Abstract
Aims
The aim of the present study was to investigate the association of high sensitivity CRP, interleukin-6 (IL-6) and lipoprotein-associated phospholipase A2 (Lp-PLA2) with the extent of calcified coronary atherosclerosis in patients with type 2 diabetes mellitus (T2DM).
Materials and results
This is a cross-sectional study of 306 subjects aged 40 years or older who were enrolled into the Veterans Affair Diabetes Trial. Calcified coronary atherosclerosis was assessed using electron beam computed tomography scored by the Agatston method. Clinical parameters, traditional cardiovascular risk factors and plasma levels of CRP, IL-6 and Lp-PLA2 were measured at the time of the scan. Coronary artery calcium scores (CAC) increased stepwise across increasing categories of IL-6, but did not change across increasing categories of CRP and Lp-PLA2. After adjustment for traditional cardiovascular risk factors, IL-6 was significantly associated with CAC scores (p= 0.05). The association between IL-6 and CAC was largely in those with lower (below the median) abdominal artery calcium (AAC) levels (p= 0.03).
Conclusions
Despite a generally higher level of systemic inflammation in T2DM, the inflammatory marker IL-6 remained significantly associated with CAC score, particularly in those subjects with lower AAC scores.
Keywords: Inflammatory markers, Type 2 Diabetes, Atherosclerosis, Vascular calcification, CAC, AAC
1. Introduction
Mounting evidence supports the contribution of inflammation to the pathophysiology of atherosclerosis. In fact, there has been remarkable consistency in the relationship between inflammatory markers and cardiovascular disease (CVD) in numerous population-based studies. Inflammatory markers such as high sensitivity CRP (CRP)[1,2], interleukin-6 (IL-6)[2], lipoprotein-associated phospholipase A2 (Lp-PLA2)[3], and white blood cells [4,5] are increased in individuals with CVD, and elevated levels of these markers frequently precede the development of CVD[1–7]. While type 2 diabetes (T2DM) has frequently been referred to as a chronic inflammatory state, and increased levels of markers of systemic inflammation have been consistently reported in individuals with diabetes, when compared to those without diabetes[8], most studies of inflammatory markers and CVD have been conducted in populations with a low prevalence of T2DM. There are few studies conducted within diabetes cohorts, and the results are not conclusive[9–13].
Similarly, only a few studies have assessed the relationship between inflammatory markers and direct measures of coronary atherosclerosis, such as that provided by coronary artery calcium [12]. Subjects with T2DM have increased levels of atherosclerosis in multiple blood vessels [12–15] and the relationship between inflammation and atherosclerosis may vary across vascular beds. This complicates understanding the association between various inflammatory markers and coronary disease, and may in part explain the inconsistent findings concerning inflammation and CVD in the few previous studies in diabetic cohorts. A unique feature of this study in individuals with T2DM was the availability of measures of calcified atherosclerosis in both the coronary and abdominal aortic vascular beds, permitting investigation of the association between inflammatory markers and coronary artery calcium (CAC), while accounting for the effect of non-coronary atherosclerosis, as assessed by abdominal aortic calcium (AAC).
2. Methods
2.1. Subjects
Data for this study derive from baseline examinations of participants in the Risk Factors, Atherosclerosis, and Clinical Events in Diabetes (RACED) study [14] which is a seven-site substudy of the Veterans Affairs Diabetes Trial (VADT). The study design, with exclusion/inclusion criteria, for the VADT has been previously described[16]. Approximately 95% of all subjects who were recruited into the VADT study at sites participating in the RACED sub-study also agreed to both undergo baseline electron-beam-computer-assisted tomography scans (EBCT) of the coronary and abdominal aortic vascular beds and provide blood samples for assessment of emerging risk factors. While in the fasting state, blood was drawn, and plasma and serum aliquots were prepared and frozen at −80°C, for measurement of novel cardiovascular risk factors including IL-6, CRP, and Lp-PLA2.
The VADT baseline examination included a medical history, physical examination, and collection of blood for measurement of traditional cardiovascular risk factors. Height and weight were measured to the nearest 0.1 cm and 0.5 kg respectively, and body mass index (kg/m2) was calculated. Information regarding current medical health status, including history of diabetes, hypertension, prior CVD, and medication, was collected by a questionnaire administered by research staff as previously described [16].
2.1. Laboratory methods
Plasma total cholesterol, triglycerides, and HDL cholesterol concentrations were measured using standard enzymatic methods, with reagents obtained from Roche Diagnostics (Indianapolis, IN) on a Hitachi 911 analyzer. Serum high sensitivity CRP levels were measured by an enzyme-linked immunosorbant assay (ELISA kit, Alpha Diagnostic International, San Antonio, Texas), that yields an intra-assay CV % of 2.1, 3, and 4.5 for low, medium, and high serum CRP samples, and an inter-assay CV% of 2.7, 5, and 7. IL-6 was measured by an ELISA kit (R&D systems) with intra-assay and inter-assay CVs ranging from 4 to 6% and from 5 to 10% respectively. Lp-PLA2 mass was measured by an enzyme immunoassay (PLAC® test, diaDexus) in plasma with intra-assay and inter-assay CVs ranging from 4 to 6% and 6 to 9 % respectively.
2.3. Assessment of coronary and abdominal artery calcium scores
Coronary and abdominal aortic calcium were determined by electron beam computed tomography cardiac scanning using Imatron C150XL scanners (GE Imatron, South San Francisco, CA) as previously described[14]. Readers performing calcium scoring at the centralized reading center were blinded to the demographic and clinical information. A threshold of 4 contiguous pixels and 130 Hounsfield units were used to identify calcified lesions. Calcified lesions were scored according to the method described by Agatston and colleagues[17]. The total coronary calcium score was determined by summing individual scores from each of four coronary arteries (left main, left anterior descending, circumflex, and right coronary arteries). Abdominal aortic scans were obtained by scanning a continuous section of the aorta extending from the upper pole of the right kidney down to the aortic bifurcation with 6 mm slice thickness. A calibration phantom was scanned under the chests and abdomens of each participant at each scanning center to allow calibration of the images to identical standards, as previously described[14].
2.4. Data Analysis
Statistical analyses were performed with the SAS statistical package (The SAS Institute, Inc., Cary, NC, release 9.1). The means ± SD are reported for continuous variables, except for variables with highly skewed distributions, where medians and interquartile ranges are reported. Proportions are reported for categorical variables. For the purpose of analysis, the logarithmic transformation of CAC+1 was used to reduce the effect of the skewness of the calcium data, and to permit inclusion in the analyses of those individuals with a CAC score of zero. Predictor variables with skewed distribution such as AAC, IL-6, and CRP were examined both untransformed as well as after log transformation. Although log transformation of IL-6 yielded more significant p-values, we chose to use the untransformed variables for ease of interpretation and clinical application. Two subjects with values of IL-6 > 25 pg/mL, and 15 subjects with values of CRP > 20 mg/L were excluded as extreme outliers, as these high measured levels may be due to transitory events such as acute infections. For initial assessment of inflammatory markers, we first just compared differences in the calcium scores (logarithmic transformed values of CAC+1) among categories of inflammatory markers. For ease of interpretation, data are back transformed and presented graphically as geometric means with 95% confidence intervals. To further investigate the association between these risk factors and CAC, multiple linear regression analysis was performed. Stepwise variable selection was performed to find the set of statistically significant traditional and nontraditional risk factors (age, hypertension, AAC and IL-6) to include along with several biologically relevant variables that had significant univariate associations with CAC to provide a comprehensive but relatively parsimonious model. A more complete model that included other available variables not selected in this final model did not appreciably change results and is not presented. Gender was not included as one of covariates in the multivariable model, since only 6% (n= 17) of the study population were women. However, we evaluated the effect of gender by also analyzing the data after excluding women from the analyses.
3. Results
Our study population included 306 subjects, with a mean ± SD age of 61 ± 9 years and duration of T2DM of 12 ± 8 years (Table 1). The majority of subjects were male (94%), non-Hispanic white (66%), had a history of hypertension (78%) and of past or current smoking (72%), and had laboratory evidence of dyslipidemia (total cholesterol/HDL cholesterol ratio of 5.2 ± 1.8). In addition, as indicated above our study was a substudy to VADT study and non-responsiveness to at least one oral agent and/or daily insulin injections was one of the eligibility criteria for enrollment in the study. Therefore, the majority of subjects had poor glycemic control as reflected by a mean HbA1c 9.3% ± 1.4. However, in contrast to other studies[18, 19], older subjects in our study had lower HbA1c levels, despite longer diabetes duration.
Table 1.
Clinical Characteristics of the Population
| Characteristic | |
|---|---|
| Age (years) | 61.2 ± 8.9 |
| BMI (kg/m2) | 31.4 ± 4.3 |
| Male (%) | 94 |
| Non-Hispanic whites (%) | 66 |
| Diabetes duration (years) | 12 ± 8 |
| Hypertension (%) | 78 |
| Statin use (%) | 60 |
| TZD use (%) | 11 |
| Ever smoker (%) | 72 |
| HbA1c (%) | 9.3 ± 1.4 |
| Total cholesterol/HDL cholesterol | 5.2 ± 1.8 |
| CAC score | 274 (23 – 875) |
| AAC score | 1217 (184 – 4018) |
| CRP (mg/L) | 2.9 (1.3 – 5.7) |
| Lp-PLA2 (ng/mL) | 292 (241 – 351) |
| IL-6 (pg/mL) | 3.1 (2.1 – 4.6) |
Data are presented as mean ± SD, median (25%–75%) or percent (%).
BMI: body mass index; TZD: thiazolidinedione; HbA1c: hemoglobin A1c; HDL: high density lipoprotein; CAC: coronary artery calcium; AAC: abdominal aortic calcium; CRP: high sensitivity C reactive protein; Lp-PLA2: lipoprotein-associated phospholipase A2; IL-6: interleukin-6.
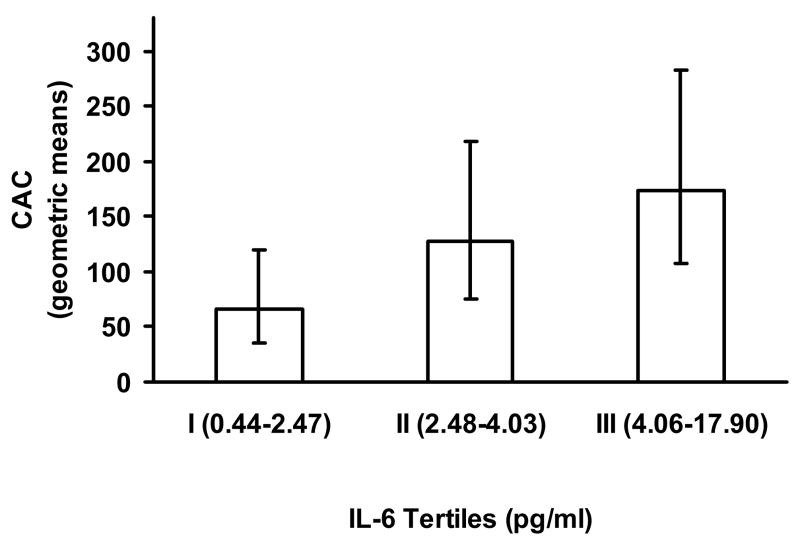
To investigate a potential relationship between inflammatory markers and CAC, we first compared CAC levels across increasing categories of CRP, Lp-PLA2 and IL-6. Coronary calcium levels did not vary significantly across CRP and Lp-PLA2 categories. However, as shown in Figure 1, CAC Agatston scores increased in a stepwise fashion across increasing tertiles of IL-6 [geometric means and (95% CIs): 66 (36–119), 128 (75–218), 174 (107–282)].
Figure 1. Coronary artery calcium levels by tertiles of IL-6.
Geometric means and 95% CI are shown for CAC levels by IL-6 tertiles for subjects with all data available (n =273). P-value= 0.11 from Kruskal-Wallis test.
To identify other relevant correlates of CAC in our study sample, univariate regression analyses were performed. As shown in table 2, age, duration of diabetes, ethnicity, hypertension, statin use, and AAC were positively associated with increasing CAC, while HbA1c was inversely associated with CAC. Older subjects, who generally have higher CAC also had lower HbA1c, which contributed to the unexpected inverse univariate association between CAC and HbA1c in our cohort. As shown in Table 3 in the multivariable model, after adjustment for other covariates including age, the association between HbA1c and CAC was substantially weaker and did not remain significant.
Table 2.
Univariate relationship between dependent variable Ln (CAC+1) and independent variables
| Independent Variable | β | SE of β | P-value |
|---|---|---|---|
| Age (years) | 0.12 | 0.02 | < 0.01 |
| BMI (kg/m2) | 0.01 | 0.04 | 0.68 |
| Waist circumference (cm) | 0.02 | 0.01 | 0.11 |
| Non-Hispanic whites (vs. others) | 1.30 | 0.31 | < 0.01 |
| Diabetes duration (years) | 0.08 | 0.02 | < 0.01 |
| Hypertension (yes/no) | 1.18 | 0.37 | < 0.01 |
| Statin use (yes/no) | 0.72 | 0.31 | 0.02 |
| TZD use (yes/no) | 0.27 | 0.49 | 0.58 |
| Ever smoker (yes/no) | 0.37 | 0.34 | 0.28 |
| HbA1c (%) | −0.42 | 0.11 | < 0.01 |
| Total cholesterol/HDL cholesterol | −0.01 | 0.09 | 0.94 |
| AAC score | 0.00036 | 0.000036 | < 0.01 |
| CRP (mg/L) | −0.04 | 0.04 | 0.38 |
| Lp-PLA2 (ng/mL) | 0.000052 | 0.00195 | 0.98 |
| IL-6 (pg/mL) | 0.11 | 0.06 | 0.07 |
BMI: body mass index; TZD: thiazolidinedione; HbA1c: hemoglobin A1c; HDL: high density lipoprotein; CAC: coronary artery calcium; AAC: abdominal aortic calcium; CRP: high sensitivity C reactive protein; Lp-PLA2: lipoprotein-associated phospholipase A2; IL-6: interleukin-6.
Table 3.
Multivariable regression models searching for independent correlates of Ln (CAC+1)
| Model Without Interaction | Nested Interaction Model | |||||
|---|---|---|---|---|---|---|
| Independent Variable | β | SE of β | P-value | β | SE of β | P-value |
| Age (years) | 0.06 | 0.02 | < 0.01 | 0.06 | 0.02 | < 0.01 |
| Non-Hispanic whites (vs. others) | 0.44 | 0.30 | 0.15 | 0.45 | 0.30 | 0.14 |
| Diabetes duration (years) | 0.02 | 0.02 | 0.33 | 0.02 | 0.02 | 0.37 |
| Hypertension (yes/no) | 0.81 | 0.34 | 0.02 | 0.86 | 0.33 | 0.01 |
| HbA1c (%) | −0.15 | 0.10 | 0.16 | −0.16 | 0.10 | 0.13 |
| Statin use (yes/no) | 0.27 | 0.28 | 0.34 | 0.07 | 0.28 | 0.81 |
| Continuous AAC | 0.00024 | 0.000042 | < 0.01 | |||
| IL-6 (pg/mL) | 0.10 | 0.05 | 0.05 | |||
| Higher AAC (vs. lower AAC) | 2.42 | 0.51 | < 0.01 | |||
| IL-6 within higher AAC | 0.01 | 0.07 | 0.86 | |||
| IL-6 within lower AAC | 0.16 | 0.07 | 0.04 | |||
IL-6 was treated as a continuous variable, while AAC was treated as a continuous variable in the model without the interaction term, but dichotomized at the median in the nested model. Number of subjects with all data = 273.
Consistent with the data presented in Figure 1, there was an indication of a borderline significant (p= 0.07) association between IL-6 and CAC in the univariate analysis (Table 2). After log transformation of the skewed IL-6 data, this association was statistically significant (p < 0.01). To determine if the association of IL-6 with CAC persisted after adjustment for other traditional risk factors, a series of multiple linear regression models were performed. Controlling for potential confounding variables (age, duration of diabetes, ethnicity, HbA1c, AAC and hypertension, statin use) that were individually associated with CAC, IL-6 remained significantly (p= 0.05) associated with CAC (Table 3-model without interaction). The risk factor-adjusted association between IL-6 and CAC was even more significant when the analyses were limited to men (p= 0.03). Addition of other variables not included in this final more parsimonious model, such as BMI, cigarette smoking, medication use, and lipid levels were neither significantly associated with CAC nor appreciably changed the relationship of IL-6 with CAC (Supplemental Table).
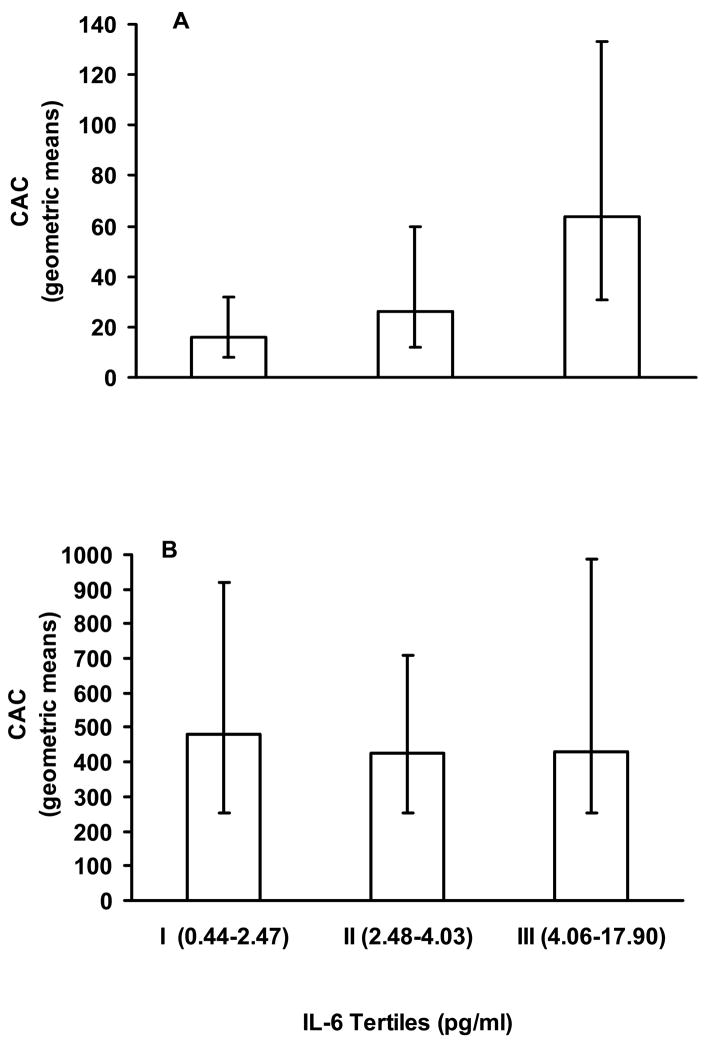
As calcified atherosclerosis in the abdominal aorta typically occurs earlier than, and is highly correlated with, calcified atherosclerosis in other vascular beds[20], AAC provides a reasonable measure of non-coronary atherosclerotic burden. It therefore seemed plausible that subjects with extensive non-coronary atherosclerosis (as estimated by AAC) could have high IL-6 levels regardless of the CAC scores, and this could influence the association between CAC and IL-6. This speculation was supported by the fact that after removing AAC from the multiple regression model, the association between IL-6 and CAC was no longer significant (p= 0.11). When we dichotomized AAC (at the median value of 1217) we noted that the median CAC was considerably lower in those with lower AAC than for those in the higher AAC group (p < 0.0001). Furthermore, as shown in Figure 2, mean CAC is significantly different among the tertiles of IL-6 in those with lower AAC (p=0.03), but not in those with higher AAC (p=0.76). Similarly, when IL-6 was parameterized as being nested within lower or higher AAC levels, as shown in Table 3, after adjustment for age, diabetes duration, ethnicity (non-Hispanic whites vs. others), HbA1c and hypertension, the association between IL-6 and CAC remained statistically significant (p= 0.03) in those with lower AAC (below the median) but not (p= 0.86) in those with higher AAC (above the median).
Figure 2. Coronary artery calcium levels by tertiles of IL-6 according to AAC levels.
Geometric means and 95% CI are shown for CAC levels by tertiles of IL-6 as defined for the entire group (see Fig. 1).
Panel A: CAC values by tertiles of IL-6 in those with lower AAC (< median value of 1217). Numbers of subjects in categories I, II and III were 53, 39 and 44, respectively. P-value= 0.02 from Kruskal-Wallis test.
Panel B: CAC values by tertiles of IL-6 in those with higher AAC (≥ median). Numbers of subjects in categories I, II and III were 38, 51 and 48, respectively. P-value= 0.76 from Kruskal-Wallis test.
4. Discussion
This study demonstrated that despite high levels of markers of systemic inflammation in T2DM patients, the inflammatory marker IL-6 but not CRP or Lp-PLA2 was associated with CAC. The association between IL-6 and CAC remained significant after adjustment for other traditional cardiovascular risk factors. In order to avoid overfitting of the regression model, we limited the total number of predictor variables tested in any regression model. We therefore presented a final relatively parsimonious model comprised of the most relevant variables. However, other variables, not included in this final model, such as BMI, waist circumference, cigarette smoking, medication use, and lipid levels were also evaluated in similar multiple regression models and were not found to be significantly associated with CAC nor to appreciably change the model results (Supplemental Table).
It was also notable that this association was substantially more evident in those with less extensive systemic atherosclerosis. As shown in Figure 2, CAC generally increased with increasing IL-6 in those with lower AAC, while the IL-6: CAC relationship was much less obvious in those with higher AAC. Similar results were obtained when other cut-offs for AAC (such as tertiles of AAC or a commonly used clinical cut-off score of 1000) were used to divide the groups into low and high categories. In fact, the interaction effect between AAC and IL-6 was observed using dichotomized AAC (low/high) categories and IL-6 as tertiles, quartiles or a continuous variable (data not shown). As described above and demonstrated in Figure 2, stratifying subjects by lower AAC values identified subjects with substantially lower CAC values, with median or geometric mean values 10–20 times lower than in those with higher AAC values. This is consistent with many studies which indicate that aortic calcification usually precedes coronary artery calcium deposition[20].
One may speculate that the group with lower CAC and lower AAC may represent those with earlier stages of atherosclerosis, in which ongoing formation of new plaque and higher inflammatory activity is present, thus displaying the stronger relationship with IL-6. In contrast, the group with higher AAC presumably includes many individuals with extensive and more advanced atherosclerosis. Much of the calcium deposition in these advanced lesions may reflect vascular healing and remodeling, leading to more stable plaque and less ongoing vascular inflammation, and may not contribute to further elevation of plasma inflammatory markers.
The limitations of this study also need to be considered. As this was a cross-sectional study, it is not possible to draw conclusions about causal relationships and the direction of this association between IL-6 and CAC. Although IL-6 has been found to predict future CVD events[21], it is certainly possible that the association may have resulted from IL-6 being released from the artery wall as a consequence of plaque formation. In addition, T2DM is commonly associated with a cluster of risk factors, such as obesity, hyperglycemia, insulin resistance and hypertension, as well as with derangements of the immune system [8,22,23] that contribute individually or in combination to the high risk of both atherosclerosis and a heightened level of systemic inflammation. Many inflammatory markers, such as IL-6, are produced not only by a variety of vascular cell types [24,25] but also by myocardial cells [26] and adipose tissue[27]. Thus, with so many potential contributing metabolic factors and nonvascular sources of inflammation, discerning the relationship between inflammatory markers and coronary atherosclerosis can be difficult in individuals with T2DM. It is therefore not surprising that not all inflammatory markers examined were significantly associated with CAC. Although recent findings suggest that higher plasma concentrations of CRP and Lp-PLA2 mass and activity are associated with CVD events and plaque vulnerability[2,3], they were not associated with CAC in our study. The association between Lp-PLA2 (mass and activity) and atherosclerosis per se in humans has not been well investigated and the association between CRP and atherosclerosis has been inconsistent[28]. Although IL-6 is a primary stimulus for hepatic CRP production and other inflammatory cytokines, concentrations of IL-6 and CRP may not always change in parallel. In fact, the correlation between IL-6 and CRP (r= 0.47) in our study, consistent with other reports[2,29], suggests that IL- 6 accounted for less than 25% of the variation in CRP levels. Notably, this study only included several potentially relevant inflammatory CVD risk factors, and there certainly may be other novel factors that may be associated with CAC in diabetic populations. It is also important to recognize that although EBCT is a highly sensitive method for detecting vascular calcification and that it strongly related to plaque burden and CVD events, it is not possible to distinguish between the contribution of intimal vs. medial calcification to the coronary and abdominal aortic scores. Finally, this cohort was comprised mainly of men with T2DM in poor control, and these results may not extend to a more diverse diabetes population.
In summary, our results demonstrate that despite a generally higher level of systemic inflammation in T2DM, the inflammatory marker IL-6 remained significantly associated with coronary atherosclerosis, particularly in those subjects with evidence of less systemic atherosclerosis. The results of this study also raise the possibility that a better characterization of systemic atherosclerosis may uncover relationships between risk factors and site-specific atherosclerosis that were not previously appreciated.
Supplementary Material
Acknowledgments
We would like to thank the VADT study participants, study staff and the investigators at the Phoenix, San Diego, Long Beach, Hines, Pittsburgh, Tucson, and Miami Veteran Affairs Medical Centers for the participation in this study. We would also like to acknowledge the contributions of the Hines VA Cooperative Studies Program Coordinating Center, the Tufts Lipid Metabolism Laboratory and the Harbor UCLA EBCT Reading Center. This work was supported by the Office of Research and Development, Medical Research Service and Cooperative studies program, Department of Veteran Affairs, and by NIH grants RO1067690 (P.D.R), P01 HL076491, P01 HL77107, HL70621, Kronos Research Institute, and a clinical research award from the American Diabetes Association (P.D.R).
Footnotes
Duality of interest The authors attest that no conflict of interest or duality exists related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 2.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb Haemost. 2006;95:511–518. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study 1. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 4.Sabatine MS, Morrow DA, Cannon CP, et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 (Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy- Thrombolysis in Myocardial Infarction 18 trial)substudy. J Am Coll Cardiol. 2002;40:1761–1768. doi: 10.1016/s0735-1097(02)02484-1. [DOI] [PubMed] [Google Scholar]
- 5.Stewart RA, White HD, Kirby AC, et al. White blood cell count predicts reduction in coronary heart disease mortality with pravastatin. Circulation. 2005;111:1756–1762. doi: 10.1161/01.CIR.0000160924.73417.26. [DOI] [PubMed] [Google Scholar]
- 6.Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. doi: 10.1016/s0021-9150(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, He J, Chen J, Fonseca V, Whelton PK. Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III) Ann Epidemiol. 2004;14:686–695. doi: 10.1016/j.annepidem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Malik S, Wong ND, Franklin S, Pio J, Fairchild C, Chen R. Cardiovascular disease in U.S. patients with metabolic syndrome, diabetes, and elevated C-reactive protein. Diabetes Care. 2005;28:690–693. doi: 10.2337/diacare.28.3.690. [DOI] [PubMed] [Google Scholar]
- 10.Tuttle HA, vis-Gorman G, Goldman S, Copeland JG, McDonagh PF. Proinflammatory cytokines are increased in type 2 diabetic women with cardiovascular disease. J Diabetes Complications. 2004;18:343–351. doi: 10.1016/S1056-8727(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 11.Moussavi N, Renier G, Roussin A, Mamputu JC, Buithieu J, Serri O. Lack of concordance between plasma markers of cardiovascular risk and intima-media thickness in patients with type 2 diabetes. Diabetes Obes Metab. 2004;6:69–77. doi: 10.1111/j.1463-1326.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- 12.Bowden DW, Lange LA, Langefeld CD, et al. The relationship between C-reactive protein and subclinical cardiovascular disease in the Diabetes Heart Study (DHS) Am Heart J. 2005;150:1032–1038. doi: 10.1016/j.ahj.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe ML, Iqbal N, Gefter W, Mohler ER, III, Rader DJ, Reilly MP. Coronary artery calcification at electron beam computed tomography is increased in asymptomatic type 2 diabetics independent of traditional risk factors. J Cardiovasc Risk. 2002;9:369–376. doi: 10.1097/01.hjr.0000049242.21319.a7. [DOI] [PubMed] [Google Scholar]
- 14.Reaven PD, Sacks J. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia. 2005;48:379–385. doi: 10.1007/s00125-004-1640-z. [DOI] [PubMed] [Google Scholar]
- 15.Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care. 2001;24:335–338. doi: 10.2337/diacare.24.2.335. [DOI] [PubMed] [Google Scholar]
- 16.Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003;17:314–322. doi: 10.1016/s1056-8727(02)00277-5. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Furuya H, Nagaoka T, Mizushima S, et al. Associations between glycated hemoglobin levels and atherosclerotic risk factors in urban communities. Nippon Koshu Eisei Zasshi. 2002;49:729–738. [PubMed] [Google Scholar]
- 19.Jorde R, Hagen T. Screening for diabetes using HbA1c in elderly subjects. Acta Diabetol. 2006;43:52–56. doi: 10.1007/s00592-006-0212-8. [DOI] [PubMed] [Google Scholar]
- 20.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 21.Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–148. doi: 10.1053/j.ajkd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Reaven GM. Banting Lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 23.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 24.Resch U, Winsauer G, Hofer-Warbinek R, de MR. X-linked inhibitor of apoptosis protein regulates human interleukin-6 in umbilical vein endothelial cells via stimulation of the nuclear factor-kappaB and MAP kinase signaling pathways. Pharmacol Rep. 2006;58 (Suppl):111–117. [PubMed] [Google Scholar]
- 25.Giulietti A, van EE, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007 Jul;77(1):47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko K, Kanda T, Yokoyama T, et al. Expression of interleukin-6 in the ventricles and coronary arteries of patients with myocardial infarction. Res Commun Mol Pathol Pharmacol. 1997;97:3–12. [PubMed] [Google Scholar]
- 27.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 28.Hamirani YS, Pandey S, Rivera JJ, et al. Markers of inflammation and coronary artery calcification: A systematic review. Atherosclerosis. 2008 May 13; doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.