Abstract
One of the most conspicuous and stereotyped activities of social insects such as ants and honey bees is necrophoresis, the removal of dead colony members from the nest. Previous researchers suggested that decomposition products such as fatty acids trigger necrophoric behavior by ant workers. However, fatty acids elicit both foraging and necrophoric responses, depending on the current nest activities (e.g., feeding or nest maintenance). Furthermore, workers often carry even freshly killed workers (dead for <1 h) to refuse piles before significant decomposition has a chance to occur. Here, we show that the cuticular chemistry of Argentine ant workers, Linepithema humile, undergoes rapid changes after death. When the workers are alive or freshly killed, relatively large amounts of 2 characteristic ant-produced compounds, dolichodial and iridomyrmecin, are present on the ants' cuticle. However, these compounds disappear from the cuticle within about 1 h after death. We demonstrate how this phenomenon supports an alternative mechanism of ant necrophoresis in which the precise recognition and rapid removal of dead nestmates are elicited by the disappearance of these chemical signals associated with life.
Keywords: chemical communication, dolichodial, iridomyrmecin, Linepithema humile, social insects
Dead individuals pose a potential hazard to other members of social groups because of the risk of exposure to pathogens (1). Animals typically recognize other individuals as dead based on the absence of signals or cues associated with life (e.g., lack of movement, lack of response to stimuli) and modify their behavior accordingly (1). Necrophoresis (the removal of dead colony members from the nest) is a characteristic behavior exhibited by ant and honey bee species that live in large colonies in enclosed nests, and it is considered one of the most important innate hygienic behaviors of these eusocial insects (2–8). Chemical stimuli associated with dead nestmates have been reported to be the most important cues for elicitation of necrophoric behaviors by nestmates (3–8), with previous studies hypothesizing that increasing titers of decomposition products in the corpses, particularly fatty acids (e.g., myristoleic, palmitoleic, oleic, linoleic acids), were the major stimuli eliciting such behaviors (3, 5, 6).
However, fatty acids have been shown to elicit both foraging and necrophoric responses in ants, depending on the nest activities at any given time (e.g., feeding, nest maintenance) (7). The fatty acid decomposition product hypothesis also does not adequately explain certain aspects of necrophoresis. For example, in the red imported fire ant, Solenopsis invicta, the latency period between death and release of necrophoric behavior is too brief to attribute necrophoresis to decomposition products, because elicitation of necrophoresis increases rapidly after death, reaching a plateau within 1 h (8). Similarly, in the honey bee, Apis mellifera, freshly killed individuals placed in an experimental hive were removed by nestmates within 1 h (4). To explain this rapid recognition and removal of dead adults by nestmates, it has been suggested that some chemical signature associated with living adult insects might nullify or mask preexisting necrophoresis-releasing stimuli (4, 8).
In preliminary studies with the Argentine ant, Linepithema humile, we observed that freshly killed L. humile workers placed in a foraging arena were carried to refuse piles within 1–2 h, suggesting that workers recognized dead nestmates before significant decomposition could have occurred. This led us to test the hypothesis that L. humile workers recognize and remove dead adult nestmates based on the rapid decrease of ant-produced compounds associated with life rather than the accumulation of compounds signaling death. Here, we provide experimental evidence (bioassays and chemical analyses) to show that the chemical stimuli that elicit removal of nestmate corpses by workers are present in both live and dead workers but that additional chemicals associated with live ants inhibit necrophoric behaviors. Our results resolve a conundrum of long standing in animal behavior and correct a misinterpretation of previous results that has become both popular and widespread in the literature. For example, the concept of a “fatty acid death cue” releasing necrophoric response in ants and honey bees has been embraced by disciplines as diverse as insect physiology (9), plant-insect interactions (10), robotics (11), and computer simulation studies dealing with self-organization theory (12). In contrast, our results demonstrate that it is the dissipation of chemical signals associated with life rather than the increase of a “death cue” that triggers necrophoric behavior by Argentine ant workers.
Results
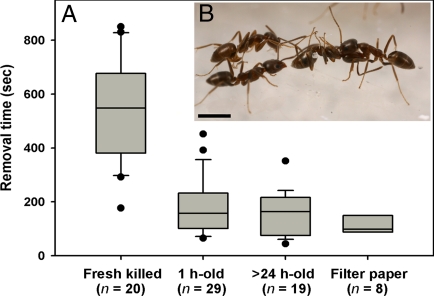
We examined the responses of workers to nestmate corpses at various times post mortem by dropping corpses into the nest entrance in an experimental arena [supporting information (SI) Fig. S1]. Workers took significantly longer to remove freshly killed ants and carry them to the refuse pile than corpses that were either 1 h or >24 h old (Fig. 1), whereas there was no difference in worker behavior toward corpses that were 1 h or >24 h old. Furthermore, although workers also promptly removed small inanimate objects (pieces of filter paper) dropped into the nest, the behavioral responses to paper fragments were different from those elicited by ant corpses, with the papers simply being dropped outside the nest entrance rather than being carried to the refuse pile.
Fig. 1.
Responses of Argentine ant workers to dead ants and inanimate objects. (A) Box plots of the times between introduction and removal of different test items from the nest. Boxes are bounded by the first quartile, median, and third quartile; whiskers (error bars) above and below the box indicate the 90th and 10th percentiles; and points outside the whiskers are outliers. A group of 10 items was observed for 15 min after introducing the items into the nest entrance. Freshly killed ants were removed more slowly by workers than other test items (Kruskal-Wallis ANOVA for the 4 groups: H = 40.6, df = 3, P < 0.0001). Removal times for corpses 1 h and >24 h old and pieces of filter paper were not significantly different at α = 0.05. All the dead ants were eventually carried to refuse piles, whereas the filter papers were dropped around the nest entrance. (B) A typical necrophoric response of workers to a nestmate corpse. (Scale bar, 1 mm.)
L. humile workers readily picked up and retrieved their own pupae when pupae were placed in the foraging arena, returning them to the nest. This innate behavior was exploited in a bioassay in which we applied extracts of worker ant corpses to pupae to determine whether chemical cues on ants that were dead for 1 h elicited necrophoric behaviors. When extracts of ants that were dead for 1 h were applied to live pupae, workers carried 90% (18 of 20) of the treated pupae to the refuse pile (Fig. 2), whereas all (20 of 20) the solvent-treated control pupae were promptly carried into the nest. In contrast, only 2 of 20 pupae treated with extracts of freshly killed ants were carried to the refuse pile (Fisher's exact test between fresh-killed and 1-h-old dead ant extracts; P < 0.0001, one-tailed), with the remaining 18 treated pupae being left where they had been placed initially, without being returned to the nest. Overall, these bioassays confirmed that solvent-extractable chemicals on dead ants mediated the necrophoric response.
Fig. 2.
Worker responses to live pupae treated with cuticular extracts from 1-h-old dead ants. (A) A worker carrying a pupa to the refuse pile. (Scale bar, 1 mm.) (B) Live pupae treated with 1-h-old dead ant extract being carried to the refuse pile (arrows show locations of 9 pupae). (Scale bar, 10 mm.)
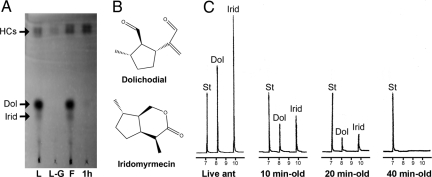
TLC analyses of extracts of live, freshly killed, and 1-h-old dead ants showed that a subset of cuticular chemicals disappeared within 1 h after death (Fig. 3A). This was verified by analyzing extracts of small groups of ants (n = 15) from different nest origins by coupled GC-MS. These analyses confirmed that extracts of live ants contained substantial amounts of trans,trans-dolichodial (referred to as “peruphasmal” in ref. 13) and cis,trans-iridomyrmecin, whereas these compounds were virtually absent in extracts of 1-h-old dead ants (Fig. 3B). These 2 compounds also were not found in cuticular extracts of pupae.
Fig. 3.
Chemical analyses of ant extracts. (A) TLC plate showing differences in cuticular extracts of live and dead L. humile workers at different times post mortem. L, live ant; L-G, live ant without gaster; F, freshly killed ant; 1h, 1-h-old dead ant. Hydrocarbons (HCs) migrated to the top of the plate. Dolichodial (Dol) and iridomyrmecin (Irid) were found in extracts of live or freshly killed ants but not in extracts from 1-h-old corpses. The absence of Dol and Irid in L-G indicates that these compounds originated from the gasters of workers. All extracts were made from 30 ants extracted in 0.3 mL of methylene chloride, with 10 μL of each extract being applied to the silica gel TLC plate. (B) Chemical structures of dolichodial and iridomyrmecin from Argentine ants, with relative configurations as shown (15, 32). The absolute configurations were not determined. (C) Gas chromatogram showing dissipation/degradation of dolichodial (Dol) and iridomyrmecin (Irid) on the body of ants (split injection, DB-5 column). Extracts were prepared by extracting live or dead ants (n = 30) at different times post mortem (10, 20, 30, 40, 50, and 60 min old) in 0.3 mL of methylene chloride. The amount of Dol and Irid decreased quickly after death, resulting in >50% reduction within the first 10 min after death. The compounds were not detected in extracts of 40-min-old dead ants, whereas the internal standard (St, n-dodecane) in the solvent remained unchanged. Retention times are expressed in minutes.
To assess the stability and/or volatility of dolichodial and iridomyrmecin, aliquots of the dolichodial/iridomyrmecin fraction were placed on glass coverslips held under ambient conditions. Both compounds were recovered when the coverslips were extracted immediately following deposition and evaporation of the aliquots, but neither compound was detectable in extracts of the coverslips made 1 h later. A more detailed study of the time course of disappearance of these 2 chemicals from ant cuticle was made by extracting workers at different times post mortem. Analyses of the extracts by GC indicated that the amount of dolichodial and iridomyrmecin on the body surface began to decrease immediately after death, reaching <50% of the original amount in 10 min (i.e., 40% and 32% for dolichodial and iridomyrmecin, respectively). Forty minutes after death, neither compound was detectable in extracts (Fig. 3C). However, the titers of dolichodial and iridomyrmecin stored internally in the pygidial glands of 1-h-old corpses were similar to those of freshly killed ants.
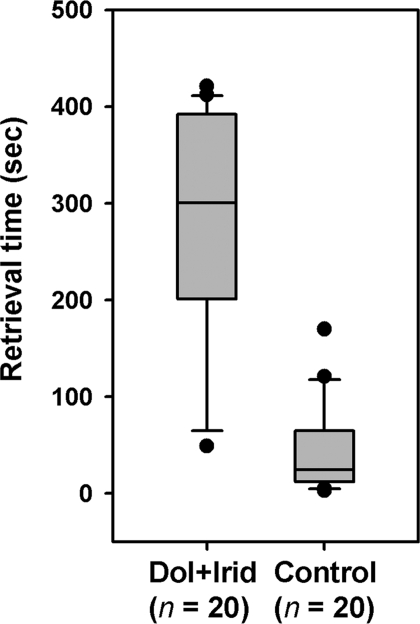
To examine the effects of these chemicals on worker behavior, a fraction containing dolichodial and iridomyrmecin was isolated from extracts of freshly killed ants by TLC and applied to pupae. Less than 5 ant-equivalents of the dolichodial/iridomyrmecin fraction applied to a pupa significantly delayed its retrieval (Fig. 4). Workers initially investigated treated pupae or, more often, simply ignored them (Movie S1) before eventually carrying all the treated pupae into the nest, as they did with control pupae. These data supported the results of the first and second bioassays and suggested that the relatively high titer of dolichodial/iridomyrmecin on freshly killed ants (or treated pupae) was responsible for their delayed removal by nestmates.
Fig. 4.
Box plots of retrieval times for pupae treated with the dolichodial/iridomyrmecin (Dol+Irid) fraction or solvent controls. Five pupae were observed in each replicate until all had been removed. Workers took longer to retrieve pupae treated with Dol+Irid than solvent-treated controls (Wilcoxon rank-sum test: z = 4.96, P < 0.0001).
Other groups of compounds in the ant extracts were isolated and tested for their possible effect on worker behavior by applying them to pupae. Extracts of 1-h-old corpses (composite extract of 2,149 workers) contained larger amounts of hydrocarbons than did extracts of freshly killed ants (composite extract of 2,296 workers) (see fraction a in Fig. S2). However, all the pupae (n = 10) treated with the hydrocarbon fraction from either 1-h-old corpses or freshly killed ants were carried into the nest within 25 min, indicating that the hydrocarbons of dead ants did not affect retrieval of pupae by workers. In contrast, for both freshly killed and 1-h-old corpse extracts, the compounds eluting between the hydrocarbons and the dolichodial/iridomyrmecin fraction on TLC (see fraction b in Fig. S2) elicited aggressive behaviors from workers, including biting and spread-eagling (Movie S2). In the bioassays using the fraction from extracts of 1-h-old corpses, all the treated pupae were carried to the refuse pile within 79 min in the first trial (n = 10) and within 168 min in the second trial (n = 10). NMR spectroscopy analysis indicated that this fraction consisted primarily of triglycerides, which are the major internal lipid components of most insects (14). The triglyceride fraction isolated from crushed live ants was also very effective in causing workers to attack the treated pupae (n = 10) vigorously and carry them to refuse piles within 10 min.
Discussion
Our study showed that the chemical stimuli that elicit necrophoric behavior by L. humile workers are continually present, but additional chemicals associated with living workers inhibit this behavior. Shortly after death, these inhibitory chemicals dissipate or are degraded, allowing the latent necrophoric behavior to be triggered. In distinct contrast to previous hypotheses, our results demonstrate that the decrease in chemical compounds associated with living ants, rather than an increase in compounds associated with decomposition of dead ants, is the crucial cue that elicits necrophoric behavior. Dolichodial and iridomyrmecin are produced and stored in the pygidial gland of L. humile (15, 16), and this gland occurs in all ant subfamilies except the Formicinae, where it may have been lost secondarily (17, 18). Volatile chemicals produced by the pygidial glands of dolichoderine and myrmicine ants are commonly thought to have alarm or defensive functions (19–21), and the less volatile constituents such as iridodials, dolichodials, and related compounds were assumed to retard the evaporation of the more volatile repellent constituents (18, 20) or to act as viscous defensive agents (20–22). However, to date, the possible functions of the pygidial gland chemicals of L. humile have been largely ignored, because L. humile workers do not display alarm reactions to crushed workers (19, 23).
Our results showed that the iridomyrmecin/dolichodial fraction inhibited the grasping and removal behaviors typical of necrophoresis by L. humile workers. The intraspecific function of these semiochemicals has parallels with other biological systems, because there are several precedents for these chemicals being used to suppress ant aggression. For example, iridomyrmecin-like compounds are found in the mandibular gland secretion of the aphid hyperparasitoid Alloxysta brevis, discouraging aggression by aphid-tending ants (Lasius sp.) (24). Furthermore, iridodials and iridomyrmecin in the pygidial gland secretion of Tapinoma sp. ants suppress aggression by other ant species when individuals are daubed with the secretion (22). Iridomyrmecin is also known to have an insecticidal knockdown effect (25).
Although we were not able to determine exactly how dolichodial and iridomyrmecin on the cuticle of live or freshly killed L. humile dissipated within 1 h post mortem, both dolichodial and iridomyrmecin disappeared from the cuticle of dead worker ants that were held under ambient conditions for 1 h, suggesting that the 2 chemicals degrade or evaporate quickly. Dolichodial is known to be particularly reactive, being readily oxidized and polymerized when exposed to air (15, 16). The chemical changes or deterioration of dolichodial/iridomyrmecin was limited to the amounts on the ants' cuticular surfaces at death, because the titers of these 2 chemicals stored internally in the pygidial glands remained unchanged over the first hour after death.
Physical impediments caused by inanimate objects placed in or near an ant colony elicit removal responses by ant workers (3, 7, 26, 27). However, the responses to such “clean” objects are markedly different from responses elicited by the same objects when they have been treated with chemicals such as fatty acids, diglycerides, and triglycerides, with the treated objects eliciting carrying behaviors more rapidly and being carried over greater distances (3, 26, 27). In addition, our results showed that the triglyceride fraction from extracts of live or recently killed ants released aggressive as well as necrophoric responses, suggesting that workers do not carry live nestmates to a refuse pile, because live nestmates have chemical signals that mask or inhibit chemical stimuli that, by themselves, elicit aggression. Furthermore, our results may explain previous bioassay results (7) that appeared to contradict the fatty acid decomposition product hypothesis, in which 92% (22 of 24) of live ants treated with a “drop” of pure oleic acid were antennated and then ignored, without being carried to refuse piles by nestmates.
Recognition of dead nestmates based on the rapid disappearance of a chemical vital sign has several distinct advantages over recognition systems based on increasing titers of compounds associated with death. Ants can recognize and remove dead nestmates quickly before substantial decomposition of the corpse occurs, thus limiting the risk of infection of the colony by pathogens from the corpse. Various insect pathogens are commonly found on or in workers (28), and worker corpses that remain in the nest represent a continuing threat to the nest, particularly because frequent contact between infected and uninfected individuals in the close confines of the nest would accelerate the dissemination of the pathogen (29). This threat is exacerbated by the high humidity and relatively stable temperature inside the nest that enhance growth of microbial pathogens in the dead ants (2).
In addition, the use of signals associated with life allows workers to distinguish live and dead ants without confusion, particularly if live foragers become contaminated with fatty acids, glycerides, or other common chemical constituents that may be shared by both conspecifics and various plant seeds or prey items. We suggest that analogous chemical vital signs may occur in other ant species, given that various related iridoids are found in the pygidial gland secretions of many dolichoderine and myrmicine ants (18).
Materials and Methods
Study Animals.
L. humile colonies were collected from a citrus grove on the University of California, Riverside campus. Large laboratory colonies were maintained in plastic boxes (26.5-cm × 30-cm × 10-cm height), and each colony box was provided with 2 or 3 artificial nests constructed from plaster-filled Petri dishes (9-cm diameter × 1.5-cm depth) formed with a cylindrical area (5-cm diameter × 1-cm depth) in the center of the dish to serve as a nesting space (30). Colonies were provisioned with water, 25% (wt/vol) sucrose in water, dead western drywood termites (Incisitermes minor), and dead American cockroaches (Periplaneta americana).
Round Experimental Arena.
The conical end of a 50-mL polypropylene culture tube was connected to a round plastic container (19-cm diameter × 8-cm depth) with vinyl tubing (0.6-cm diameter × 5-cm length) (Fig. S1). The round container and conical tube comprised the foraging arena and nest, respectively. About half of the workers, queens, and brood from the larger laboratory colony were anesthetized with CO2 and placed in the foraging arena (the other half of the colony was used for preparation of extracts or as a source of pupae). As the ants recovered from anesthesia, they moved into the conical tube nest with their brood (eggs, larvae, and pupae) within ≈1 h.
Responses to Dead Ants and Inanimate Objects.
Ants (≈100) were anesthetized with CO2 and subsequently killed by freezing them in dry ice for 1 min. To examine worker responses to freshly killed ants, the frozen ants were tested immediately after being left at room temperature for 2 min to thaw out. Dead ants tested at different periods post mortem were stored in covered Petri dishes until used for bioassays (22–27 °C, 20–28% relative humidity). To examine worker responses to inanimate objects encountered in the nest, clean pieces of filter paper (1 mm × 3 mm) were used as test objects. Ten items were dropped into the nest entrance of the foraging arena, and the time intervals between introduction and removal of the items were recorded. Data were collected for 15 min after introduction, and each trial was replicated 1 to 3 times. Removal times were compared using Kruskal-Wallis one-way ANOVA (31).
Responses to Extracts of Ants.
Ants [≈100 pupae and 800 workers (337 and 346 workers were used for freshly killed and 1-h-old dead ant extracts, respectively)] were collected from colonies anesthetized with CO2. Workers were killed, and the corpses were aged using the methods described previously. The dead ants were loaded into a glass pipette plugged with glass wool and briefly extracted (5–10 sec) by eluting the pipette with 1–1.5 mL of methylene chloride. The extracts were transferred to a 2-mL glass vial, and the solvent was removed under a flow of N2. Thirty pupae then were placed in the vial and gently moved around with a fine brush, allowing each pupa to pick up a similar amount of chemical on its cuticle. For controls, 30 pupae were treated in the same fashion in a vial in which an identical volume of solvent had been evaporated. For bioassays, 10 treated or control pupae were placed in the foraging arena, and the fate of each pupa was recorded. Data were collected for 30 min after introduction, and each trial was replicated twice.
Responses to Fraction Containing Dolichodial and Iridomyrmecin.
A methylene chloride extract (brief extraction for <30 sec) of 1.0 g of freshly killed workers (≈2,200 individuals) was subjected to flash liquid chromatography (0.4-cm diameter × 27-cm long column packed with 230–400 mesh silica gel) by successive elution with hexane and ethyl acetate. The ethyl acetate fraction was further fractionated by preparative silica gel TLC (5 × 20 cm, 250-μm layer) eluting with hexane/ethyl acetate (3:1, vol:vol). Purified fractions containing dolichodial and iridomyrmecin were taken up in methylene chloride at a concentration such that 5-μL aliquots applied to the inner surface of a glass test tube bottom (12-mm diameter × 75-mm depth) provided 25 ant-equivalents. Five pupae were coated with the fraction in the glass tube, as described previously. The treated pupae were positioned at the center of a circular paper disk (9-cm diameter) placed in the larger colony box, and the retrieval times from the disk were recorded. Each trial was replicated 4 times. The retrieval times for test and control treatments were compared using the Wilcoxon rank-sum test (31).
Responses to Other Fractions.
Fractions containing hydrocarbons or triglycerides were isolated from methylene chloride extracts of 1.0 g of workers using flash liquid chromatography and TLC as described in the previous section. Both fractions initially were bioassayed using the method described in the section on responses to extracts of ants. Worker responses to the fraction containing triglycerides were further examined by using the method described in the section on responses to fraction containing dolichodial and iridomyrmecin, whereby 5 ant-equivalents were applied to each test pupa and the treated pupae were bioassayed. Internal triglycerides were extracted from 1.1 g of ants squashed in methylene chloride and subsequently purified by flash liquid chromatography and preparative TLC.
Chemical Analyses.
Dead ants (n = 30 and n = 15 for TLC and GC analyses, respectively) at different times post mortem (freshly killed and 1 h post mortem) were briefly (5–10 sec) extracted in a glass pipette plugged with glass wool using 0.3 mL of methylene chloride. For live ant extracts (with or without gaster), ants were anesthetized with CO2 and immediately extracted. Live (n = 30) or freshly killed (n = 30, killed by freezing in dry ice for 1 min) pupae were also extracted in the same manner. As an internal standard (0.05 mg mL−1) before extraction, n-dodecane was added to the extraction solvent. TLC plates were developed with hexane/ethyl acetate (3:1, vol:vol), and the spots were visualized by spraying with 5% (wt/vol) phosphomolybdic acid in ethanol, followed by heating with a heat gun. GC analyses were conducted with a Hewlett-Packard 5890 gas chromatograph equipped with a DB-5 30-m × 0.25-mm i.d. column (J&W Scientific), using helium carrier gas. The instrument was programmed from 40 to 280 °C at 15 °C min−1. Extracts also were analyzed by coupled GC-MS, in which electron impact mass spectra (70 eV) were taken with a Hewlett-Packard 5973 mass selective detector interfaced to a Hewlett-Packard 6890 gas chromatograph fitted with a Hewlett-Packard 5MS 30-m × 0.25-mm i.d. column. Extracts were analyzed in both splitless and split modes, with a temperature program of 40 °C for 1 min and then 10 °C min−1 to 280 °C and injector and transfer line temperatures of 250 °C and 280 °C, respectively. Compounds in the extracts were identified by comparison of retention times and mass spectra with those of authentic standards of natural or synthetic origin (dolichodial: A.T. Dossey, University of Florida, Gainesville, FL; iridomyrmecin: K.R. Chauhan, US Department of Agriculture-Agricultural Research Service, Beltsville, MD). The relative configurations of dolichodial (15) and iridomyrmecin (32) produced by Argentine ants have been unequivocally determined by previous researchers, but the absolute configurations remain unknown. 1H NMR spectra of the triglyceride fractions were obtained as CDCl3 solutions on a Varian INOVA-400 instrument equipped with a NALORAC 5-mm 4-nucleus gradient probe.
Dissipation/Degradation of Dolichodial and Iridomyrmecin.
Approximately 1 ant-equivalent of the purified dolichodial/iridomyrmecin mixture (extracted and purified from freshly killed ants as described previously) in 10 μL of methylene chloride was spotted onto each of 6 glass coverslip pieces (3 mm × 5 mm). After the solvent evaporated, 3 of the treated glass pieces (controls) were immediately extracted with 50 μL of methylene chloride containing n-dodecane as an internal standard. The other 3 were aged for 1 h on the laboratory bench under ambient conditions (23 °C, 36% RH) and subsequently extracted with 50 μL of the methylene chloride solution (treatment). All the extracts were analyzed by GC to compare the amounts of dolichodial/iridomyrmecin between control and 1-h aged treatments.
To examine the dissipation of dolichodial and iridomyrmecin from the cuticle, live ants or dead ants (n = 30) at different times post mortem (10, 20, 30, 40, 50, and 60 min) were briefly (5–10 sec) extracted using 0.3 mL of methylene chloride containing n-dodecane as an internal standard, and the extracts were analyzed by GC. Internally stored pygidial gland secretions of freshly killed (n = 30) and 1-h-old (n = 30) corpses were collected in micropipettes by taking up the droplets of secretion from the pygidium while gently milking the gland reservoir by depressing the preceding tergum (20). The secretions were dissolved in 0.5 mL of methylene chloride and quantified by TLC and GC.
Supplementary Material
Acknowledgments.
We thank L. Greenberg, J. Klotz, J. Silverman, and P. Kirk Visscher for reading an earlier version of the manuscript. We thank A.T. Dossey for providing dolichodial standards and K.R. Chauhan for providing iridomyrmecin standards. This work was partially funded by a Carl Strom/Western Exterminator Scholarship, a Pi Chi Omega Scholarship, and a Bayer Young Scientist of the Year 2008 Scholarship (to D.-H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901270106/DCSupplemental.
References
- 1.Allen C, Hauser MD. Chapter 4. Concept Attribution in Nonhuman Animals: Theoretical and Methodological Problems in Ascribing Complex Mental Processes. In: Bekoff M, Jamieson D, editors. Readings in Animal Cognition. Cambridge, MA: MIT Press; 1999. pp. 47–62. [Google Scholar]
- 2.Oi DH, Pereira RM. Ant behavior and microbial pathogens (Hymenoptera: Formicidae) Florida Entomologist. 1993;76:63–74. [Google Scholar]
- 3.Wilson EO, Durlach NI, Roth LM. Chemical releasers of necrophoric behavior in ants. Psyche. 1958;65:108–114. [Google Scholar]
- 4.Visscher PK. The honey bee way of death: Necrophoric behaviour in Apis mellifera colonies. Anim Behav. 1983;31:1070–1076. [Google Scholar]
- 5.Blum MS. The Chemical Basis of Insect Sociality. In: Beroza M, editor. Chemicals Controlling Insect Behavior. New York: Academic; 1970. pp. 61–94. [Google Scholar]
- 6.Haskins CP, Haskins EF. Notes on necrophoric behavior in the archaic ant Myrmecia vindex (Formicidae: Myrmeciinae) Psyche. 1974;81:258–267. [Google Scholar]
- 7.Gordon DH. Dependence of necrophoric response to oleic acid on social context in the ant, Pogonomyrmex badius. J Chem Ecol. 1983;9:105–111. doi: 10.1007/BF00987774. [DOI] [PubMed] [Google Scholar]
- 8.Howard DF, Tschinkel WR. Aspects of necrophoric behavior in the red imported fire ant, Solenopsis invicta. Behaviour. 1976;56:157–180. [Google Scholar]
- 9.López-Riquelme GO, Malo EA, Cruz-López L, Fanjul-Moles ML. Antennal olfactory sensitivity in response to task-related odours of three castes of the ant Atta mexicana (Hymenoptera: Formicidae) Physiol Entomol. 2006;31:353–360. [Google Scholar]
- 10.Agosta W. Thieves, Deceivers and Killers: Tales of Chemistry in Nature. Princeton: Princeton Univ Press; 2001. [Google Scholar]
- 11.Purnamadjaja AH, Russell RA. Pheromone communication in a robot swarm: Necrophoric bee behaviour and its replication. Robotica. 2005;23:731–742. [Google Scholar]
- 12.Kennedy J, Eberhart RC, Shi Y. Swarm Intelligence. Inc., San Francisco: Morgan Kaufmann Publishers; 2001. [Google Scholar]
- 13.Dossey AT, Walse SS, Rocca JR, Edison AS. Single insect NMR: A new tool to probe chemical biodiversity. ACS Chem Biol. 2006;1:511–514. doi: 10.1021/cb600318u. [DOI] [PubMed] [Google Scholar]
- 14.Beenakkers AMT, Van der Horst DJ, Van Marrewijk WJA. Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res. 1985;24:19–67. doi: 10.1016/0163-7827(85)90007-4. [DOI] [PubMed] [Google Scholar]
- 15.Cavill GWK, Houghton E, McDonald FJ, Williams PJ. Isolation and characterization of dolichodial and related compounds from the Argentine ant, Iridomyrmex humilis. Insect Biochem. 1976;6:483–490. [Google Scholar]
- 16.Cavill GWK, Houghton E. Volatile constituents of the Argentine ant, Iridomyrmex humilis. J Insect Physiol. 1974;20:2049–2059. doi: 10.1016/0022-1910(74)90112-7. [DOI] [PubMed] [Google Scholar]
- 17.Jackson BD, Morgan ED. Insect chemical communication: Pheromones and exocrine glands of ants. Chemoecology. 1993;4:125–144. [Google Scholar]
- 18.Davidson DW, Clark DA, Jones TH. Gastral exocrine products of a myrmicine ant strongly overlap pygidial gland products of Dolichoderinae. Insectes Sociaux. 2005;52:305–308. [Google Scholar]
- 19.Wilson EO, Pavan M. Glandular sources and specificity of some chemical releasers of social behavior in dolichoderine ants. Psyche. 1959;66:70–76. [Google Scholar]
- 20.Kugler C. Alarm and defense: A function for the pygidial gland of the myrmicine ant, Pheidole biconstricta. Ann Entomol Soc Am. 1979;72:532–536. [Google Scholar]
- 21.Hefetz A, Lloyd HA. Identification of new components from anal glands of Tapinoma simrothi pheonicium. J Chem Ecol. 1983;9:607–613. doi: 10.1007/BF00990412. [DOI] [PubMed] [Google Scholar]
- 22.Tomalski MD, et al. Chemistry and functions of exocrine secretions of the ants Tapinoma melanocephalum and T. erraticum. J Chem Ecol. 1987;13:253–263. doi: 10.1007/BF01025886. [DOI] [PubMed] [Google Scholar]
- 23.Van Vorhis Key SE, Gaston LK, Baker TC. Effects of gaster extract trail concentration on the trail following behaviour of the Argentine ant, Iridomyrmex humilis (Mayr) J Insect Physiol. 1981;27:363–370. [Google Scholar]
- 24.Völkl W, Hübner G, Dettner K. Interactions between Alloxysta brevis (Hymenoptera, Cynipoidea, Alloxystidae) and honeydew-collecting ants: How an aphid hyperparasitoid overcomes ant aggression by chemical defense. J Chem Ecol. 1994;20:2901–2915. doi: 10.1007/BF02098397. [DOI] [PubMed] [Google Scholar]
- 25.Cavill GWK, Clark DV. Chapter 7. Ant Secretions and Cantharidin. In: Jacobson M, Crosby DG, editors. Naturally Occurring Insecticides. New York: Marcel Dekker; 1971. pp. 271–305. [Google Scholar]
- 26.Skidmore BA, Heithaus ER. Lipid cues for seed-carrying by ants in Hepatica americana. J Chem Ecol. 1988;14:2185–2196. doi: 10.1007/BF01014024. [DOI] [PubMed] [Google Scholar]
- 27.Brew CR, O'Dowd DJ, Rae ID. Seed dispersal by ants: Behaviour-releasing compounds in elaiosomes. Oecologia. 1989;80:490–497. doi: 10.1007/BF00380071. [DOI] [PubMed] [Google Scholar]
- 28.Baird R, Woolfolk S, Watson CE. Survey of bacterial and fungal associates of black/hybrid imported fire ants from mounds in Mississippi. Southeastern Naturalist. 2007;6:615–632. [Google Scholar]
- 29.Kelley-Tunis KK, Reid BL, Andis M. Activity of entomopathogenic fungi in free-foraging workers of Camponotus pennsylvanicus (Hymenoptera: Formicidae) J Econ Entomol. 1995;88:937–943. [Google Scholar]
- 30.Hooper-Bui LM, Rust MK. Oral toxicity of abamectin, boric acid, fipronil, and hydramethylnon to laboratory colonies of Argentine ants (Hymenoptera: Formicidae) J Econ Entomol. 2000;93:858–864. doi: 10.1603/0022-0493-93.3.858. [DOI] [PubMed] [Google Scholar]
- 31.Analytical Software. STATISTIX 9 User's Manual. Tallahassee, FL: Analytical Software; 2008. [Google Scholar]
- 32.McConnell JF, Mathieson AM, Schoenborn BP. Conformation of iridomyrmecin and isoiridomyrmecin. Tetrahedron Lett. 1962;10:445–448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.