Abstract
Leukotrienes (LTs) are lipid mediators of inflammation formed by enzymatic oxidation of arachidonic acid. One intriguing aspect of LT production is transcellular biosynthesis: cells expressing 5-lipoxygenase (5LO) form LTA4 and transfer it to cells expressing LTA4 hydrolase (LTA4H) or LTC4 synthase (LTC4S) to produce LTB4 or LTC4. This process has been demonstrated in vivo for LTB4, but not for cysteinyl LTs (cysLTs). We examined transcellular cysLT synthesis during zymosan-induced peritonitis, using bone marrow transplants with transgenic mice deficient in key enzymes of LT synthesis and analyzing all eicosanoids by liquid chromatography/tandem mass spectrometry. WT mice time-dependently produced LTB4 and cysLTs (LTC4, LTD4, and LTE4). 5LO−/− mice were incapable of producing LTs. WT bone marrow cells restored this biosynthetic ability, but 5LO−/− bone marrow did not rescue LT synthesis in irradiated WT mice, demonstrating that bone marrow-derived cells are the ultimate source of all LTs in this model. Total levels of 5LO-derived products were comparable in LTA4H−/− and WT mice, but were reduced in LTC4S−/− animals. No differences in prostaglandin production were observed between these transgenic or chimeric mice. Bone marrow cells from LTA4H−/− or LTC4S−/− mice injected into 5LO−/− mice restored the ability to synthesize LTB4 and cysLTs, providing unequivocal evidence of efficient transcellular biosynthesis of cysLTs. These results highlight the potential relevance of transcellular exchange of LTA4 for the synthesis of LTs mediating biological activities during inflammatory events in vivo.
Keywords: chimeric mice, leukotriene B4, mass spectrometry, leukotriene E4, 5-lipoxygenase
Leukotrienes (LTs) are biologically active lipid mediators that play important roles in inflammation and are involved in pathological states with an inflammatory component, such as asthma, cardiovascular disease, or cancer (1–5). They derive from arachidonic acid (AA) through the action of 5-lipoxygenase (5LO) (6), an enzyme expressed in a limited number of cells, including neutrophils, eosinophils, monocytes, macrophages, mast cells, and basophils (7, 8). Considerable work has led to our current understanding of events regulating the formation of these mediators. AA is released from phospholipids by cytosolic phospholipase A2α (cPLA2α) after this enzyme translocates from cytosol to perinuclear membranes of cells, usually following an increase in calcium ion concentration (9). 5LO, a soluble protein in the cytosol or nucleoplasm of resting cells, also translocates to perinuclear membranes when calcium levels increase (10). 5LO oxidizes free AA to 5-hydroperoxyeicosatetraenoic acid. This intermediate can be reduced by peroxidases to 5-hydroxyeicosatetraenoic acid (5-HETE) or dehydrated, in a second 5LO-catalyzed reaction, to leukotriene A4 (LTA4). 5LO action is facilitated by 5LO-activating protein (FLAP), an integral nuclear membrane protein, and the assembly of a multienzymatic machine to make LTs (10–12). Thus, LTA4 is formed near the nuclear envelope, and this chemically reactive molecule must find its way either to leukotriene C4 synthase (LTC4S), another nuclear membrane protein that conjugates LTA4 with glutathione to form leukotriene C4 (LTC4), or to cytosolic LTA4 hydrolase (LTA4H), which stereospecifically opens the epoxide ring and controls the addition of water at carbon-12 to form leukotriene B4 (LTB4). LTC4 can be metabolized by sequential proteolytic hydrolysis to leukotriene D4 (LTD4) and leukotriene E4 (LTE4); these 3 compounds are collectively termed cysteinyl LTs (cysLTs). The final LT products derived from LTA4 play important roles in inflammation, such as inducing neutrophil chemotaxis (LTB4) or causing edema and smooth muscle contraction (LTC4, LTD4) (1, 2). The effects of these eicosanoids are mediated by G-protein coupled receptors. Two have been identified for LTB4 (BLT1 and BLT2) (13, 14), and 3 for cysLTs (cysLT1, cysLT2, and GPR17) (15–17). Although the protein has not been identified, a novel receptor showing specificity for LTE4 has been recently described (18). CysLT1 receptor antagonists are currently used to help control asthma symptoms, and drugs that interfere with LT biosynthesis or action are candidates for preventing cardiovascular disease (19, 20).
LT production is regulated at different levels (8), including methylation of the 5LO promoter (21) and posttranslational control of 5LO activity through phosphorylation, interaction with FLAP (11, 12), and translocation induced by calcium or free AA (22). Additionally, experimental evidence suggests that cells cooperate in the synthesis of LTB4 and LTC4. For example, human platelets, which express no 5LO, can interact with activated neutrophils, which do not express LTC4S, to generate LTC4 (23). Many other instances of transcellular LT biosynthesis have been described (24). The chemical half-life of LTA4 in buffer is less than 5 s (25), which implies some protective mechanism of the epoxide ring during transfer from the donor cell to the acceptor cell.
Mice deficient in enzymes responsible for LT synthesis are valuable tools to test the extent of transcellular formation of LTs. Koller and colleagues illustrated this value by transplanting bone marrow from LTA4H−/− mice into lethally irradiated 5LO−/− mice (26). In a peritoneal inflammation model, LTB4 was found to be synthesized, proving that transcellular biosynthesis did occur in vivo. Production of LTB4 in these chimeric animals was significantly lower than in WT controls or 5LO−/− mice rescued with WT bone marrow. This raised questions about the extent of transcellular biosynthesis, whether this was a minor pathway for in vivo LT generation, or whether LTA4 was converted into other products besides LTB4.
In the present study, our main aim was to find evidence of the possible transcellular biosynthesis of cysLTs using LTC4S−/− bone marrow cells, while identifying and quantifying all of the major COX- and 5LO-derived eicosanoids.
Results
Generation of Eicosanoids in Zymosan-Induced Peritonitis.
Injection of zymosan into the peritoneal cavity of mice resulted in production of eicosanoids, as revealed by liquid chromatography/tandem mass spectrometry (LC/MS/MS) (Fig. 1). After 2 h, eicosanoids derived from the 5LO and COX pathways of AA oxidation were detected. The most abundant metabolites in peritoneal lavage were LTE4, LTB4, and 5-HETE. Also present were LTC4, LTD4, Δ6-trans-LTB4 isomers, 5,6-dihydroxyeicosatetraenoic acid (diHETE) isomers, and small amounts of COX-derived thromboxane B2 (TXB2) and prostaglandin E2 (PGE2). Levels of 5,6-diHETEs were consistently higher (2–8 times) than levels of Δ6-trans-LTB4s. In subsequent experiments, Δ6-trans-LTB4s and 5,6-diHETEs are reported together as nonenzymatic products of LTA4. 6-keto-PGF1α and PGF2α were barely detected above background (10–20% of the PGE2 signal). PGD2, PGJ2, PGA2, 20-OH-LTB4, and 20-COOH-LTB4 were below the limit of detection. 12-HETE and 15-HETE were also found, but their levels were similar in control mice injected with PBS. None of the other eicosanoids was detected in mice injected with PBS or not treated at all (Fig. 2, 0 h time point).
Fig. 1.
LC/MS/MS profile of eicosanoids in peritoneal lavage. Mice were treated with 1-mg zymosan for 2 h, and eicosanoids analyzed by LC/MS/MS, monitoring the specific m/z transitions indicated. Retention times of deuterated internal standards (described in the text) coincided with their cognate metabolites.
Fig. 2.
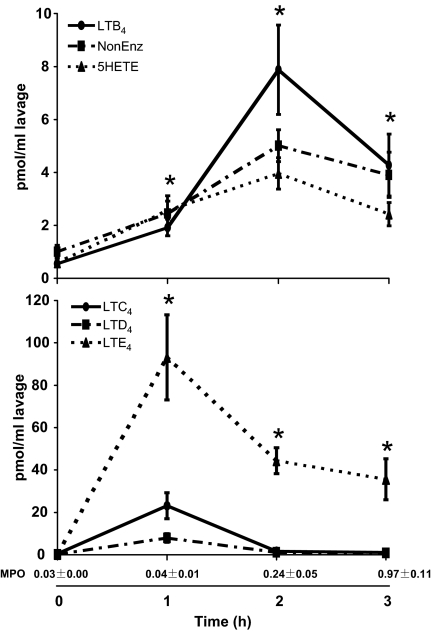
Time-course of production of 5LO-derived metabolites and myeloperoxidase (MPO) activity. Mice were treated with 1-mg zymosan for the times indicated, and eicosanoids were analyzed by LC/MS/MS and quantitated by isotopic dilution of the corresponding internal standards. NonEnz: sum of 5,6-diHETEs and Δ6-trans-LTB4s. *, P < 0.05 vs. 0 h, for LTB4 and LTE4. Also shown is the MPO activity (Abs 570 nm) in peritoneal lavage at the same time points. Results are average ± SEM of at least 4 experiments.
Zymosan-induced LT release was time-dependent. LTB4 increased at 1 h and peaked at 2 h (see Fig. 2, Top). 5-HETE and nonenzymatic LTA4 metabolites followed similar time courses. In contrast, cysLTs peaked at 1 h and were decreased at 2 h, with LTE4 remaining elevated at 3 h (see Fig. 2, Bottom). Subsequent experiments were carried out at 2 h to assess the effects of genetic manipulation in both LTB4 and LTE4 levels.
The initial release of eicosanoids was likely the result of zymosan phagocytosis by resident peritoneal macrophages or mast cells, the predominant 5LO-expressing cells recovered from peritoneal cavity of unchallenged mice (27). LTB4 generated in the first hour of stimulation possibly helped recruit neutrophils to the peritoneal cavity, which would explain LTB4 release at later time points. Indeed, zymosan increased MPO activity in the peritoneal lavage (see Fig. 2). At 3 h, MPO was maximal, but LTB4 synthesis was lower than at 2 h, probably because of zymosan depletion. It has been shown that a second injection of zymosan 6 h after stimulation results in a second, more pronounced, burst of LTB4 production in the peritoneal cavity of mice, consistent with increased numbers of neutrophils (28). Further evidence linking cell population changes and LT production was provided by stimulating peritoneal lavage cells with calcium ionophore A23187 (Table 1). In unchallenged mice, peritoneal cells produced mainly LTC4, consistent with the presence of macrophages and mast cells. Upon zymosan treatment, there was a time-dependent decrease of cysLT production and an increase in LTB4 production, likely because of macrophages being replaced by neutrophils.
Table 1.
Eicosanoid production by peritoneal lavage cells
| 0 h | 1 h | 2 h | 3 h | |
|---|---|---|---|---|
| LTB4 | 1.17 ± 0.27 | 0.50 ± 0.03 | 2.38 ± 0.33 | 7.80 ± 1.67 |
| Δ6t-LTB4s | 3.29 ± 0.74 | 0.43 ± 0.02 | 1.21 ± 0.18 | 3.77 ± 0.77 |
| 5,6-diHETEs | 2.20 ± 0.47 | 0.34 ± 0.04 | 0.77 ± 0.11 | 1.88 ± 0.13 |
| 5-HETE | 6.59 ± 1.17 | 0.88 ± 0.06 | 3.15 ± 0.47 | 8.18 ± 0.57 |
| LTC4 | 19.11 ± 3.51 | 0.92 ± 0.16 | 0.54 ± 0.12 | 0.48 ± 0.27 |
| TXB2 | 2.20 ± 0.44 | 0.14 ± 0.02 | 0.36 ± 0.06 | 1.31 ± 0.11 |
| PGE2 | 2.55 ± 0.58 | 0.19 ± 0.04 | 1.47 ± 0.27 | 3.99 ± 0.55 |
WT mice were treated with 1-mg zymosan for the times indicated, and peritoneal lavage cells (106 in 1 ml) were stimulated with 0.5 μM A23187. All eicosanoid products were measured by LC/MS/MS. Results (ng/106 cells) are average ± SEM of at least 5 experiments.
Production of Leukotrienes in Transgenic Mice Deficient in Leukotriene Biosynthetic Enzymes.
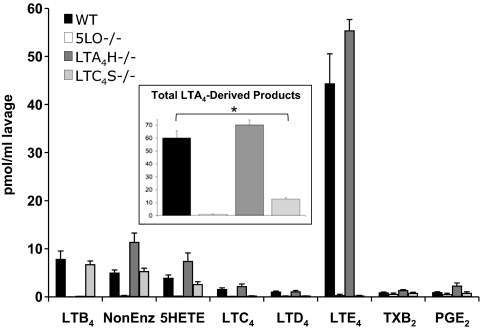
Production of eicosanoids by WT and transgenic mice was investigated (Fig. 3). As expected, 2 h after zymosan injection the peritoneal lavage of 5LO−/− mice showed no LTB4 and barely detectable amounts of cysLTs. The levels of COX-derived TXB2 and PGE2 were similar to WT mice. No other COX-derived metabolite was increased in 5LO−/− animals, indicating that no shunting of AA substrate between the 2 oxygenases occurred in this model. These animals showed decreased MPO activity (indicative of neutrophil infiltration) and protein concentration (indicative of plasma extravasation) in the peritoneal lavage (Table 2), in line with sharply reduced LT levels. In LTA4H−/− mice, no LTB4 was produced, but levels of cysLTs, 5-HETE and nonenzymatic products of LTA4 were slightly increased compared to WT animals. Total production of LTA4 (the sum of all LTA4-derived eicosanoids) was comparable between WT and LTA4H−/− mice (see Fig. 3, Inset). In the case of LTC4S−/− mice, normal levels of LTB4, nonenzymatic LTA4 products and 5-HETE were measured. Virtually no cysLTs were detected, and the total levels of LTA4 produced were significantly lower than in WT mice. The reasons for this are not known at this time, but they could not be explained by differences in cell populations in the peritoneal cavity. In additional experiments with unchallenged mice, both the total number of peritoneal cells recovered and the relative content of monocytes/macrophages and mast cells were very similar between WT and LTC4S−/− mice.
Fig. 3.
Production of eicosanoids in peritoneal lavage of transgenic mice deficient in LT synthesis enzymes. WT or transgenic mice with the indicated genotypes were treated with 1-mg zymosan for 2 h, and the indicated eicosanoids were analyzed by LC/MS/MS and quantitated by isotopic dilution of the corresponding internal standards. Data in the inset represent the sum of all LTA4-derived metabolites (Δ6-trans-LTB4s and 5,6-diHETEs, LTB4, and cysLTs). Results are average ± SEM of at least 3 experiments. *, P < 0.05 vs. WT.
Table 2.
Protein concentration and MPO activity in peritoneal lavage
| Protein (mg/ml) | MPO (Abs 570 nm) | |
|---|---|---|
| WT | 2.52 ± 0.07 | 0.23 ± 0.05 |
| 5LO−/− | 1.38 ± 0.07* | 0.03 ± 0.01 |
| LTA4H−/− | 3.56 ± 0.38* | 0.11 ± 0.01 |
| LTC4S−/− | 2.16 ± 0.08* | 0.37 ± 0.11 |
| WT → 5LO−/− | 2.61 ± 0.17 | 0.40 ± 0.13 |
| 5LO−/− → WT | 1.17 ± 0.06* | 0.12 ± 0.05 |
| LTA4H−/− → 5LO−/− | 2.41 ± 0.13 | 0.24 ± 0.12 |
| LTC4S−/− → 5LO−/− | 3.03 ± 0.40 | 0.41 ± 0.19 |
Mice were treated with 1-mg zymosan for 2 h. Protein concentration and MPO activity were determined in the peritoneal lavage. Results are expressed as average ± SEM of at least 3 experiments.
*, P < 0.05 vs. WT.
Generation of Leukotrienes in Chimeric Mice.
A major strategy of this study was to restrict the presence of 5LO to cells of bone marrow origin, as opposed to cells of nonhematopoietic origin present in the peritoneal cavity. This was accomplished by lethally irradiating 5LO−/− mice and rescuing the animals with bone marrow cells from mice that did express 5LO, either WT, LTA4H−/−, or LTC4S−/−. The success of the transplants was confirmed by the ability of the regenerated bone marrow cells to synthesize eicosanoids upon stimulation with A23187 (Table 3). In 42 out of 43 chimeric animals, the eicosanoid profile showed that, after the 8-week period of recovery, the bone marrow population exhibited the expected phenotype of the donor rather than the recipient mouse, validating the irradiation and implantation protocols. Bone marrow cells from LTA4H−/− → 5LO−/− mice released abundant 5LO-derived products but, compared to WT or LTC4S−/− cells, very small amounts of LTB4, possibly through transcellular metabolism of LTA4 by stromal cells within the bone marrow cell preparation. In stimulated bone marrow cells from LTC4S−/− → 5LO−/−, LTC4 was undetectable, whereas WT or LTA4H−/− cells produced measurable levels of this cysLT, consistent with previous results (29).
Table 3.
Eicosanoid production by bone marrow cells in chimeric mice
| WT → 5LO−/− | 5LO−/− → WT | LTA4H−/− → 5LO−/− | LTC4S−/− → 5LO−/− | |
|---|---|---|---|---|
| LTB4 | 7.39 ± 1.20 | n.d. | 0.65 ± 0.24 | 5.90 ± 2.80 |
| Δ6t-LTB4s | 2.03 ± 0.30 | n.d. | 4.46 ± 0.67 | 1.47 ± 0.62 |
| 5,6-diHETEs | 1.49 ± 0.18 | n.d. | 1.40 ± 0.29 | 1.05 ± 0.76 |
| 5-HETE | 7.33 ± 1.19 | n.d. | 6.62 ± 1.32 | 5.10 ± 2.59 |
| LTC4 | 0.20 ± 0.04 | n.d. | 0.39 ± 0.05 | n.d. |
| TXB2 | 1.79 ± 0.22 | 3.10 ± 1.39 | 1.40 ± 0.17 | 1.76 ± 0.25 |
| PGE2 | 9.77 ± 1.25 | 10.05 ± 0.81 | 9.71 ± 2.50 | 9.28 ± 0.75 |
Bone marrow cells (106 in 1 ml) from chimeric mice (donor → acceptor) were stimulated with 0.5 μM A23187. All eicosanoid products were measured by quantitative LC/MS/MS. Results (ng/106 cells) are average ± SEM of at least 6 experiments. n.d., not detected.
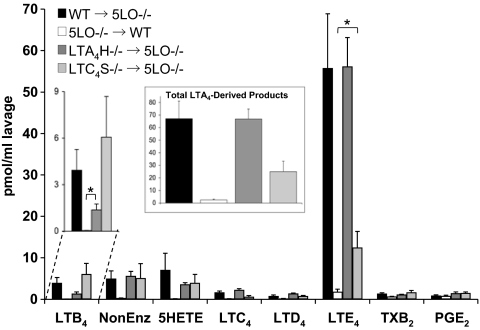
When irradiated 5LO−/− mice were rescued with WT cells, zymosan triggered abundant production of both LTB4 and LTE4 (Fig. 4). Nonenzymatic hydrolysis products of LTA4 were also detected, as were 5-HETE, LTC4, and LTD4. Phagocytic stimulation clearly activated the 5LO pathway, resulting in formation of LTA4. The amounts of LTB4 and LTE4 were not significantly different from WT animals that had not undergone irradiation or transplantation. Neutrophil infiltration (as indicated by MPO activity) and protein concentration were also similar to those observed in nonirradiated WT animals (see Table 2).
Fig. 4.
Production of eicosanoids in peritoneal lavage of chimeric mice. Chimeric mice (bone marrow donor → irradiated acceptor) were treated with 1-mg zymosan for 2 h, and the indicated eicosanoids were analyzed by LC/MS/MS and quantitated by isotopic dilution of the corresponding internal standards. The LTB4 section is expanded for clarity. The inset shows the sum of all LTA4-derived metabolites (Δ6-trans-LTB4 isomers and 5,6-diHETE isomers, LTB4 and cysLTs). Results are average ± SEM of at least 3 experiments. *, P < 0.05.
When peritonitis was induced in chimeric animals with a 5LO−/− background and bone marrow from LTA4H−/− animals, significant amounts of LTB4 were observed (see Fig. 4). Unequivocally, this eicosanoid could only be produced in these animals through transcellular metabolism, because no cell could express both 5LO and LTA4H (Fig. 5). However, the levels of LTB4 were not as high as those in WT mice or WT → 5LO−/− chimeras. This suggested the possibility that LTA4H−/− → 5LO−/− chimeras may exhibit decreased 5LO activity, but nonenzymatic LTA4 products, 5-HETE, and cysLTs, were measured at levels comparable to WT animals (see Fig. 4, Inset), indicating that absence of LTA4H did not affect other components of LT synthesis. In these chimeric mice, MPO levels were higher than in LTA4H−/− animals (see Table 2), consistent with LTB4 being present in the peritoneal lavage.
Fig. 5.
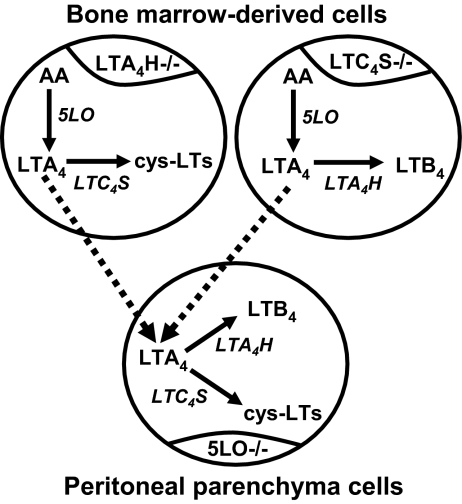
Experimental model of transcellular leukotriene biosynthesis. 5LO−/− mice were lethally irradiated, then rescued with bone marrow cells from LTA4H−/− or LTC4S−/− mice. Upon zymosan stimulation, cells of a bone marrow origin could synthesize either cysLTs (if donors were LTA4H−/−) or LTB4 (if donors were LTC4S−/−), but no single cell could synthesize both. Parenchymal cells could not synthesize either, as they did not express 5LO. To produce both types of LTs, LTA4 needed to be exported from bone marrow-derived cells and imported into parenchymal cells, then converted to LTB4 and cysLTs. LTA4H and LTC4S were both expressed in the parenchymal cell population, although not necessarily within the same cell.
For 5LO−/− animals transplanted with LTC4S−/− cells, the most striking observation was the significant production of LTE4, only possible through transcellular exchange of LTA4 (see Fig. 4). In these animals, no single cell expressed both 5LO and LTC4S (see Fig. 5). To our knowledge, this report of transcellular synthesis of cysLTs in an in vivo model of inflammation is unique. Moreover, this process was very efficient, as total production of cysLTs (almost 14 pmol of LTC4, LTD4, and LTE4) was more than 50% of the total LTA4 production (around 25 pmol of cysLTs, LTB4, and nonenzymatic LTA4 products). Protein concentration in the peritoneal lavage of LTC4S−/− → 5LO−/− mice was higher than LTC4−/− animals, and similar to WT, correlating with the presence of cysLTs. This suggested that cysLT transcellular biosynthesis may be sufficient to induce plasma extravasation in this experimental model.
In contrast with the production of LTB4 and cysLTs in the peritoneal cavity of WT animals, chimeric animals of a WT background, lethally irradiated and rescued with 5LO−/− cells, were found to generate no LTB4 and very low levels of cysLTs. There was a small but reproducible amount of LTE4, suggesting that a small number of cells in the peritoneal cavity of WT animals survived irradiation and remained able to generate cysLTs. It has been reported that macrophages and mast cells are relatively radioresistant (30, 31). Compared to the robust restoration of LT synthesis in the chimeric animals described above, these experiments with 5LO−/− bone marrow provide solid support for the hypothesis that the overwhelming majority of LTA4 generated during zymosan-induced peritonitis in the mouse is derived from bone marrow hematopoietic cells.
Discussion
Eicosanoids, such as PGs and LTs, are potent lipid mediators involved in inflammatory responses. It is not surprising that their synthesis is tightly regulated through a variety of mechanisms, some of which are not well understood, including transcellular biosynthesis. In this study, we revisited the zymosan-induced peritonitis model used to demonstrate transcellular LTB4 synthesis (26), using additional transgenic mice and comprehensive measurement of eicosanoids to help deepen our understanding of this phenomenon.
When acute peritoneal inflammation was induced in mice by zymosan, the major LTs observed after 1 h were cysLTs, predominantly LTE4. We did not analyze lavages at shorter times, but it has been shown that LTC4 is the main LT after 15 min of i.p. injection of either opsonized (32) or nonopsonized (33) zymosan, with LTE4 becoming more abundant after 1 h. After 2 h, LTB4 was also abundant in peritoneal lavage, most likely because of neutrophil infiltration. Assessment of the peritoneal lavage cell ability to produce eicosanoids upon stimulation and MPO activity provided evidence of a change in cell population in agreement with previous reports on this model (27, 28, 34, 35). All these cells have a hematopoietic origin, and while other cells in the peritoneal cavity might possibly express 5LO, data from irradiated WT mice rescued with 5LO−/− cells demonstrate that none of the LTA4 in this peritonitis model originates in cells not derived from bone marrow. Likely sources of LTA4 are peritoneal monocytes/macrophages, mast cells, or influxing neutrophils. Although mast cells are known to release LTs in response to zymosan (36), previous reports suggest that they are involved in LTB4 rather than cysLT production (28, 33). The results presented here also show that parenchyma cells of nonhematopoietic origin express downstream enzymes in the LT synthesis pathway, such as LTA4H and LTC4S.
The mechanism by which LTA4 is transported between cells is poorly understood. This chemically reactive epoxide must be stabilized in a manner that shields it from the aqueous environment, because water rapidly hydrolyzes LTA4 in a nonenzymatic process leading to the production of Δ6-trans-LTB4s (6-trans-LTB4 and 6-trans-12-epi-LTB4) and, to a lesser extent, 5,6-diHETEs (37). In this model, however, higher levels of 5,6-diHETEs were observed, indicating preferential attack of water at carbon-6 of LTA4 rather than at carbon-12, the thermodynamically most stable position for water to attack the delocalized carbocation after the epoxide ring is opened. This suggests that some protein is binding LTA4 through a hydrophobic pocket, which protects carbon-12 to a larger extent than carbon-6.
When irradiated 5LO−/− mice were rescued with LTA4H−/− bone marrow, LTB4 was produced transcellularly in quantities significantly lower than in WT animals, in agreement with the report from Koller and colleagues (26). A possible explanation would be that most of the LTA4 was hydrolyzed nonenzymatically and did not reach a cell that had LTA4H. Alternatively, chimeras may have generated less LTA4 than WT mice. In this study, total production of LTs in LTA4H−/− → 5LO−/− chimeric mice was found to be quite comparable to WT mice, suggesting that there was not a major change in the cell population or the activity of 5LO. The loss of LTB4 was balanced by an increase in cysLTs and nonenzymatic LTA4 products. In contrast, LTC4S−/− mice, and chimeras generated with their bone marrow, showed reduced levels of LTA4 and other metabolites derived from 5LO. The reasons for this surprising finding may include decreased expression of 5LO or FLAP, or some unknown mechanism regulating 5LO activity in peritoneal cells. It is interesting to note that physical interaction between 5LO, FLAP, and LTC4S has been reported (12), and the absence of one of these enzymes during cell differentiation may affect the stability of the others. As an additional conclusion, the fact that LTC4S−/− bone marrow cells were unable to synthesize LTC4 upon A23187 stimulation, indicates that LTC4S was the predominant enzyme able to convert LTA4 into LTC4 in this model, suggesting that microsomal GSTs (38, 39) are not involved.
Transcellular synthesis of cysLTs has been shown in heterologous perfusion models with human neutrophils and rabbit hearts or guinea pig brains (40, 41). This report provides unequivocal evidence of transcellular cysLT production in an in vivo model of inflammation in a single organism. A very surprising result was that production of cysLTs in LTC4S−/− → 5LO−/− chimeric mice accounted for more than half the total LTA4 produced. Because the only way in which cysLTs could be generated was transcellular biosynthesis, it is reasonable to conclude that transcellular production of cysLTs is a very efficient process in this model. This is consistent with the fact that more than 50% of LTA4 produced by human neutrophils is exported from the cell (42).
Transcellular synthesis of LTB4 and cysLTs represents only a fraction of the LTs produced in WT mice (≈20–25%). These results are remarkably similar to those published by Koller and colleagues (26), where transcellular synthesis of LTB4 accounted for a significant portion of plasma extravasation and edema during AA-induced cutaneous inflammation. Our data on MPO activity and protein concentration suggest that LTB4 and cysLTs produced transcellularly in this peritonitis model contribute substantially to neutrophil recruitment and plasma extravasation, respectively, supporting the potential biological relevance of transcellular LT synthesis.
In summary, this study provides evidence for the occurrence of transcellular biosynthesis of cysLTs in vivo. Given the efficiency of transfer of the reactive intermediate LTA4 to generate cysLTs, it appears that transcellular synthesis could be a sizable component of cysLT production during inflammation and contribute to the overall inflammatory response. The separation of critical enzymes in different cells probably constitutes an additional regulatory mechanism controlling production of these important mediators, and as such it provides a potentially unique target for pharmacological intervention.
Materials and Methods
Chemicals.
All chemicals and solvents were purchased from Fisher Scientific. Zymosan, BSA, hexadecyltrimethylammonium bromide (HTAB), and 3,3′,5,5′-tetramethylbenzidine (TMB) were from Sigma-Aldrich. Stable isotope-labeled internal standards [d4]LTB4 (≥97 atom %D), [d5]LTC4 (97%D), [d8]5-HETE (≥98%D), [d4]TXB2 (≥98%D), [d4]PGE2 (≥99%D), as well as synthetic LTs used in stable isotope dilution curves, were purchased from Cayman Chemical.
Animals.
5LO−/− and LTA4H−/− mice (129S6 background) were a generous gift from B.H. Koller (University of North Carolina) (43, 34). LTC4S−/− mice (C57BL background) were a generous gift from K.F. Austen, B.K. Lam, and Y. Kanaoka (Harvard Medical School) (44). 129S6 mice were from Taconic. C57BL mice were from The Jackson Laboratory. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver.
Mouse Peritoneal Inflammation Model.
Zymosan was boiled for 5 min in HBSS, resuspended at 1 mg/ml, and injected (1 ml) into the peritoneal cavity of mice. After 1, 2, or 3 h, mice were killed and the peritoneal cavity washed with 5 ml HBSS. One milliliter of the lavage fluid was added to 1 ml of methanol for eicosanoid measurement by LC/MS/MS. Separate aliquots (200 μl) were used for protein measurement (bicinchoninic acid assay, Pierce) and MPO assay. For the assay, cells were resuspended in 50-mM phosphate buffer, pH 6, 0.1% gelatin, 0.5% HTAB, and incubated with TMB Liquid Substrate for 20 min, before monitoring absorbance at 570 nm.
Cells in the remaining lavage were pelleted, resuspended in HBSS, and stimulated at a concentration of 106/ml (1 ml) with 0.5 μM calcium ionophore A23187 at 37 °C. After 10 min, 1 ml of methanol was added for extraction and LC/MS/MS analysis.
Eicosanoid Analysis by LC/MS/MS.
After addition of stable isotope-labeled internal standards, eicosanoids in peritoneal lavages or stimulated cells were extracted and analyzed essentially as previously described (29), with some modifications: [d5]LTC4 (5 ng) was used as an internal standard for cysLTs; solid phase cartridges were Strata-X, 33 μm Polymeric Reversed Phase (Phenomenex); chromatography was performed on an Ascentis 150 × 2 mm, 5 μm column (Supelco), at 200 μl/min with a linear gradient from 45% solvent B to 75% in 12 min, 75% to 98% in 2 min, 11 min hold and re-equilibration for 10 min; and m/z transitions were included for additional eicosanoids. Transitions monitored were: 335→195, LTB4 and Δ6-trans-LTB4s; 351→195, 20-OH-LTB4; 365→195, 20-COOH-LTB4; 335→115, 5,6-diHETEs; 624→272, LTC4; 495→177, LTD4; 438→333, LTE4; 319→115, 5-HETE; 319→179, 12-HETE; 319→219, 15-HETE; 351→271, PGE2 and PGD2; 353→309, PGF2α; 333→189, PGJ2 and PGA2; 369→163, 6-keto-PGF1α; 369→169, TXB2; 629→272, [d5]LTC4; 327→116, [d8]5-HETE; 339→197, [d4]LTB4; 373→173, [d4]TXB2; and 355→275, [d4]PGE2.
Generation of Chimeric Animals.
After fasting for 16 h, mice were lethally irradiated (RS-2000 X-ray Biological Irradiator, Rad Source Technologies) in a ventilated container, using 2 cycles of 300 rads with a 3-h interval. Bone marrow cells were isolated from femurs and tibias of donor mice as previously described (29). The cell suspension was filtered through a 40-μm nylon cell strainer and centrifuged at 160 × g for 10 min. Cells were resuspended in PBS containing 0.5% BSA at a density of 107/ml. Immediately after irradiation, recipient animals were injected through the tail vein with 200 μl of bone marrow cell suspension using a syringe with a 29-gauge needle. Mice were then fed and housed normally for 8 weeks. To verify the irradiation/transplantation protocol, bone marrow cells from each transplanted animal were harvested after collection of the peritoneal lavage. Cells (106 in 1 ml) were stimulated with 0.5 μM A23187, and eicosanoids measured as described above.
Data Analysis.
Eicosanoids were quantitated with the help of MultiQuant software (Applied Biosystems/MDS SCIEX). Data are expressed as pmol/ml peritoneal lavage (or ng for stimulated cells) and are reported as the average ± SEM, with statistical significance considered as P < 0.05. Unpaired Student's t test was performed using GraphPad InStat 3.
Acknowledgments.
We thank Dr. Beverly H. Koller (University of North Carolina) and Drs. K. Frank Austen, Bing K. Lam, and Yoshihide Kanaoka (Harvard Medical School) for kindly providing transgenic mice. Dr. Koller and Dr. Peter Henson (National Jewish Health) contributed valuable discussion regarding the manuscript. This work was supported by National Institutes of Health Grant HL025785. A.S. received partial support from the European Community (LSHM-CT-2004–005033) and was the recipient of a William Fulbright Research Scholarship.
Footnotes
The authors declare no conflict of interest.
References
- 1.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;18:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 2.Nicosia S, Capra V, Rovati GE. Leukotrienes as mediators of asthma. Pulm Pharmacol Ther. 2001;14:3–19. doi: 10.1006/pupt.2000.0262. [DOI] [PubMed] [Google Scholar]
- 3.De Caterina R, Zampolli A. From asthma to atherosclerosis–5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004;350:4–7. doi: 10.1056/NEJMp038190. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, Funk CD. Lipoxygenase pathways in atherogenesis. Trends Cardiovasc Med. 2004;14:191–195. doi: 10.1016/j.tcm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Fürstenberger G, Krieg P, Müller-Decker K, Habenicht A. What are cyclooxygenases and lipoxygenases doing in the driver's seat of carcinogenesis? Int J Cancer. 2006;119:2247–2254. doi: 10.1002/ijc.22153. [DOI] [PubMed] [Google Scholar]
- 6.Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 7.Steinhilber D. 5-Lipoxygenase: Enzyme expression and regulation of activity. Pharm Acta Helv. 1994;69:3–14. doi: 10.1016/0031-6865(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 8.Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Clark JD, Schievella AR, Nalefski EA, Lin LL. Cytosolic phospholipase A2. J Lipid Mediat Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- 10.Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene biosynthesis. Am J Respir Crit Care Med. 2000;161:S36–S40. doi: 10.1164/ajrccm.161.supplement_1.ltta-8. [DOI] [PubMed] [Google Scholar]
- 11.Woods JW, et al. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal AK, et al. The nuclear membrane organization of leukotriene synthesis. Proc Natl Acad Sci USA. 2008;105:20434–20439. doi: 10.1073/pnas.0808211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 14.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch KR, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 16.Heise CE, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 17.Ciana P, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci USA. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 20.Evans JF, Ferguson AD, Mosley RT, Hutchinson JH. What's all the FLAP about?: 5-lipoxygenase-activating protein inhibitors for inflammatory diseases. Trends Pharmacol Sci. 2008;29:72–78. doi: 10.1016/j.tips.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Uhl J, et al. The 5-lipoxygenase promoter is regulated by DNA methylation. J Biol Chem. 2002;277:4374–4379. doi: 10.1074/jbc.M107665200. [DOI] [PubMed] [Google Scholar]
- 22.Flamand N, Lefebvre J, Surette ME, Picard S, Borgeat P. Arachidonic acid regulates the translocation of 5-lipoxygenase to the nuclear membranes in human neutrophils. J Biol Chem. 2006;281:129–136. doi: 10.1074/jbc.M506513200. [DOI] [PubMed] [Google Scholar]
- 23.Maclouf JA, Murphy RC. Transcellular metabolism of neutrophil-derived leukotriene A4 by human platelets. A potential cellular source of leukotriene C4. J Biol Chem. 1988;263:174–181. [PubMed] [Google Scholar]
- 24.Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: From cell-cell interactions to in vivo tissue responses. Pharmacol Rev. 2006;58:375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick FA, Morton DR, Wynalda MA. Albumin stabilizes leukotriene A4. J Biol Chem. 1982;257:4680–4683. [PubMed] [Google Scholar]
- 26.Fabre JE, et al. Transcellular biosynthesis contributes to the production of leukotrienes during inflammatory responses in vivo. J Clin Invest. 2002;109:1373–1380. doi: 10.1172/JCI14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullaly SC, Kubes P. Mast cell-expressed complement receptor, not TLR2, is the main detector of zymosan in peritonitis. Eur J Immunol. 2007;37:224–234. doi: 10.1002/eji.200636405. [DOI] [PubMed] [Google Scholar]
- 28.Rao TS, Currie JL, Shaffer AF, Isakson PC. In vivo characterization of zymosan-induced mouse peritoneal inflammation. J Pharmacol Exp Ther. 1994;269:917–925. [PubMed] [Google Scholar]
- 29.Gijón MA, Zarini S, Murphy RC. Biosynthesis of eicosanoids and transcellular metabolism of leukotrienes in murine bone marrow cells. J Lipid Res. 2007;48:716–725. doi: 10.1194/jlr.M600508-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Prakash H, Bala M, Ali A, Goel HC. Modification of gamma radiation induced response of peritoneal macrophages and splenocytes by Hippophae rhamnoides (RH-3) in mice. J Pharm Pharmacol. 2005;57:1065–1072. doi: 10.1211/0022357056668. [DOI] [PubMed] [Google Scholar]
- 31.Soule BP, et al. Effects of gamma radiation on FcεRI and TLR-mediated mast cell activation. J Immunol. 2007;179:3276–3286. doi: 10.4049/jimmunol.179.5.3276. [DOI] [PubMed] [Google Scholar]
- 32.Whelan J, Golemboski KA, Broughton KS, Kinsella JE, Dietert RR. Characterization of leukotriene production in vivo and in vitro in resident and elicited peritoneal macrophages in chickens and mice. Prostaglandins Leukot Essent Fatty Acids. 1997;56:41–49. doi: 10.1016/s0952-3278(97)90523-8. [DOI] [PubMed] [Google Scholar]
- 33.Kolaczkowska E, Shahzidi S, Seljelid R, van Rooijen N, Plytycz B. Early vascular permeability in murine experimental peritonitis is co-mediated by resident peritoneal macrophages and mast cells: crucial involvement of macrophage-derived cysteinyl-leukotrienes. Inflammation. 2002;26:61–71. doi: 10.1023/a:1014837110735. [DOI] [PubMed] [Google Scholar]
- 34.Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Koller BH. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J Immunol. 1999;163:6810–6819. [PubMed] [Google Scholar]
- 35.Takeshita K, Sakai K, Bacon KB, Gantner F. Critical role of histamine H4 receptor in leukotriene B4 production and mast cell-dependent neutrophil recruitment induced by zymosan in vivo. J Pharmacol Exp Ther. 2003;307:1072–1078. doi: 10.1124/jpet.103.057489. [DOI] [PubMed] [Google Scholar]
- 36.McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: Distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170:1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 37.Borgeat P, Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: Unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci USA. 1979;76:3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakobsson PJ, Mancini JA, Ford-Hutchinson AW. Identification and characterization of a novel human microsomal glutathione S-transferase with leukotriene C4 synthase activity and significant sequence identity to 5-lipoxygenase-activating protein and leukotriene C4 synthase. J Biol Chem. 1996;271:22203–22210. doi: 10.1074/jbc.271.36.22203. [DOI] [PubMed] [Google Scholar]
- 39.Scoggan KA, Jakobsson PJ, Ford-Hutchinson AW. Production of leukotriene C4 in different human tissues is attributable to distinct membrane bound biosynthetic enzymes. J Biol Chem. 1997;272:10182–10187. doi: 10.1074/jbc.272.15.10182. [DOI] [PubMed] [Google Scholar]
- 40.Sala A, Zarini S, Folco G, Murphy RC, Henson PM. Differential metabolism of exogenous and endogenous arachidonic acid in human neutrophils. J Biol Chem. 1999;274:28264–28269. doi: 10.1074/jbc.274.40.28264. [DOI] [PubMed] [Google Scholar]
- 41.Di Gennaro A, et al. Cysteinyl-leukotrienes receptor activation in brain inflammatory reactions and cerebral edema formation: A role for transcellular biosynthesis of cysteinyl-leukotrienes. FASEB J. 2004;18:842–844. doi: 10.1096/fj.03-0599fje. [DOI] [PubMed] [Google Scholar]
- 42.Sala A, Bolla M, Zarini S, Müller-Peddinghaus R, Folco G. Release of leukotriene A4 versus leukotriene B4 from human polymorphonuclear leukocytes. J Biol Chem. 1996;271:17944–17948. doi: 10.1074/jbc.271.30.17944. [DOI] [PubMed] [Google Scholar]
- 43.Goulet JL, Snouwaert JN, Latour AM, Coffman TM, Koller BH. Altered inflammatory responses in leukotriene-deficient mice. Proc Natl Acad Sci USA. 1994;91:12852–12856. doi: 10.1073/pnas.91.26.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]