targets the spinal cord white matter, TAG-1 tEAE showed a preferential targeting of cortex and spinal cord gray matter (Figs. 3 and 4). This is in harmony with previous observations in EAE mediated by MOG-specific T cells (32–34).
Table 1.
Clinical characteristics of animals after transfer of TAG-1– or MOG-specific T cells
| T-cell line | Cell dose | n | Onset of weight loss, dpt, mean ± SD | % weight, 4 dpt | Incidence of weight loss | Incidence of clinical signs | Cumulative clinical score, mean ± SD |
|---|---|---|---|---|---|---|---|
| TAG1 | 1×107 | 9 | 3.00 ± 0.28 | 98.93 ± 0.48 | 100% | 33% | 1.25 ± 2.0 |
| MOG | 1×107 | 5 | 3.20 ± 0.45 | 86.43 ± 0.89 | 100% | 100% | 5.3 ± 1.57 |
dpt, days post-transfer.
Fig. 3.
TAG-1–specific T cells induce inflammation in spinal cord gray and white matter and cerebral cortex. Histology of spinal cord (A–F) and cortex (G–L) showing H&E (A, D, G, and J), T-cell staining with W3/13 (B, E, H, and K), and macrophage staining with ED1 (C, F, I, and L) in serial sections of TAG-1–mediated EAE. The boxed area in A is shown in D–F at higher magnification. Perivascular infiltrates (arrows in D and J) are seen in close contact to neurons (arrowheads in D and J) in both the spinal cord and cortex. (D) The border between gray and white matter is indicated as a dashed line. (Magnification: A–C, G–I, ×80; D–F, J–L, ×320.)
Fig. 4.
Quantitative analysis of MOG– and TAG-1–mediated EAE. Dark Agouti (DA) rats with TAG-1 T-cell–induced disease and 3 DA rats with MOG T-cell–induced disease were killed 5 days after T-cell transfer. Tissue sections of brain and spinal cord were stained for inflammatory infiltrates with H&E, for macrophages with ED1, and for T cells with W3/13. For quantification of inflammatory infiltrates, the number of perivascular infiltrates per square millimeter is given. For quantification of macrophages and T cells, the number of cells per square millimeter is given. In the cortex of TAG-1 T-cell–transferred animals, there were significantly more perivascular infiltrates (P < 0.05), macrophages (P < 0.005), and T cells (P < 0.05) compared with the MOG-induced disease. In the spinal cord of TAG-1 T-cell–transferred animals, inflammation was pronounced in gray matter (GM) versus white matter (WM) (P < 0.05 for H&E stain, P < 0.05 for ED1 counts). In contrast MOG T-cell–transferred animals showed more prominent inflammation in the white matter of spinal cord (P < 0.05 for ED1 counts, P < 0.05 for W3/13 counts).
In isolation, this inflammatory response induced in the cortex and spinal cord by the adoptive transfer of TAG-1–specific T cells was insufficient to induce either demyelination or axonal injury. However, additional transfer of the demyelinating MOG-specific mAb Z2 (i.p.) 4 days after T-cell transfer triggered a marked exacerbation of clinical disease (Fig. S3). On day 6 after transfer of TAG-1–specific T cells, all animals that received additional MOG-specific antibodies showed hind limb paralysis (Fig. S3). This was associated with demyelination in gray and white matter and deposition of Ig and complement, indicating that the TAG-1–specific T cells were sufficient to open the blood-brain barrier (Fig. 5). These antibody-mediated demyelinating lesions were smaller and more circumscribed in the cortex than in the spinal cord and reproduced the gross pathological features of small intracortical lesions described in patients with early and fulminant MS (35, 36). As reported previously, passive transfer of MOG-specific mAb into naive animals failed to initiate any clinical deficit (37). Similarly, passive transfer of an irrelevant IgG2a myeloma protein into animals with TAG-1 tEAE failed to influence disease severity or pathology, as has been reported in other EAE models (25). We also cotransferred TAG-1–specific mAbs [4D7 (IgM) and 3.1C12 (IgG1)] into animals with TAG-1 tEAE, but these also failed to have any effect on disease severity. Although both TAG-1–specific mAbs stain the surface of live rat TAG-1–transfected cell lines in vitro (Figs. S4 and S5), the transferred antibodies did not alter the pathology of the inflammatory lesions, suggesting that TAG-1 is not available to bind antibody in vivo.
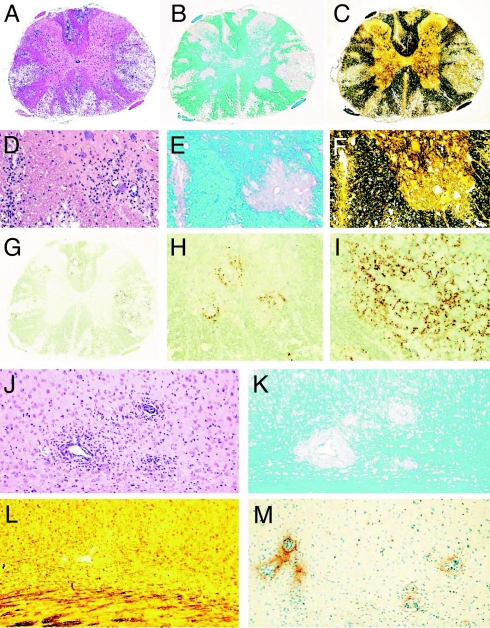
Fig. 5.
Massive demyelination induced by anti-MOG antibodies in animals with TAG-1 T-cell induced encephalomyelitis. (A–I) Spinal cord lesions after cotransfer of TAG-1 reactive T cells and anti-MOG antibodies. H&E staining shows profound inflammation and large confluent lesions in the white and gray matter (A), staining for myelin (Luxol fast blue) confirms complete loss of myelin in the lesions (B), and reduced axonal density in the lesional areas is seen in sections stained by Bielschowsky silver impregnation (C). Images D–F represent higher magnifications of the lesion in the anterior horn gray matter shown in A–C documenting inflammation (D, H&E), demyelination (E, Luxol fast blue), and axonal loss (F, Bielschowsky silver impregnation). Immunocytochemistry for complement C9 shows massive complement deposition in the white matter (G and I) and lower extent of complement deposition in gray matter lesions (H). (Magnification: A–C and G, ×25; D–F, H, and I, ×75.) Cortex with perivascular inflammation (J, H&E) and demyelination (K, Luxol fast blue) but little axonal loss (L, Bielschowsky silver impregnation) and only moderate complement C9 deposition (M, immunocytochemistry for C9). (Magnification: J–M, ×75.)
Discussion
Gray matter pathology is seen from early in the course of MS and is implicated in brain atrophy, cognitive impairment, and fatigue (38). The extent of this gray matter involvement increases with time, and quantitative analysis reveals that up to 68% of cerebral cortex (10) and 33% of spinal cord gray matter (17) may be demyelinated in individual patients. The causes of this extensive injury and why lesions develop in gray matter in patients with MS are currently unclear, although roles have been discussed for mechanisms, including chronic meningeal inflammation, secondary degeneration, and antineuronal immunity (7, 38). In the current study, we used a proteomics approach to identify contactin-2 as an autoantigen in MS and demonstrate that contactin-2/TAG-1 autoimmunity preferentially mediates gray matter encephalitis and paves the way for antibody-mediated demyelination in the gray and white matter.
Contactin-2–specific T-cell proliferation was higher in MS patients than controls, and this antigen-specific response was associated with secretion of IFN-γ and IL-17. This cytokine phenotype is compatible with an active role in neuroinflammation. Increased mRNA transcripts for IL-17 are present in MS lesions (39), and IL-17 has also been identified in infiltrating T cells in MS lesions by immunohistochemistry (28). Animal models provide convincing evidence that both IFN-γ– and IL-17–producing T cells induce organ-specific autoimmunity (27, 40). Not unexpectedly, contactin-2–specific T-cell responses were also seen in control donors, reflecting the presence of a multitude of autoimmune specificities within the normal healthy repertoire (41). Clearly, further studies are needed to characterize the contactin-2–specific T-cell response in MS patients, particularly with respect to activation status, dependence on costimulation, and correlation to lesional topography.
On testing the functional relevance of contactin-2/TAG-1 T cells in EAE, we found that they induced lesions in both gray and white matter, and of particular note was the involvement of the cortex. This was especially striking when we compared these findings with the distribution of lesions in animals with disease induced by the adoptive transfer of MOG-specific T cells in which cortical involvement was minimal. Preferential involvement of gray matter is not commonly seen in actively induced models of EAE (42–44), and, until now, preferential involvement of the cortex had not been reported in tEAE. This raises the question as to why gray matter involvement, particularly cortical involvement, is such a prominent feature of EAE induced by TAG-1–specific T cells. We suggest that this simply reflects the distribution of this axoglial autoantigen in the CNS, as previously discussed in other EAE models in which the identity of the target antigen influences the distribution of lesions. Unlike “classical” encephalitogens such as MBP, PLP, or MOG, which are all components of the myelin sheath, contactin-2/TAG-1 is also expressed by the myelinated axon itself. Contactin-2/TAG-1 is sequestered within the juxtaparanodal domain of myelinated fibers both on the axolemma and myelin sheath, where it is believed to be involved in the local clustering of axonal voltage-gated potassium channels (21, 22). In addition, contactin-2/TAG-1 is expressed by a variety of neuronal subsets within the adult CNS (23) and spinal cord (24).
As in other rat models of EAE, the inflammatory response in the CNS was itself insufficient to trigger demyelination; however, as demonstrated by cotransfer of an anti-MOG mAb, the inflammatory response in gray matter was associated with blood-brain barrier damage, allowing the antibody to penetrate into the CNS to initiate demyelination. In this case, the TAG-1–specific T-cell response is paving the way for antibody-dependent pathomechanisms to mediate tissue damage. The 2 applied TAG-1–specific mAbs failed to induce any clinical exacerbation of disease or to modify lesion pathology. This indicates that in the context of the intact myelin sheath, contactin-2/TAG-1 sequestered within the juxtaparanodal domain is inaccessible to antibody. This is reminiscent of the in vivo features of antibodies to neurofascin (25). These antibodies selectively targeted the nodes of Ranvier but did not bind to the myelin form of neurofascin at the paranodes.
In summary, we identify contactin-2 as an autoantigen for both antibodies and T cells in MS patients and demonstrate in an animal model that contactin-2–specific T cells induce cortical lesions. Our findings (i) provide further support for the existence of an antineuronal adaptive immune response, which is supported by combined evidence from EAE and MS; (ii) provide emerging evidence for the heterogeneity of the antiaxonal response, raising the intriguing possibility that paranodal antigens shared between myelin and neurons might play a special role in MS pathogenesis; and (iii) indicate that contactin-2–specific T cells may contribute to the development of gray matter pathology in MS.
Materials and Methods
Patients and Control Donors.
Sera from 153 donors were analyzed. These donors included 56 patients with MS, 45 patients with OIND, 12 patients with OND, and 40 healthy blood donors. The group of MS patients included 36 patients with clinically definite MS and 20 patients with a clinical isolated syndrome suggestive of MS. Because these 2 patient groups did not significantly differ in the various experiments, they are combined in this study. The sources of the immunoadsorption eluates are described in detail in a previous publication (26). T-cell assays were performed using PBMCs prepared from 15 untreated patients with MS and 14 healthy controls (Tables S1 and S2). This study was approved by the local ethical committee, and all patients gave their informed consent for the study.
Purification and Characterization of Myelin Glycoproteins.
In this study, we used lentil lectin-binding glycoproteins purified from human myelin. This protein preparation contains purely myelin proteins and proteins shared by myelin and axons (25). For characterization, individual proteins were identified by Western blotting using a range of different antibodies and antisera (see SI Methods).
ELISA.
Recombinant human contactin-2 (produced in a mouse myeloma cell line) was purchased from R&D Systems. Coating was performed at a concentration of 3 μg/mL in carbonate buffer on MaxiSorp plates (Nunc) for 12 h at 4 °C. Subsequently, plates were blocked using 3% wt/vol for 2 h at room temperature. Sera (diluted 1:400 in 1% wt/vol) were incubated for 2 h at room temperature. Bound antibodies were detected using goat anti-human IgG (Fc-γ fragment specific) biotin-conjugated antibodies diluted 1:3,000 (Jackson ImmunoResearch), followed by streptavidin-peroxidase diluted 1:5,000 (Jackson ImmunoResearch). For determining isotype usage and intrathecal IgG production, see SI Methods.
2D Gels, Western Blots, and Mass Spectrometry.
These are essentially as described elsewhere (25).
ELISPOT Assay and ELISA for IFN-γ and IL-17.
For T-cell assays, PBMCs from 15 untreated MS patients (mean age = 55 years, female/male = 8/7, relapsing-remitting/secondary-progressive = 5/10) and from 14 healthy controls (mean age = 47 years, female/male = 8/6) were isolated using Pancoll (Pan-Biotech). The ex vivo ELISPOT assay was performed using 2 × 105 PBMCs seeded into 96-well plates and stimulated using SEB (1 μg/mL; Sigma), contactin-2 (3, 12, or 50 μg/mL), or MBP (7 or 50 μg/mL; Serotec) for 21 h. ELISPOT assays for IFN-γ and IL-17 were performed according to the manufacturer's guidelines (R&D Systems, eBioscience). All ELISPOT assays were performed in quintuplicate. To enhance sensitivity, an in vitro restimulation protocol was performed as described by McCutcheon et al. (45) and in SI Methods.
Proliferation Assay.
PBMCs from 9 MS patients and 8 healthy controls were labeled at a density of 1 × 106 cells/mL using 1 μM 5-chloromethylfluorescein diacetate (Invitrogen). The cells were stimulated with contactin-2 (50 μg/mL), MBP (50 μg/mL), tetanus toxoid (20 μg/mL), or SEB (10 μg/mL) and kept in culture for 7 days. Proliferation was measured as a decrease in fluorescence in a FACS. The specific proliferation was calculated as the difference between stimulated cells and unstimulated control.
Generation of Antigen-Specific T-Cell Lines and Induction of EAE.
Short-term antigen-specific T-cell lines were generated using antigen-primed donors essentially as described previously (46), utilizing recombinant antigens corresponding to either amino acids 31–240 of rat TAG-1 (TAG-1 amino acid sequence according to GenBank accession no. NM_012884) or the entire extracellular domain of rat MOG (47). EAE was induced by the adoptive transfer of freshly restimulated antigen-specific T blasts followed in specific experiments by the cotransfer (i.p.) of 1 mg of mAb specific for MOG (mAb Z2; ref. 48) or TAG-1 (4D7 and 3.1C12). Control animals were treated with appropriate isotype mouse myeloma proteins (IgM, M2521; IgG1, M7894; and IgG2a, M7769; all from Sigma Aldrich). Animals were perfused with 4% vol/vol paraformaldehyde in PBS under terminal anesthesia; the spinal cord and brain were then postfixed with 4% vol/vol paraformaldehyde in PBS for 24 h at 4 °C.
Histology and Immunohistochemistry.
See SI Methods.
Supplementary Material
Acknowledgments.
We thank Drs. G. Krishnamoorthy and M. Kerschensteiner for carefully reviewing our manuscript. We are grateful to Robert Bittner for technical assistance. This work was funded by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 571); United Kingdom Multiple Sclerosis Society; Verein zur Therapieforschung für Multiple Sklerose-Kranke; Hermann und Lilly Schilling Foundation; and Excellency Initiative of the Ludwig-Maximilian-University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. NM_012884).
See Commentary on page 8083.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901496106/DCSupplemental.
References
- 1.Steinman L. Multiple sclerosis: A two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 2.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 3.Weiner HL. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol (Chicago) 2004;61:1613–1615. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- 4.Kanter JL, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12:138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- 5.Robinson WH, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 6.Quintana FJ, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci USA. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trapp BD, Nave KA. Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 8.Stadelmann C, Albert M, Wegner C, Brück W. Cortical pathology in multiple sclerosis. Curr Opin Neurol. 2008;21:229–234. doi: 10.1097/01.wco.0000318863.65635.9a. [DOI] [PubMed] [Google Scholar]
- 9.Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 10.Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 11.Kutzelnigg A, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 12.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 13.Geurts JJ, et al. Intracortical lesions in multiple sclerosis: Improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236:254–260. doi: 10.1148/radiol.2361040450. [DOI] [PubMed] [Google Scholar]
- 14.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: A longitudinal study. Ann Neurol. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- 15.Fisniku LK, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–254. doi: 10.1002/ana.21423. [DOI] [PubMed] [Google Scholar]
- 16.Geurts JJ, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66:819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- 17.Gilmore CP, et al. Spinal cord gray matter demyelination in multiple sclerosis—A novel pattern of residual plaque morphology. Brain Pathol. 2006;16:202–208. doi: 10.1111/j.1750-3639.2006.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wegner C, Matthews PM. A new view of the cortex, new insights into multiple sclerosis. Brain. 2003;126:1719–1721. doi: 10.1093/brain/awg238. [DOI] [PubMed] [Google Scholar]
- 19.Kidd D, et al. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- 20.Kutzelnigg A, et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17:38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci. 2002;22:3016–3024. doi: 10.1523/JNEUROSCI.22-08-03016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girault JA, Peles E. Development of nodes of Ranvier. Curr Opin Neurobiol. 2002;12:476–485. doi: 10.1016/s0959-4388(02)00370-7. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Dolado M, et al. Thyroid hormone regulates TAG-1 expression in the developing rat brain. Eur J Neurosci. 2001;14:1209–1218. doi: 10.1046/j.0953-816x.2001.01745.x. [DOI] [PubMed] [Google Scholar]
- 24.Soares S, et al. Neuronal and glial expression of the adhesion molecule TAG-1 is regulated after peripheral nerve lesion or central neurodegeneration of adult nervous system. Eur J Neurosci. 2005;21:1169–1180. doi: 10.1111/j.1460-9568.2005.03961.x. [DOI] [PubMed] [Google Scholar]
- 25.Mathey EK, et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moldenhauer A, et al. Immunoadsorption patients with multiple sclerosis: An open-label pilot study. Eur J Clin Invest. 2005;35:523–530. doi: 10.1111/j.1365-2362.2005.01518.x. [DOI] [PubMed] [Google Scholar]
- 27.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 28.Tzartos JS, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiber H, Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: Sensitive and specific detection of antibody synthesis in brain. Clin Chem. 1991;37:1153–1160. [PubMed] [Google Scholar]
- 31.Brehm U, Piddlesden SJ, Gardinier MV, Linington C. Epitope specificity of demyelinating monoclonal autoantibodies directed against the human myelin oligodendrocyte glycoprotein (MOG) J Neuroimmunol. 1999;97:9–15. doi: 10.1016/s0165-5728(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 32.Berger T, et al. Experimental autoimmune encephalomyelitis: The antigen specificity of T lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab Invest. 1997;76:355–364. [PubMed] [Google Scholar]
- 33.Linington C, et al. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol. 1993;23:1364–1372. doi: 10.1002/eji.1830230627. [DOI] [PubMed] [Google Scholar]
- 34.Shao H, Huang Z, Sun SL, Kaplan HJ, Sun D. Myelin/oligodendrocyte glycoprotein-specific T-cells induce severe optic neuritis in the C57BL/6 mouse. Invest Ophthalmol Visual Sci. 2004;45:4060–4065. doi: 10.1167/iovs.04-0554. [DOI] [PubMed] [Google Scholar]
- 35.Brück W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(Suppl 5):v3–v9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- 36.Kutzelnigg A, Lassmann H. Cortical demyelination in multiple sclerosis: A substrate for cognitive deficits? J Neurol Sci. 2006;245:123–126. doi: 10.1016/j.jns.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- 38.Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008;7:841–851. doi: 10.1016/S1474-4422(08)70191-1. [DOI] [PubMed] [Google Scholar]
- 39.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 40.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 41.Cohen IR. The cognitive principle challenges clonal selection. Immunol Today. 1992;13:441–444. doi: 10.1016/0167-5699(92)90071-E. [DOI] [PubMed] [Google Scholar]
- 42.Storch MK, et al. Cortical demyelination can be modeled in specific rat models of autoimmune encephalomyelitis and is major histocompatability complex (MHC) haplotype-related. J Neuropathol Exp Neurol. 2006;65:1137–1142. doi: 10.1097/01.jnen.0000248547.13176.9d. [DOI] [PubMed] [Google Scholar]
- 43.Pomeroy IM, Matthews PM, Frank JA, Jordan EK, Esiri MM. Demyelinated neocortical lesions in marmoset autoimmune encephalomyelitis mimic those in multiple sclerosis. Brain. 2005;128:2713–2721. doi: 10.1093/brain/awh626. [DOI] [PubMed] [Google Scholar]
- 44.Huizinga R, Gerritsen W, Heijmans N, Amor S. Axonal loss and gray matter pathology as a direct result of autoimmunity to neurofilaments. Neurobiol Dis. 2008;32:461–470. doi: 10.1016/j.nbd.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 45.McCutcheon M, et al. A sensitive ELISPOT assay to detect low-frequency human T lymphocytes. J Immunol Methods. 1997;210:149–166. doi: 10.1016/s0022-1759(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 46.Stefferl A, et al. Disease progression in chronic relapsing experimental allergic encephalomyelitis is associated with reduced inflammation-driven production of corticosterone. Endocrinology. 2001;142:3616–3624. doi: 10.1210/endo.142.8.8292. [DOI] [PubMed] [Google Scholar]
- 47.Adelmann M, et al. The N-terminal domain of the myelin oligodendrocyte glycoprotein (MOG) induces acute demyelinating experimental autoimmune encephalomyelitis in the Lewis rat. J Neuroimmunol. 1995;63:17–27. doi: 10.1016/0165-5728(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 48.Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol. 1993;143:555–564. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.