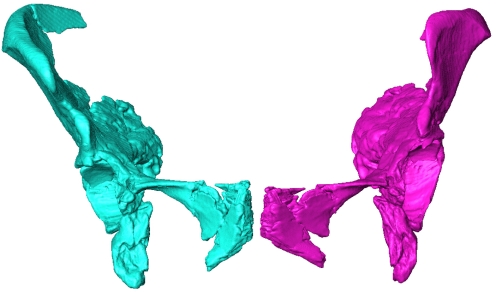Abstract
Childbirth is complicated in humans relative to other primates. Unlike the situation in great apes, human neonates are about the same size as the birth canal, making passage difficult. The birth mechanism (the series of rotations that the neonate must undergo to successfully negotiate its mother's birth canal) distinguishes humans not only from great apes, but also from lesser apes and monkeys. Tracing the evolution of human childbirth is difficult, because the pelvic skeleton, which forms the margins of the birth canal, tends to survive poorly in the fossil record. Only 3 female individuals preserve fairly complete birth canals, and they all date to earlier phases of human evolution. Here we present a virtual reconstruction of a female Neandertal pelvis from Tabun, Israel. The size of Tabun's reconstructed birth canal indicates that childbirth was about as difficult in Neandertals as in present-day humans, but the canal's shape indicates that Neandertals had a more primitive birth mechanism. A significant shift in childbirth apparently occurred quite late in human evolution, during the last few hundred thousand years. Such a late shift underscores the uniqueness of human childbirth and the divergent evolutionary trajectories of Neandertals and the lineage leading to present-day humans.
Keywords: climate, geometric morphometrics, obstetrics, pelvis, virtual reconstruction
Because of the need to shorten the anteroposterior distance between the sacrum and the acetabulum for efficient bipedal gait, humans and other hominins are the only primates for which the pelvic inlet, or entrance to the birth canal, is larger transversely (mediolaterally) than anteroposteriorly (1, 2). Further down the birth canal, at the pelvic midplane and outlet, humans, like other primates, have larger anteroposterior dimensions. The result is a twisted birth canal in humans, in which the largest dimension is first transverse and then anteroposterior (1, 2). Because of the constricted human birth canal, the neonate must be oriented so that the largest dimensions of its head and shoulders align with the most spacious parts of the birth canal to be able pass through it successfully. Consequently, a human neonate enters the birth canal facing sideways, so that its larger anteroposterior head dimensions match up with the wider transverse dimensions of the inlet. On entering the midplane, the neonate rotates so that its head length is aligned anteroposteriorly, and continues in this way until it exits the outlet. One final rotation then occurs so that the neonate's shoulders can pass anteroposteriorly although the midplane and outlet. Typically, the neonate exits the birth canal facing behind its mother, because its occiput tends to pass alongside the outlet's more spacious anterior part (1, 2).
To investigate the evolution of human childbirth, we reconstructed the size and shape of a Neandertal birth canal. For simplicity, here we use “human” to refer to present-day humans and fossil specimens more closely related to present-day humans than to Neandertals. Our Neandertal birth canal reconstruction is based on the fragmentary pelvic remains of the Tabun C1 skeleton that was discovered during Garrod's 1929–1934 excavation of a site at Mugharet et-Tabun, Israel (3). Fragments of Tabun's left pubis and ilium and right pubis, ischium, and ilium have been preserved. Whether the skeleton originates from archaeological layer C or layer B is uncertain; thus, its geologic age could be closer to ≈60,000 or ≈100,000 years ago (3–5). Although the skeleton's exact age is somewhat in doubt, there is broad consensus regarding its Neandertal taxonomic designation and female sex (6, 7). The Tabun pelvis was originally described and partially reconstructed by McCown and Keith in 1939 (8). Later, Ponce de León, et al. (9) attempted another reconstruction, but they assumed a priori that Neandertals had a similar birth mechanism and cephalopelvic proportions as humans when making their reconstruction, which precludes using their work to assess whether in fact this is the case. They claimed that the preservation of the specimen forced them to make these assumptions (9), but, using different methods, we found that these assumptions are not required.
Results
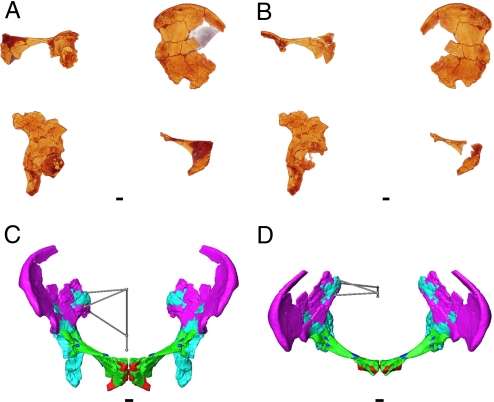
In brief, we created our virtual reconstruction (Fig. 1) as follows. We obtained computed tomography (CT) scans of the original pelvic fragments; virtually disassembled parts reconstructed by McCown and Keith and separated the femoral head from the right acetabulum; fit together right- and (mirrored) left-sided fragments by matching overlapping anatomy both manually and using a surface alignment computer algorithm; estimated sacral and other missing anatomical landmarks using an expectation-maximization (E-M) computer algorithm; oriented the right hemipelvis in the standard anatomical position; and mirrored about the midline to produce the left side.
Fig. 1.
Virtual reconstruction of the Tabun pelvis. Original fragments (A) and after mirroring of the left-sided ilium and acetabular-pubic fragments and segmentation (B). Colors reflect higher (yellow) to lower (red) density. Anterior (C) and anterosuperior (D) views of the completed reconstruction. Each fragment is shown in a different color. The spheres indicate the locations of sacral landmarks, which are connected by links to create a stick representation of the form of the estimated sacrum. Sacral landmark definitions are given in Table 1. (Scale bar = 1 cm.)
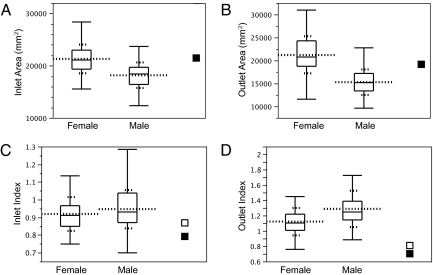
Because adequate maternal pelvic areas are crucial for successful childbirth (10), we first compared these dimensions in Tabun and a comparative sample of humans (Fig. 2 A and B). Tabun's inlet area (21,485 mm2, based on anteroposterior and transverse diameters of 104 mm and 131 mm, respectively) is nearly the same as the human female mean inlet area, and Tabun's outlet area (19,176 mm2, based on anteroposterior and transverse diameters of 93 mm and 132 mm, respectively) is slightly smaller than the human female mean outlet area, but well within 1 SD of it.
Fig. 2.
Pelvic metrics: inlet area (A), outlet area (B), inlet index (C), and outlet index (D). The long dashed line represents the mean, and the shorter dashed lines represent SDs. The line in the middle of the box is the median, the box ends span the 25th–75th quantiles, and the whiskers denote the range. The filled-in square represents Tabun; the open square represents the hypothetical female based on Kebara.
Although Tabun and humans have similar pelvic areas, their birth canal shapes differ considerably (Fig. 2 C and D). Both female and male humans typically have transversely oval inlets (pelvic inlet index <1) and anteroposteriorly oval outlets (pelvic outlet index >1), but human females tend to have lower inlet and outlet indices than males (Fig. 2 C and D). Tabun has quite a low inlet index (0.79) and an extremely low outlet index (0.70) compared with both female and male humans. Tabun's outlet index is completely outside the range of variation of that of our comparative sample of 231 humans. Unlike humans, who have a anteroposteriorly oval outlet, Tabun has a transversely oval outlet. Tabun's midplane also appears to be transversely oval, but poor preservation of the ischial spine leaves open the possibility that it is round or perhaps anteroposteriorly oval. Nevertheless, because neonatal rotations are mediated by physical resistance within the birth canal, this transversely oval outlet indicates that Tabun has a different birth mechanism than humans. On reaching the outlet, a neonate passing through Tabun's birth canal would align its anteroposterior head dimensions transversely, leading to an occiput transverse exit position.
How secure is this description of Tabun's birth mechanism given the various reconstruction steps? One possible source of error is the estimation of the sacral landmarks. To evaluate the magnitude of this error, we estimated sacral landmarks for each individual in our comparative sample of humans using the same methods that we applied to Tabun. We then compared the outlet dimensions based on the estimated sacral landmarks with the actual dimensions. Adding the median error (0.10) to Tabun's outlet index gives a value of 0.80, which is still very low. In addition, given the error distribution, we investigated what percentage of the comparative sample had errors of sufficient magnitude and direction (from higher to lower indices) to generate Tabun's outlet index if the actual shape were round (outlet index = 1). We chose a round outlet as the starting point, because it has the lowest index that does not imply an occiput transverse exit position. Only <3% of the comparative sample exhibit errors capable of producing a round outlet from Tabun's outlet index. If anything, this percentage an overestimate, because the multiple regression step of the E-M algorithm tends to make outlying cases such as Tabun more, not less, similar to the mean human outlet shape.
Another possible source of error is the orientation of the pubis fragment relative to the rest of the acetabulum, ischium, and ilium. We used 4 anatomical relationships to check the accuracy of this alignment. First, the contours of the arcuate line match up closely across the different fragments [Fig. 1 C and D, supporting information (SI) Fig. S1A]. Second, the base of the ischial tuberosity appears to align well with the pubic body (Fig. S1B). Third, the pubic symphyseal faces lie in a plane very close to sagittal (Fig. 1D). If the pubic fragment were to be rotated to produce a more gently curving arcuate line, then the sacrum would need to be wider than currently reconstructed to maintain the pubic symphyseal faces in a roughly sagittal plane, resulting in increased transverse birth canal dimensions. Fourth, the pubic part and the rest of of the acetabulum align well both superoinferiorly and anteroposteriorly (Fig. S1C). We attempted to create a transversely narrower outlet by rotating the ischial tuberosity fragment medially in a coronal plane relative to the pubic fragment, but found that even small changes cause the pubic section of the acetabular rim to pull apart superiorly from the other parts of the rim or lead to mismatching of the arcuate line. The allowable changes move the ischial tuberosity medially by a few millimeters, which is much less than the 2 cm on each side required to produce a round outlet (Fig. S1D).
Unfortunately, in no other female Neandertal is enough of the pelvis preserved to allow reconstruction of the birth canal. However, we further checked our findings for Tabun against a well-preserved male Neandertal pelvis from Kebara, Israel (11) (see SI Text and Fig. S2 for details about this specimen). To do this, we investigated, assuming the same pelvic sexual dimorphism in Neandertals and humans, what the dimensions of a female Neandertal pelvis would be based on Kebara's shape. More specifically, we created a hypothetical female Neandertal pelvis by overlaying human patterns of sexual dimorphism on Kebara's shape. Importantly, our Tabun reconstruction is not based on Kebara in any way, so that results from Tabun and the hypothetical female are completely independent of each other. The hypothetical female has a transversely oval inlet (Fig. 2C) and, like Tabun and unlike humans, a transversely oval outlet (Fig. 2D). Kebara has a transversely oval outlet, and human females have lower outlet indices than males (Fig. 2D), so a hypothetical female based on Kebara necessarily has an even more transversely oval outlet than Kebara (12). The pelvic shapes of Tabun and Kebara are consistent in indicating that Neandertal females had transversely oval outlets.
The hypothetical female based on Kebara has similar pelvic indices as Tabun, but these indices reflect only birth canal shape. In other aspects of pelvic shape, the hypothetical female differs noticeably from Tabun (Fig. 3), indicating that Tabun and Kebara do not follow average patterns of sexual dimorphism seen in humans. For example, compared with Tabun, the hypothetical female has a longer pubic bone (relative to other pelvic dimensions), a wider pubic body, a less laterally flared iliac blade, and a more medially pointing anterosuperior iliac spine (Fig. 3). Importantly, although Tabun has a long pubic bone in absolute dimensions (6), the hypothetical female shows that Tabun's pubic length actually is shorter than would be expected if Neandertals followed human patterns of sexual dimorphism.
Fig. 3.
Anteroinferior view of Tabun and the hypothetical female based on Kebara. Tabun is shown in cyan, and the shape produced by warping Tabun to match the landmark configuration of the hypothetical female is shown in magenta. Both innominates are approximately in standard anatomical position. The figure depicts shape, which is scale-free.
Discussion
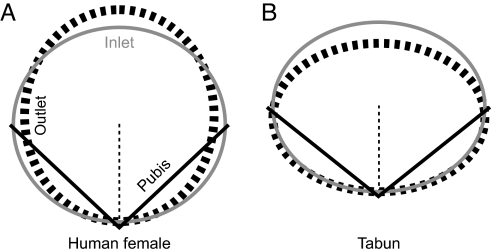
If we assume that the pelvic shapes of Tabun and Kebara are representative of Neandertal female and male averages, respectively, why would Neandertals and humans have different patterns of pelvic sexual dimorphism? The explanation may be related to differences in birth canal shape. For successful childbirth, both human and Neandertal females need transversely wider inlets than are found in males, which can be achieved either by having pubic bones that are about the same length as those in males but more coronally oriented or by having pubic bones oriented similarly to those in males but longer. Human females follow the second option (Fig. 4A), whereas Tabun's pelvis (Fig. 4B) suggests that Neandertals follow the first option. Part of the reason for this must be that a twisted birth canal makes the first option infeasible for humans. A more coronal pubic bone orientation not only would increase transverse inlet dimensions, but also would decrease anteroposterior outlet dimensions by moving the pubic symphyses closer to the sacrum. The only way to increase both transverse inlet and anteroposterior outlet dimensions is to increase pubic length, so with a twisted birth canal, females must have longer pubic bones than males. On the other hand, the first option would not be a problem for Neandertals, because they seem to maximize transverse rather than anteroposterior dimensions at both the inlet and the outlet. As a result, Neandertals would not be expected to be sexually dimorphic in pubic length. This pattern of Neandertal sexual dimorphism is consistent with the observation that female Neandertals do not appear to have longer pubic lengths than males (6). (In the preserved specimens, pubic lengths are actually longer in males, but this could be a product of small sample sizes.)
Fig. 4.
Schematic comparing mean birth canal shape in humans (A) and Tabun (B). The gray and dashed black ovals depict pelvic inlet and outlet shapes, respectively, based on the pelvic indices given in Fig. 2. All ovals are constrained to have the same maximum diameter, which occurs either transversely (mediolaterally) or anteroposteriorly. Black lines represent pubic length and orientation with respect to the midline (dashed black line).
Because the pelvic bones are fragile, there are only a handful of fossils geologically older than Tabun and Kebara that are sufficiently well preserved to allow assessment of outlet shape for comparisons with humans and Neandertals. Pelvic aperture dimensions have been measured on A.L. 288–1 and Sts 14, Pliocene australopith specimens from Ethiopia and South Africa, respectively. Both of these specimens are generally considered to be female (2, 10), although some researchers have argued that A.L. 288–1 is male (13). The pelvis of A.L. 288–1 has been reconstructed 3 times. One reconstruction has a transversely oval outlet (2), another has a round outlet (13, 14), and the third has an anteroposteriorly oval outlet (13). Both of the 2 published reconstructions of the Sts 14 pelvis have a transversely oval outlet (13, 15). Although the reconstructions do not all agree, the consensus seems to be that the pre-Homo outlet is transversely oval. All 5 reconstructions have transversely oval inlets, which is consistent with other anatomical adaptations for bipedal gait.
Two specimens, KNM-WT 15000 from Kenya and BSN49/P27 from Ethiopia, have been used to reconstruct birth canal dimensions for Early Pleistocene Homo. Although KNM-WT 15000 preserves fragments of the pelvis, it is not ideal for reconstructing birth canal dimensions. It comes from a subadult and is thought to be from a male (16). In addition, the pubic regions are almost entirely missing, and only small pieces of the sacrum are preserved (17), making it unclear how to align the individual fragments in anatomical position (18, 19). Based on traits known to be sexually dimorphic in humans, BSN49/P27 is thought to come from a female. This specimen has a transversely oval midplane (19), and even though the apex of the sacrum is not preserved, it is likely that it also had a transversely oval outlet. In humans, because the sacrum is curved, the anteroposterior dimensions of the midplane are always more spacious those of the outlet (12). If this were the case for BSN49/P27, then this specimen's outlet index would have been <0.84.
The only other specimen for which outlet dimensions have been measured is Pelvis 1, which almost certainly comes from a male individual, from the Middle Pleistocene site of Sima de los Huesos, Spain (20). Fossils from this site generally are considered ancestral, at least broadly, to Neandertals, because they exhibit multiple derived Neandertal features (20–22). The site is thought to date to around the time of origination of the Neandertal lineage (21–24), so Pelvis 1 may quite closely resemble the last common ancestor of humans and Neandertals. Similar to other hominin fossils, but unlike humans, Pelvis 1 has a transversely oval outlet (20). On the other hand, the midplane of this specimen has been reconstructed to be anteroposteriorly oval, which led the describers to suggest neonatal rotation at the midplane. Although we agree that this conclusion is plausible, 2 caveats should be kept in mind. First, the pelvic aperture is a birth canal only in females, so it is difficult to evaluate the significance of a male specimen with an anteroposteriorly oval midplane. In humans, females have lower midplane indices than males, so it is possible that a female counterpart to Pelvis 1 would have had a transversely oval midplane even if males did not. Second, as we noted earlier, reconstructing midplane dimensions is problematic, because the ischial spine often is poorly preserved. In the case of Pelvis 1, the describers noted that they reconstructed the ischial spine to be “large and pointing” (20), which would have the effect of minimizing transverse midplane dimensions, resulting in a higher midplane index.
Based on A.L. 288–1, Sts 14, and BSN49/P27 it appears that a transversely oval outlet was the primitive condition for hominins. Sima Pelvis 1 has a transversely oval outlet, suggesting that the last common ancestor of humans and Neandertals also would have had a transversely oval outlet. Brain size relative to body size increased substantially during the Middle Pleistocene (25). These changes in encephalization would have had obstetrical consequences for both the human and Neandertal evolutionary lineages. Neandertals apparently adapted to increased obstetrical constraints by further expanding their outlet transverse dimensions, as earlier hominins had done, whereas in the human lineage there was a shift to expanding the outlet anteroposteriorly.
Why did humans change their birth mechanism when Neandertals did not? One possible explanation is that the need to dissipate heat when living close to the equator led to pelvic narrowing in the African-centered human lineage, and when human brain size expanded in the Middle Pleistocene (25), natural selection produced a solution to increased obstetrical constraints that did not result in a wider outlet. Whereas outlet breadth is somewhat independent of overall pelvic (bi-iliac) breadth, wide outlets are closely linked to wide biacetabular distances, which tend to result in more flared ilia to maintain the biomechanical advantage of the hip abductors (18, 26). Consequently, the combination of climate and biomechanics may have constrained transverse outlet expansion in the human lineage. In contrast, Neandertals tended to live in cold climates, where wide trunks are advantageous for thermoregulation, so maintaining the primitive pattern of transversely wide outlets would not have interfered with their climatic adaptations.
One potential problem with this explanation is that the BSN49/P27 pelvis is quite wide, which may indicate that thermoregulatory constraints were not important factors in shaping the pelvis of early Homo (19). However, there is abundant evidence that climate has influenced present-day human patterns of variation in pelvic breadth (27–29), and by ≈100,000 years ago, members of the human lineage had “warm-adapted” body proportions (30, 31). So although a wide pelvis may be the primitive condition for Homo and was perhaps found in the last common ancestor of humans and Neandertals, pelvic width clearly decreased in the African-centered human lineage, and thermoregulatory constraints provide the current best explanation for this decrease, as well as for the retention of a wide pelvis by Neandertals. After humans expanded from Africa ≈50,000 years ago, groups inhabiting higher latitudes adapted to colder climates with increased pelvic width (27–29), but evidently they did not redevelop the primitive (Neandertal) condition of transversely oval outlets (Table S1). They were able to maintain anteroposteriorly oval outlets because, although a transversely oval outlet is incompatible with a narrow pelvis, a anteroposteriorly oval outlet is compatible with either a narrow or a wide pelvis. We argue that the combination of similar adaptations to cold climates but a different birth mechanism explains why Neandertals resemble “cold-adapted” humans in some, but not all, pelvic features (29, 32).
Even though Neandertals appear to have a different birth mechanism than humans, Tabun's pelvic areas are similar to those of human females (Fig. 2 A and B), suggesting that a human-sized neonate would have been able to pass through Tabun's birth canal. This perhaps is not surprising, given that Neandertals had similar neonatal (9) and adult brain sizes (25) as humans. In addition, the neonate's anteroposterior head dimensions have 132 mm of space in Tabun's outlet (Tabun's transverse outlet dimensions), compared with 122 mm in a human outlet (human female mean anteroposterior outlet dimensions). Hormonal relaxation of ligaments during human childbirth enlarges the anteroposterior outlet dimensions by ≈10%–20%, but the transverse dimensions by only ≈5%–7% (33, 34). Assuming that ligament relaxation was the same in Neandertals as in humans, the neonate's anteroposterior head dimensions during childbirth would have 139–141 mm of space in Tabun, compared with 134–146 mm in humans. From these comparisons, we conclude that childbirth was about as difficult in Neandertals as in humans.
Materials and Methods
Sample.
We collected 28 pelvic landmarks (Table 1) from a comparative sample of 231 adult present-day humans (110 females and 123 males) from Africa, Australia, Europe, New Guinea, North America, Oceania, and South America (32) and Rak and Arensburg's reconstruction of the Kebara 2 pelvis (11) with a Microscribe 3DX digitizer (Immersion Corp). We used Amira (Mercury Computer Systems) to collect the preserved subset of these landmarks from a surface rendering of our Tabun reconstruction. This landmark set describes a right hemipelvis, and we mirrored all specimens for which preservation forced us to collect landmarks on the left side.
Table 1.
Pelvic landmarks
|
Tabun Reconstruction.
CT scans of the original Tabun pelvic fragments were done using a Siemens medical scanner with a reconstructed slice thickness of 0.5 mm (actual slice thickness of 1 mm with a 0.5-mm overlap). We removed filling material and metal rods from the original reconstruction, the femoral head from the right acetabulum, parts of the left femur, and distorted portions of the left acetabulum by manually segmenting each slice in Amira. After mirroring the left-sided ilium and acetabular-pubic fragments, we used virtual reality tools to manually fit the fragments together by matching anatomical features. We then used a surface alignment algorithm in RapidForm XO (INUS Technology) to refine the alignment of the (mirrored) left ilium, right acetabulum-ischium, (mirrored) left acetabulum-pubis, and right superior pubic ramus fragments.
We used an E-M algorithm (35) to estimate the locations of sacral and other missing landmarks based on Tabun's preserved anatomy and the comparative human sample. The E-M algorithm proceeds as follows. First, use generalized Procrustes analysis (GPA) (36) to superimpose all of the specimens using only the landmarks preserved on Tabun. Second, estimate Tabun's missing landmarks using multiple regression. Third, use GPA to superimpose all of the specimens again, using the complete set of landmarks. Finally, repeat the second and third steps iteratively until the sum of squares error of the GPA stabilizes. Basing our sacrum reconstruction on patterns of variation found in humans seems reasonable, because Neandertal and human sacra appear to be morphologically similar (37, 38). Tabun's estimated sacral breadth of 107 mm, measured as twice the distance to the midline from landmark 3 (Table 1), is similar to the human female mean (102.0 mm; SD ± 8.7 mm) in our comparative sample.
To orient the reconstructed right hemipelvis in standard anatomical position, we aligned landmarks 4 and 24 in a sagittal plane, aligned landmarks 24 and 25 in a sagittal plane, and aligned landmarks 4 and 15 in a coronal plane. Finally, we mirrored the anatomically oriented right hemipelvis about the midline to produce the left side.
A STL file containing the triangular mesh for our Tabun reconstruction and an ASCII (.txt) file with the landmark locations (including the estimated landmarks) are available online at either http://anthropology.ucdavis.edu or http://www.eva.mpg.de/evolution.
Statistical Analyses.
We calculated pelvic inlet and outlet areas and indices following the approach of Tague (39). We used landmark 25 and the midsagittal plane to measure the mediolateral inlet dimensions, landmarks 4 and 26 for the anteroposterior inlet, landmark 21 and the midsagittal plane for the mediolateral outlet, and landmarks 5 and 28 for the anteroposterior outlet.
We used cross-validation to assess the impact of sacral estimation error on the outlet index. More specifically, we used the E-M algorithm to estimate sacral landmarks for all of the humans in our comparative sample and then, for each individual, calculated the difference in outlet index between the estimated and actual configurations. Because each individual's sacral landmarks were estimated based on all of the other individuals in the comparative sample, the error estimates are virtually unbiased (40). Based on the error distribution, we determined the median error and the percentage of the comparative sample with errors of sufficient magnitude and direction to generate Tabun's outlet index if the actual shape were round (outlet index = 1).
We estimated the hypothetical female Neandertal as follows. First, we used GPA to superimpose all of the specimens in the sample and, for each specimen, calculated residuals from the GPA mean landmark configuration. These residuals represent shape in a Euclidean space tangent to Kendall's non-Euclidean shape space (36). The residuals describe shape as opposed to form because GPA removes size, defined as centroid size (square root of the sum of the squared deviations of the landmark coordinates from the landmark centroid for a given specimen). Second, we calculated a vector describing the direction through shape space that maximizes between-sex variation in humans (sexual dimorphism vector), projected out variation along this vector, and calculated the eigenvectors of the covariance matrix of the remaining shape variation. The sexual dimorphism vector accounted for 19% of the sample shape variation. Third, we calculated the landmark configuration of the hypothetical female Neandertal. Taken together, the sexual dimorphism vector and the eigenvectors form an orthonormal basis for shape space. Any shape can be described as scores along the basis vectors, and its landmark configuration can be generated by adding the linear combination of the basis vectors multiplied by the scores along them to the GPA mean landmark configuration (36). In this way, we created the shape of a hypothetical Neandertal female using Kebara's scores for the eigenvectors and the mean human female score for the sexual dimorphism vector. We created a visual image of the hypothetical female's shape in Amira by warping Tabun's shape to match the landmark configuration of the hypothetical female with a thin-plate spline interpolation (36, 41) of differences at the 28 pelvic landmarks.
We wrote C and Matlab (MathWorks) programs to perform the analyses or, where specified, used Amira and RapidForm.
Supplementary Material
Acknowledgments.
This work was supported by the L.S.B. Leakey Foundation, the Morrison Institute for Population and Resource Studies, the A.W. Mellon Foundation, the “EVAN” Marie Curie Research Training Network (Grant MRTN-CT-019564), and the Max Planck Society. We thank Chris Stringer for access to Tabun and Yoel Rak for access to Kebara; D. Hunt, I. Tattersall, K. Mowbray, G. Avery, A. Morris, the late C. Simon, R. Orban, P. Semal, L. Humphrey, Robert Kruszynski, R. Foley, M. Bellatti, A. Langaney, P. Mennecier, G. Spedini, G. Manzi, B. Chiarelli, and G. D'Amore for access to the comparative specimens; Robert Kruszynski, Heather Gunson, Susan Wakeling, Tarek Yousry, Marc Braun, Alex Foedisch, Heiko Temming, and Andreas Winzer for assistance with the CT scanning; and Philipp Gunz, Yoel Rak, Teresa Steele, and 2 anonymous reviewers for comments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812554106/DCSupplemental.
References
- 1.Rosenberg KR, Trevathan W. Birth, obstetrics and human evolution. BJOG. 2002;109:1199–1206. doi: 10.1046/j.1471-0528.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 2.Tague RG, Lovejoy CO. The obstetric pelvis of A.L. 288–1 (Lucy) J Hum Evol. 1986;15:237–255. [Google Scholar]
- 3.Garrod DAE, Bate DMA. The Stone Age of Mount Carmel: Excavations at the Wady El-Mughara. Vol I. Oxford: Clarendon Press; 1937. [Google Scholar]
- 4.Bar-Yosef O, Callander J. The woman from Tabun: Garrod's doubts in historical perspective. J Hum Evol. 1999;37:879–885. doi: 10.1006/jhev.1999.0368. [DOI] [PubMed] [Google Scholar]
- 5.Klein RG. The Human Career: Human Biological and Cultural Origins. Chicago: Univ Chicago Press; 1999. [Google Scholar]
- 6.Rosenberg KR. The functional significance of Neandertal pubic length. Curr Anthropol. 1988;29:595–607. [Google Scholar]
- 7.Trinkaus E. Neandertal pubic morphology and gestation length. Curr Anthropol. 1984;25:509–514. [Google Scholar]
- 8.McCown TD, Keith A. The Stone Age of Mount Carmel: The Fossil Human Remains From the Levalloiso-Mousterian. Vol II. Oxford: Clarendon Press; 1939. [Google Scholar]
- 9.Ponce de León M, et al. Neanderthal brain size at birth provides insights into the evolution of human life history. Proc Natl Acad Sci USA. 2008;105:13764–13768. doi: 10.1073/pnas.0803917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tague RG, Lovejoy CO. AL 288–1–Lucy or Lucifer: Gender confusion in the Pliocene. J Hum Evol. 1998;35:75–94. doi: 10.1006/jhev.1998.0223. [DOI] [PubMed] [Google Scholar]
- 11.Rak Y, Arensburg B. Kebara 2 Neanderthal pelvis: First look at a complete inlet. Am J Phys Anthropol. 1987;73:227–231. doi: 10.1002/ajpa.1330730209. [DOI] [PubMed] [Google Scholar]
- 12.Tague RG. Sexual dimorphism in the human bony pelvis, with a consideration of the Neandertal pelvis from Kebara Cave, Israel. Am J Phys Anthropol. 1992;88:1–21. doi: 10.1002/ajpa.1330880102. [DOI] [PubMed] [Google Scholar]
- 13.Häusler M, Schmid P. Comparison of the pelves of Sts 14 and AL 288–1: Implications for birth and sexual dimorphism in australopithecines. J Hum Evol. 1995;29:363–383. [Google Scholar]
- 14.Berge C, Orban-Segebarth R, Schmid P. Obstetrical interpretation of the Australopithecine pelvic cavity. J Hum Evol. 1984;13:573–587. [Google Scholar]
- 15.Abitbol MM. Reconstruction of the STS 14 (Australopithecus africanus) pelvis. Am J Phys Anthropol. 1995;96:143–158. doi: 10.1002/ajpa.1330960204. [DOI] [PubMed] [Google Scholar]
- 16.Walker A, Leakey R, editors. The Nariokotome Homo Erectus Skeleton. Cambridge, MA: Harvard Univ Press; 1993. [Google Scholar]
- 17.Walker A, Ruff CB. In: The Nariokotome Homo Erectus Skeleton. Walker A, Leakey R, editors. Cambridge, MA: Harvard Univ Press; 1993. pp. 221–233. [Google Scholar]
- 18.Ruff CB. Biomechanics of the hip and birth in early Homo. Am J Phys Anthropol. 1995;98:527–574. doi: 10.1002/ajpa.1330980412. [DOI] [PubMed] [Google Scholar]
- 19.Simpson SW, et al. A female Homo erectus pelvis from Gona, Ethiopia. Science. 2008;322:1089–1092. doi: 10.1126/science.1163592. [DOI] [PubMed] [Google Scholar]
- 20.Arsuaga J-L, et al. A complete human pelvis from the Middle Pleistocene of Spain. Nature. 1999;399:255–258. doi: 10.1038/20430. [DOI] [PubMed] [Google Scholar]
- 21.Hublin J-J. In: Neandertals and Modern Humans in Western Asia. Akazawa T, Aoki K, Bar-Yosef O, editors. New York: Plenum; 1998. pp. 295–310. [Google Scholar]
- 22.Bischoff JL, et al. High-resolution U-series dates from the Sima de los Huesos hominids yields 600 kyrs: Implications for the evolution of the early Neanderthal lineage. J Archaeol Sci. 2007;34:763–770. [Google Scholar]
- 23.Noonan JP, et al. Sequencing and analysis of Neanderthal genomic DNA. Science. 2006;314:1113–1118. doi: 10.1126/science.1131412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver TD, Roseman CC, Stringer CB. Close correspondence between quantitative- and molecular-genetic divergence times for Neandertals and modern humans. Proc Natl Acad Sci USA. 2008;105:4645–4649. doi: 10.1073/pnas.0709079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruff CB, Trinkaus E, Holliday TW. Body mass and encephalization in Pleistocene Homo. Nature. 1997;387:173–176. doi: 10.1038/387173a0. [DOI] [PubMed] [Google Scholar]
- 26.Lovejoy CO, Heiple KG, Burstein AH. The gait of Australopithecus. Am J Phys Anthropol. 1973;38:757–779. doi: 10.1002/ajpa.1330380315. [DOI] [PubMed] [Google Scholar]
- 27.Holliday TW. Body proportions in Late Pleistocene Europe and modern human origins. J Hum Evol. 1997;32:423–447. doi: 10.1006/jhev.1996.0111. [DOI] [PubMed] [Google Scholar]
- 28.Ruff CB. Morphological adaptation to climate in modern and fossil hominids. Yearbook Phys Anthropol. 1994;37:65–107. [Google Scholar]
- 29.Weaver TD. The shape of the Neandertal femur is primarily the consequence of a hyperpolar body form. Proc Natl Acad Sci USA. 2003;100:6926–6929. doi: 10.1073/pnas.1232340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holliday TW. Evolution at the crossroads: Modern human emergence in Western Asia. Am Anthropol. 2000;102:54–68. [Google Scholar]
- 31.Pearson O. In: The Middle Stone Age of Zambia, South Central Africa. Barham L, editor. Bristol, UK: Western Academic & Specialist Press; 2000. [Google Scholar]
- 32.Weaver TD. Anthropological Sciences. Palo Alto, CA: Stanford Univ Press; 2002. p. 221. [Google Scholar]
- 33.Björklund K, Lindgren PG, Bergström S, Ulmsten U. Sonographic assessment of symphyseal joint distention intra partum. Acta Obstet Gynecol Scand. 1997;76:227–232. [PubMed] [Google Scholar]
- 34.Russell JGB. Moulding of the pelvic outlet. J Obstet Gynaecol Br Commonw. 1969;76:817–820. doi: 10.1111/j.1471-0528.1969.tb06185.x. [DOI] [PubMed] [Google Scholar]
- 35.Gunz P, Mitteroecker P, Bookstein FL, Weber GW. BAR International Series. Vol 1227. Oxford: Archaeopress; 2004. Enter the Past: Computer Applications and Quantitative Methods in Archaeology; pp. 96–98. [Google Scholar]
- 36.Dryden IL, Mardia KV. Statistical Shape Analysis. New York: Wiley; 1998. [Google Scholar]
- 37.Rosenberg KR. Anthropology. Ann Arbor, MI: Univ Michigan Press; 1986. p. 237. [Google Scholar]
- 38.Rak Y. In: Le Squelette Moustérien de Kébara. Bar-Yosef O, Vandermeersch B, editors. Paris: CNRS Editions; 1991. pp. 147–166. [Google Scholar]
- 39.Tague RG. Do big females have big pelves? Am J Phys Anthropol. 2000;112:377–393. doi: 10.1002/1096-8644(200007)112:3<377::AID-AJPA8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 40.Krzanowski WJ. Principles of Multivariate Analysis: A User's Perspective. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 41.Bookstein FL. Principal warps: Thin-plate splines and the decomposition of deformations. IEEE Trans Pattern Anal Machine Intell. 1989;11:567–585. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.