Abstract
OBJECTIVE
The purpose of the current study was to longitudinally assess the development of automatic sound feature discrimination and compare it to behavioral discrimination in late-implanted cochlear implant users.
METHODS
Scalp-recorded auditory evoked potentials (AEPs) and behavioral discrimination of frequency, duration and intensity differences within an odd-ball paradigm using complex stimuli were recorded in three late-implanted Cochlear implant subjects beginning on turn-on day.
RESULTS
Variable results were obtained in behavioral and AEPs that were consistent with the amount of pre-implant auditory experience each subject had. The best user showed rapid development of neurophysiologic indices of change detection along with improvement in behavioral and real-world auditory skills. In contrast, there were no recordable AEPs in the poorer CI user and there was little change in behavioral outcomes.
CONCLUSION
There is evidence of utilization of usual auditory processing pathways in the AEPs of some children who receive cochlear implants late in their childhood. Some plasticity in the auditory cortical pathways may be present despite prolonged auditory deprivation in school-aged children who are late-implanted cochlear implant recipients
Keywords: Auditory event-related potentials (AEPs), Event-related potentials (ERPs), children, late-implanted, plasticity
Introduction
The option of multichannel cochlear implantation for patients who have sensory hearing loss has been available for the last two decades. This advanced clinical technology provides a unique opportunity to explore neural plasticity of the human auditory system following sensory deprivation and subsequent cochlear implantation. The objective of a cochlear implant (CI) is to stimulate central auditory system activity, in order to aid in the acquisition, use or maintenance of aurally based speech and language. Although it is optimal to implant children in early childhood [1, 2, 3, 4], it is not always possible to do so. Therefore, it is important to evaluate the efficacy of implantation at various stages of human development. One way to do this is to assess changes in the neurophysiological brain responses as a function of experience with sound, starting with the initial stimulation of the central auditory pathways via a CI.
Research supports the notion of a “sensitive period”, in which the greatest chance for benefit is when a CI is placed during that period [3, 5, 6]. However, some children who are implanted past the ‘sensitive period’ also receive significant benefit [7, 8], although much greater variation in auditory performance (and thus benefit) in late-implanted children as a group has been reported [9, 10, 11, 12, 13]. Performance variation may be due to the individual auditory experience of the implant candidate, in that some children have had little to no auditory experience, while others have some initial hearing and have benefited from long-term use of high-powered hearing aids. One goal of the current study was to determine whether such auditory experience prior to implantation might be related to efficacy of implant use in late-implanted children.
The reorganization and utilization of auditory cortex by other sensory modalities in adults with pre-lingual deafness is well documented [14, 15, 16]. Reorganized auditory cortical pathways following congenital deafness may be explain why efficacy of a CI is low in many pre-lingually deafened late-implanted adults, adolescents [17, 18], and children [19].
The activation of primary and secondary auditory cortical areas after cochlear implantation indicates preservation of some of the critical pathways for audition despite re-organization. In deaf cats, tonotopic preservation in auditory cortex has been shown with novel input via cochlear implants, despite long periods of deafness [20]. Additionally, implanted cats maintained some temporal and binaural processing pathways [20, 21]. However, as the period of deafness extended past a critical period, the implanted cats showed a reduced area of cortical activation when compared to cats implanted before the end of that critical period [22]. In contrast, increased metabolic activity in primary auditory cortex has been shown in fMRI studies comparing pre- and post-cochlear implantation in humans (both children and adults) [23]. This indicates that there is either re-direction of cortical areas back to the auditory modality through a CI’s input or some residual availability of the auditory cortical pathways for auditory processes despite prolonged periods of auditory deprivation. Auditory processing is thought to mainly occur in traditional cortical areas in early-implanted children and post-lingually deafened adults [23]. It is largely unknown whether the traditional auditory pathways can be effectively activated in late-implanted CI children.
Kral et al. suggest that there are three phases of adaptive plasticity in cochlear implantation, which can be extended from their animal data to young children with cochlear implants [24]. In the first few weeks of implantation, the changes within auditory cortex occur, with increased activity and recruitment of additional cortical regions for auditory processing. The pace of these early changes varies for early versus late implant recipients, but occurs in most, regardless of the length of deafness. During the first few months after implantation, further auditory cortical changes occur with larger amplitudes and increased areas of activation in auditory cortex. These changes are most pronounced when the implant is placed within the critical period. In the last and longest phase, higher order processing pathways are activated in response to auditory input. In addition, Kral et al. proposed that a reduction in the amplitudes of the late potential neurophysiologic responses seen in cats implanted after the critical period indicate that functional development of auditory processing pathways depends on the degree of auditory experience within the critical period [25]. They suggest that, after the critical period, some decoupling of primary auditory cortex from higher order auditory processing pathways occurs due to the re-organization by other sensory modalities, which may inhibit access to these pathways by the primary auditory cortex after cochlear implantation [25, 26].
Despite such reported and proposed physiological constraints due to reorganization of auditory processing pathways, Waltzman et al. [11] demonstrated clinical benefits for most late-implanted children, with continued improvement in performance in auditory discrimination tasks for pre-teen and teenaged implant recipients who were educated in oral or total communication environments. Although continued capacity for plasticity in the central auditory pathways in mature mammals and adults has been shown in acquired hearing impairments [27, 28, 29], such plasticity is usually the result of disruption of the normal input (e.g., lesion-induced changes) or, as seen in animal training (learning-related plasticity) [30, 31, 32]. The efficacy of a late-implanted CI may be influenced by the degree of cortical reorganization, which may in turn be altered by the degree of auditory input present in early childhood. Thus, information on higher auditory processing and potential plasticity of such processes in late-implanted CI recipients with different pre-implant auditory experiences is needed to determine the best way to maximize the auditory potential in such individuals.
Scalp-recorded auditory evoked potentials (AEPs) provide a non-invasive measure of cortical brain activity that has been shown to reflect the neurophysiological changes occurring during cortical maturation in childhood [2, 6]. The cortically generated AEPs in normal hearing individuals continue to show maturational changes up to 15 years of age [2, 33], determined by latency and amplitude of the responses, and can therefore be used to assess cortical plasticity following implantation. Moreover, the cortically generated mismatch negativity (MMN) and the P3a components of AEPs can provide neurophysiological indices of auditory change detection [34, 35] and passive orienting to sounds in the environment [36, 37], respectively. For the current study, the MMN and P3a components were used to index when changes in the tone frequency, duration, or intensity were passively detected, and when the changes were salient enough to involuntarily capture attention during the first three months after implantation.
Thus, the goal of this preliminary study was to determine whether early auditory experience in late-implanted pre-teenage children impacted on plasticity and maturation of central auditory processes. We evaluated late cortical auditory-evoked potentials in three children who received cochlear implants between the ages of nine and twelve years, each of who had different auditory experiences prior to implantation (see Methods section for full participant history description). Passive and active discrimination of sound features were tested using electrophysiological and behavioral measures, respectively. Additionally, to assess the time course of plasticity, measurements were taken on the first day (Day 1), four weeks (Month 1) later, and three months (Month 3) after initial stimulation. Physiological indices of sound onset detection (e.g. P1, N1, N2), deviance detection (MMN), and involuntary orienting (P3a) were assessed and compared with behavioral measures (hit rate and reaction time) at each time point. Identifying whether prior auditory experience in late-implanted children leads to differential outcomes in behavioral and neurophysiological indices of sound detection and feature discrimination may provide useful information to help direct rehabilitation efforts for late implanted children.
Materials and Methods
Subjects
Three children (hereafter called “S1”, “S2”, and “S3”) participated in the study from the day their cochlear implant was turned on (approximately one month after surgery), through the first three months of implant use. All subjects were prelingually deafened. Informed assent was obtained from the children after the experimental protocol and procedures were explained in signed English, and informed consent, was obtained by their parents (in English or Spanish), in accordance with the human subjects’ research protocol approved by the Committee for Clinical Investigations at the Albert Einstein College of Medicine (AECOM). Testing was conducted at the Kennedy Center Cognitive Neurophysiology Laboratory at AECOM.
Subjects
Relevant medical, auditory, family, and educational histories are presented in Table 1.
Table 1.
Relevant Subject History
| Subject # | Birth | Medical | Sensory Hearing loss (age identified by family) | Hearing aid use (age begun) | Family Hearing loss | Communication | Implanted age |
|---|---|---|---|---|---|---|---|
| 1 | Born full term healthy | Waardenberg’s Syndrome type II | Profound (<1 year) | Consistent (age 10.7) | None | Informal sign until age 10 then TC | 11yr 6 mo |
| 2 | Born at 28 weeks gestation (placenta previa) | none | Profound (14 months) | Inconsistent (age 16 months) | Two cousins | Total Communication from age 3 | 9 yr 1 mo |
| 3 | Born full term healthy | Multiple otitis media episodes | Rapid down slope: Moderately Severe to profound progressing to severe to profound (21 months) | Consistent (age 4) | Uncle and brother | Total Communication from age 3 | 11yr 8 mo |
S1 was implanted at 11 years, 6 months of age. She had bilateral profound deafness prior to implantation. Her family reported that she did not orient to sound from her first few months of life and estimated her length of deafness (LOD) at 11. 5 years. She moved to the U.S.A. from Guyana approximately one year prior to implantation. Once in the U.S.A., she was diagnosed with Waardenberg Syndrome. She was found to have no additional disabilities and normal intelligence. Because her family did not have access to a deaf educator, she had no formal educational training until she arrived in the U.S.A., having previously used rudimentary signs to communicate. She used high-powered hearing aids for about 8 months prior to implantation and had no lip-reading or spoken speech skills. Her CI was implanted on the right.
S2 was implanted at 9 years, 1 month of age. She was born in the U.S.A., and wore hearing aids inconsistently from age 16 months around the time when her family identified her as having hearing impairment. Her estimated LOD was 7 yr 11 mo. She was educated in a Total Communication (TC) school from age 3 and had normal intelligence with no additional disabilities. She communicated primarily in signed English. Prior to implantation, she had good lip-reading skills in both English and Spanish; poor environmental sound recognition; and was not able to identify sound patterns with hearing aid amplification in the auditory-only modality. Her audiogram showed bilateral profound hearing losses prior to implantation. Her CI was implanted on the right.
S3 was implanted at 11 years, 8 months of age. He was born in the U.S.A. His family suspected a hearing impairment at age 21 months and he used hearing aids consistently since age 4, when he was formally diagnosed with bilateral moderate to profound hearing loss, with a rapid down slope that progressed over the following 2 years, LOD was 7 years or greater. Even with hearing aids and auditory therapy, he did not develop auditory-based language, and was educated in a TC school from age 4. He had normal intelligence and no additional disabilities. His hearing history was complicated by multiple episodes of otitis media, and a need for tympanostomy tubes at ages 7 and 10. He communicated primarily in signed English. Prior to implantation, he had good lip-reading skills, in both English and Spanish. He was able to distinguish differences between sounds of musical instruments, but had poor aided auditory-only discrimination for speech or vowel-consonant pairs. His CI was implanted on the left.
Stimuli and Procedures
Stimuli
Complex tone stimuli were created and presented at a stimulus-onset-asynchrony (SOA) of 550 ms using Neuroscan Stim hardware and software (Compumedics, Corp., El Paso, TX), through one loudspeaker placed two meters away from the subjects (0° along the azimuth, and 30 cm above ear level). Sound level at the speaker was calibrated using a Brüel & Kjær sound level meter.
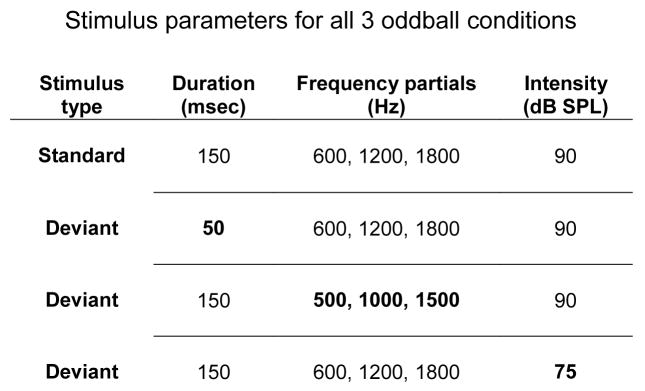
Behavioral and electrophysiological measures were obtained separately using three auditory oddball conditions (Frequency, Intensity, and Duration) to measure passive and active indices of auditory change detection. The standard tone had an f0 of 500 Hz (with two harmonic partials above f0), was 50 ms in duration, and intensity level of 90 dB SPL. ‘Oddballs’ (deviants) were randomly presented with 10% probability, separately in each condition (Figure 1). For EEG recording, 1100 stimuli (110 deviants) were presented per condition, in three blocks (367 tones per block, nine blocks in total). In the behavioral testing, 220 sounds, containing approximately 20 pseudo-random deviants (two deviants did not occur successively), were presented for each sound feature tested.
Figure 1.
Tones for the randomized oddball paradigm with all deviant tones at 10% and standard tones at 90% frequencies per block. Bold values represent changes made to the standard tone in order to create a deviant tone for each of the 3 conditions, Duration condition, Frequency condition and Intensity condition. Each tone consists of 3 partials (middle column) presented with its respective duration (left column) and intensity (right column) as indicated on each line. One deviant was presented in a condition with the standard.
Procedures
Signed English was used as the primary form of communication during the experimental procedures. Prior to each testing session, each subject had fresh batteries placed in their CI. Each subject confirmed sound sensation from his or her CI prior to beginning of each test. Subjects sat in a comfortable chair, in a sound attenuated IAC booth (Bronx, NY). EEG was recorded on all three sessions, and was always conducted first. Subjects watched a closed-captioned video of their choice during EEG recording, and had no task with the sounds. Behavioral tests were conducted in the second (Month 1) and third (Month 3) testing sessions, after the EEG recording session on the same day, also in the IAC booth. For the behavioral testing, subjects listened to the sounds and were instructed to press the response key whenever they heard a sound change. Instructions and a practice run were given prior to the test session, using larger deviant-to-standard ratios for the three features in practice than were used in the test session. If a child was unable to perceive the differences during practice (e.g., no correct button pushes after presentation of 50 tones, 5 deviants), then further behavioral testing was not conducted that day. Total session time, including electrode cap placement, snack and breaks, was 1.5 hours.
Electroencephalogram (EEG) Recording
EEG was recorded with an electrode cap using a subset of ten electrodes in the configuration of the 10–20 international system (Fz, Cz, F3, F4, C3, C4, Pz, P3, P4) and a mastoid electrode placed on the opposite side of the implant used. Vertical electro-oculogram (VEOG) was recorded between FP1 or FP2 and an external electrode placed below the eye opposite the implant. The tip of the nose was used as the reference electrode. Impedances were kept below 5 kOhms. The EEG was obtained with a Nicolet SM2000 amplifier at a gain of 1000 using a sampling rate of 500 Hz (band pass 0.05–40Hz), on a dedicated PC computer using Neuroscan Scan 4.1 software (Compumedics, Corp., El Paso Texas). EEG was monitored for movement artifacts, excessive eye blinks, and sleep spindles. The experimenter also monitored EEG for regular eye saccades that indicated that participants were reading the captions of the video.
Data Analysis
Behavioral
Hit rate (HR), false alarm rate (FAR), d′, and reaction time (RT) were measured in each condition separately. The measure d′ is derived from signal detection theory [38], measuring the separation between two classes of stimuli on a hypothetical inner perceptual dimension upon which the subject’s decision is based. In the present experiment, we calculated d′, using a yes/no model [39], as a measure of the subject’s ability to detect frequency, duration, and intensity deviants within the oddball sequences. ‘Hits’ were correct button presses to deviants when responded to within a time window of 200–1500 ms; ‘misses’ were the absence of a button press to deviant tones. “Correct rejections” were correct “no-go” responses to standard tones, and “false alarms” were any button presses to standard tones. The result was a number for each subject in each condition, on a scale from 0 – 4.65, where 0 indicates no ability to distinguish between the two sound features, and 4.65 indicates high sensitivity to discriminate the features.
EEG
EEG was analyzed off-line using Neuroscan Edit 4.1 software (Compumedics Corp., El Paso Texas). EEG data was filtered 1–15 Hz (24 dB/octave) with zero phase shift. Epochs were 600 ms, from 100 ms pre-stimulus onset to 500 ms post-stimulus onset. Epochs were baseline corrected, and then epochs with signals exceeding +/− 100 μV on any channel were rejected from further analysis. Additionally, the first 10 responses from each block were removed prior to analysis (to avoid including the possible orienting response to the onset of each new run), and the responses to the tone following a deviant stimulus were removed (to avoid potential carryover effects). The remaining epochs were sorted by stimulus type (standards and deviants), for each subject separately, in each condition. There were over 3400 standard epochs and over 190 deviant epochs, for each subject on each testing day.
To delineate the obligatory response to sound onsets, the AEP elicited by the standard tones were averaged across all oddball conditions and a grand-mean waveform was created, as the standard tone in all conditions was the same. To delineate the MMN and P3a components, a difference waveform was created by subtracting the grand-mean standard auditory evoked potential (AEP) waveform from the grand-mean deviant AEP waveform.
Results and Discussion
The behavioral results are presented in Tables 2–4, and the AEP results are displayed in Figures 2–5. Implant-generated electrical artifact in the AEPs can be observed on the implanted side of all subjects, whereas the side opposite to the implant showed little artifact.
Table 2.
S1 behavioral results
| Time | Intensity | Frequency | Duration | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | FAR | d′ | HR | FAR | d′ | HR | FAR | d′ | |
| M1 | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| M3 | 0 | 0 | - | 0 | 0.5 | - | 0.13 | 0.5 | 1.43 |
HR – Hit Rate
FAR – False Alarm Rate
d′ – d-prime
-- not able to do task
M1 – Month 1
M3 – Month 3
Table 4.
S3 behavioral results
| Time | Intensity | Frequency | Duration | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | FAR | d′ | HR | FAR | d′ | HR | FAR | d′ | |
| M1 | .20 | .4 | 0.91 | .83 | .2 | 3.01 | .70 | 0 | 3.10 |
| M3 | .44 | 0 | 2.91 | .95 | 0 | 4.22 | .86 | .5 | 3.65 |
HR – Hit Rate
FAR – False Alarm Rate
d′ – d-prime
M1 – Month 1
M3 – Month 3
Figure 2.
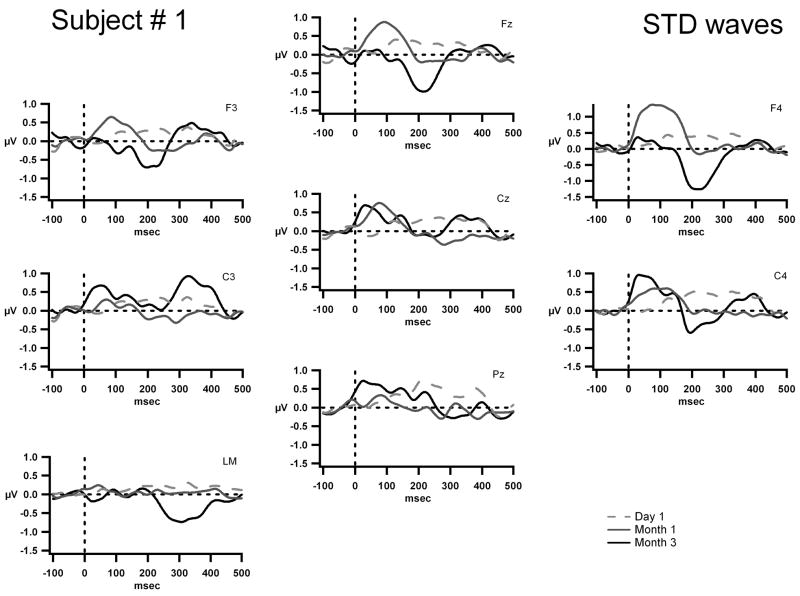
The grand-mean AEP waves to the standard tone are displayed for Fz, Cz, Pz, F3, F4, C3, C4, and the left mastoid (LM) for S1 on Day 1, (dashed gray line) Month 1, (solid gray line) and Month 3 (solid black line). The x-axis represents time in milliseconds and the y-axis represents amplitude in micro volts. The scale is five times larger than that shown in figures 3, 4 and 5. This subject’s implant is on the right side and thus there is an electrical artifact manifest as a square wave at F4 and C4.
Figure 5.
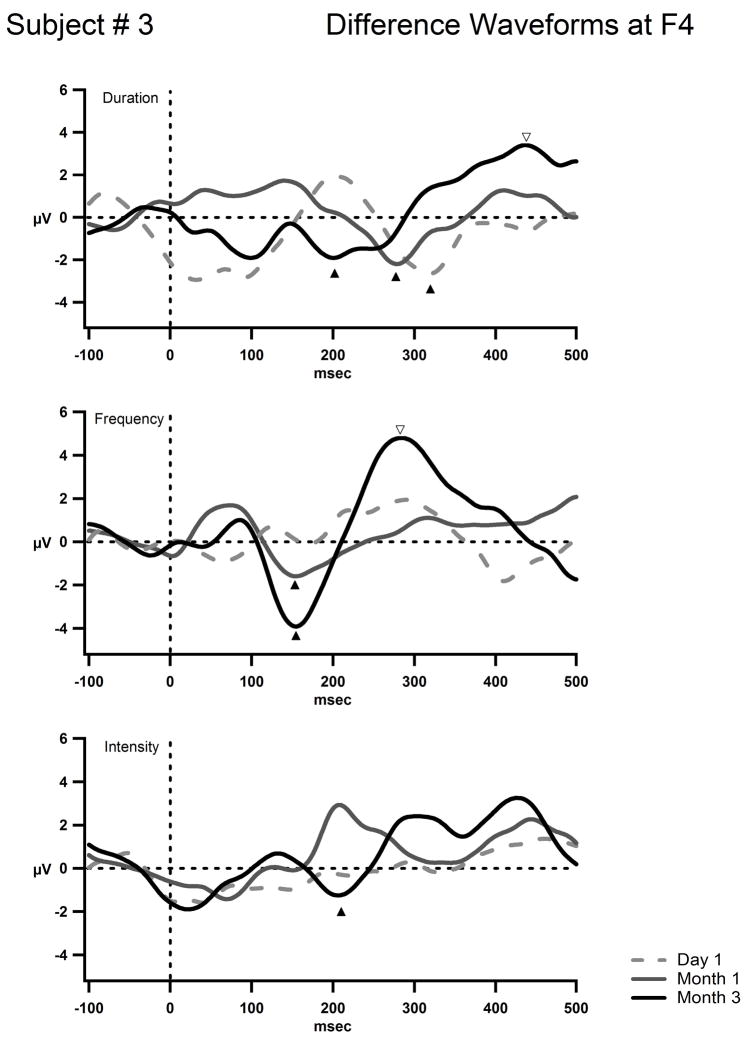
S3’s difference waves at the F4 electrode for the deviant conditions in the oddball paradigm are shown in three graphs each with its condition labeled. The three time points are plotted together for each condition as indicated in the legend (Day 1, (dashed gray line) Month 1, (solid gray line) and Month 3 (solid black line). The condition with a duration deviant is represented on the top. The frequency deviant condition is in the middle and the intensity deviant condition on the bottom. F4 was chosen as the frontal electrode furthest away from the left-sided implant magnet to minimize the electrical artifact. The x-axis represents time in milliseconds and the y-axis represents amplitude in microvolts. The scale is the same as for figures 3 and 4. The mismatch negativity (MMN) is labeled with the dark up-pointing arrowheads. The P3a waves are labeled with open down-pointing arrowheads. A mismatch negativity to the duration of the tone is present on day 1 (top row) and becomes earlier with implant usage. The MMN to frequency changes is first seen at month one and becomes more pronounced by month 3. The MMN to intensity changes is first seen at month 3 (bottom row). By month 3 a large P3a response to frequency and duration deviants were elicited (middle and top rows) and the suggestion of a P3a is seen for the duration deviant (top graph open arrowhead).
S1
Behavior and AEP’s
S1, who had little previous auditory experience prior to implantation, was unable to behaviorally detect sound changes in the first behavioral test session (M1, Table 2). In the second session, (M3, Table 2), she was able to reliably distinguish between the standard and deviant, only for tone duration (d′=1.43). This reflects a potentially emerging ability to discriminate some temporal characteristics of sound after three months of implant use.
Figure 2 displays the grand-mean AEP waveforms for S1. The presence of obligatory AEP components is negligible. The small positive peak occurring around 100 ms at Month 1, and the small negative peak occurring around 200 ms at Month 3 may provide an indication that tone onsets were being detected.
S1 was the poorest performer in this study. She had little to no auditory experience for the first ten and a half years of life. After being fitted with high-powered hearing aids at age 10.7, she wore them consistently until age 11.5, the time of CI turn-on. She was motivated to hear, and was thus also a consistent user of her implant throughout the testing period. However, her progress was slow. By Month 3 of implant use, she still relied exclusively on signing for communication. And although she demonstrated some ability to hear environmental sounds (head-turning to loud sounds), she could not subjectively distinguish between them.
Behavioral indices of simple sound feature discrimination were superior to neurophysiological indices. She began to show signs of tone duration discrimination by the third month; there was no corresponding neurophysiological index of sound change discrimination (no MMN). Moreover, there was almost no AEP index of sound onset detection. The absence of a neurophysiological response to sound onsets may be explained by re-organization of auditory cortex in pre-lingually deafened individuals that results in inadequate availability of the normal pathways for auditory processing after implantation [14–19, 22,24]. Another possibility is that there is a significant delay in early phase plasticity in late-implanted users, as described by Kral et al. [24].
S2
Behavior and AEP’s
S2, who had some auditory experience prior to implantation, was able to behaviorally discriminate tone duration differences by Month 3 (Table 3). Thus, S2 showed greater ability than S1 at discriminating temporal characteristics of the sound after three months of implant use. She was somewhat able to discriminate intensity. However, discrimination of frequency and intensity sound features did not progressively improve by month 3.
Table 3.
S2 behavioral results
| Time | Intensity | Frequency | Duration | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | FAR | d′ | HR | FAR | d′ | HR | FAR | d′ | |
| M1 | .21 | .4 | 0.95 | .14 | .8 | 0.33 | 0 | .8 | 0.17 |
| M3 | .31 | .18 | 0.98 | .11 | .16 | 0.25 | .24 | .3 | 1.43 |
HR – Hit Rate
FAR – False Alarm Rate
d′ – d-prime
M1 – Month 1
M3 – Month 3
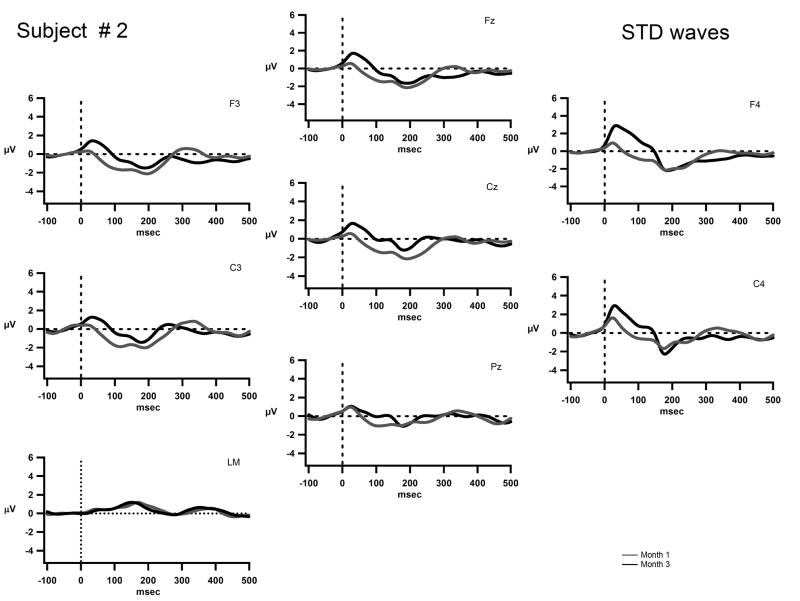
Figure 3 displays the grand means AEPs for S2. The waveforms recorded on Day 1 of implant turn-on for S2 are not displayed due to a technical error in recording on that day. A positive peak around 50 ms (P1) and a negative peak around 200 ms (N2) appear to be emerging in the waveform in Months 1 and 3. This is characteristic of obligatory responses and may indicate that sound onsets were detected. Although duration could be discriminated behaviorally at Month 3, no MMN was elicited.
Figure 3.
The grand-mean AEP waves to the standard tone are displayed for Fz, Cz, Pz, F3, F4, C3, C4, and the left mastoid (LM) for S2 on Month 1, (solid gray line) and Month 3 (solid black line). The x-axis represents time in milliseconds and the y-axis represents amplitude in micro volts. The scale is the same as in figures 4 and 5. This subject’s implant is on the right side and thus there is a positive square wave artifact seen most prominently at F4 and C4.
S2’s behavioral results reflect emerging discrimination ability of intensity at Month 1, with considerable improvement in duration discrimination at Month 3. However, there was no neurophysiological indication of sound change discrimination (no MMNs), even though sound onsets appeared to be detected (indexed by P1/N2 components). There appeared to be a slight increase in the amplitude of the “P1” component from Month 1 to Month 3, but no other indicator of a change in obligatory AEP response.
S2 was fitted with high-powered hearing aids at an early age, but was an inconsistent hearing aid user and showed little progress with auditory skills prior to implantation. She expressed herself exclusively signed English. She was also a somewhat inconsistent implant user, reporting use of the implant about 85% of the time in school (by her teacher’s report), and intermittently at home. She continued to use sign as a primary mode of expressive language during the three-month testing period. Overall, there was some progress in auditory abilities, but progress may have been slowed by initial inconsistent implant use and a possible lack of motivation to communicate orally. Although Kral et al. suggests that late-implanted CI users may have an extended “early phase of plasticity”; this potential may also have been limited by inconsistent implant use.
S3
Behavior and AEP’s
S3 had the most auditory experience prior to implantation. S3 was able to reliably distinguish frequency and duration differences between standard and deviant tones remarkably well at Month 1 (M1, Table 4). Moreover, both performance accuracy (d′) and RT improved from Month 1 to Month 3. Moreover, by Month 3, intensity deviants were also reliably discriminated (M3, Table 4).
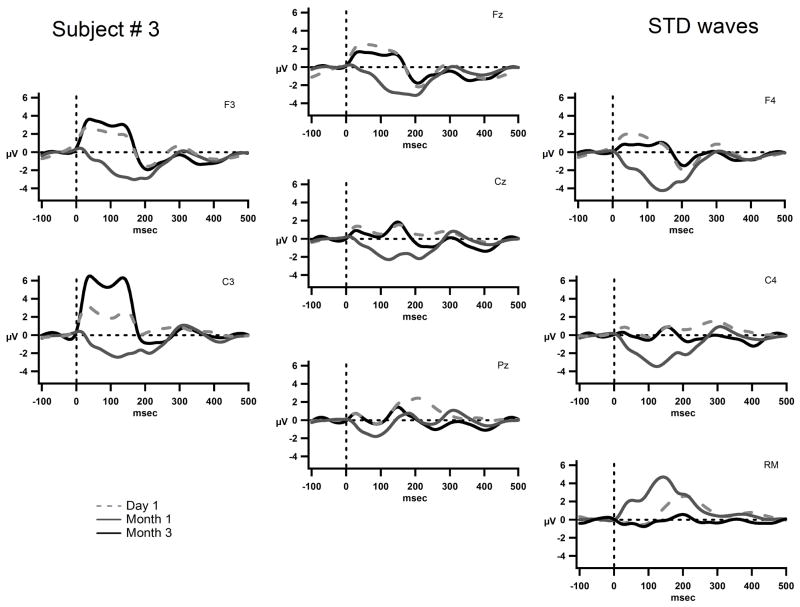
Figure 4 displays the grand-mean AEP waveforms for S3. There is some indication of a P1 component that is overlapped with implant-generated artifact, and a fairly consistent negativity peaking around 200 ms (N2) seen in all three recording times. No major changes occurred from Day 1 to Month 3 in the obligatory response to sound onset. In contrast, there was improving evidence of sound feature discrimination across the three time points. Already on the first day of CI turn-on, MMN was clearly elicited by duration deviants peaking around 300 ms (Fig. 5). As MMN is delineated by subtracting the AEP elicited by the standard, from the AEP elicited by the deviant, the resulting difference waveform subtracted out the artifact that was present in both waveforms. By Month 1, both frequency and duration deviants elicited clear MMNs, but no MMN was yet elicited by intensity deviants. Most interesting at Month 3, in addition to MMNs being elicited by all three feature deviants, P3a was also elicited for frequency and duration (Fig. 5). P3a is an index of involuntary orienting to salient sounds in the environment [37]. Thus, the presence of P3a in the third month indicates that these feature changes were now salient enough to evoke an orienting response. Given that behavioral detection of intensity differences only appeared in Month 3 along with the first appearance of MMN, P3a had not yet emerged probably indicating the intensity difference was not yet salient to S3’s passive auditory systems.
Figure 4.
The grand-mean AEP waves to the standard tone are displayed for electrodes Fz, Cz, Pz, F3, F4, C3, C4, and the right mastoid (RM) for S3 on on Day 1, (dashed gray line) Month 1, (solid gray line) and Month 3 (solid black line). The x-axis represents time in milliseconds and the y-axis represents amplitude in micro volts. The scale is the same as in figures 3 and 5. S3’s implant is on the left and thus there is a positive square wave seen most prominently at F3 and C3.
S3 consistently used high-powered hearing aids since the age of 4. He was able to distinguish some environmental sounds, but performed poorly in language-based auditory tasks prior to implantation. Of the three subjects, he had the most prior auditory experience with initial low frequency hearing at the moderate level for 125 Hz and 250 Hz and was able to utilize his implant for auditory discrimination more rapidly than the other subjects. He was able to learn to respond to auditory signals and to use his own voice for expressive communication by Month 3 of implant use. He showed evidence of sound onset detection and sound feature discrimination from the first day of implant use and began to increase the use of his voice to communicate by Month 1. By Month 3, his family reported he could distinguish his mother’s from his father’s voice when they were calling him from another room.
Unlike S1 and S2, behavioral and electrophysiological indices of sound discrimination were largely concordant in S3. The feature deviants that elicited MMN were the same feature deviants that were discriminated with a sensitivity index (d′) value greater than 2. As behavioral performance increased, evidence of involuntary orienting to the sound changes to those features appeared (P3a component). That is, greater acuity for feature discrimination evidenced by increased behavioral performance was likewise indicated by correlative neurophysiological changes. The acuity of feature discrimination, the ability to distinguish smaller feature differences than were tested in the current study, may have been developing. The appearance of the MMN on Day 1, and P3a at Month 3, may also indicate that the auditory experience prior to implantation made use of traditional auditory pathways. These results are consistent with Kral et al.’s theory of early plasticity [24], in which changes occur in a relatively rapid time frame of three months. There may have been preservation and plasticity within normal auditory pathways for sound processing due to use of hearing aids in this subject.
Limitations
This report has several limitations. With only 3 subjects, it is not possible to extrapolate to the wider range of auditory-related characteristics of pre-lingually deafened late-implanted children. Differences in the medical history and in educational environments make comparisons between the children in this study problematic. An expanded study looking at more children with similar profiles would strengthen our conclusions. Additional longitudinal data would be beneficial for assessing plastic changes that develop over longer time periods, which were not apparent within the three months of testing in the current study. The time course of auditory cortical plasticity in late-implanted prelingually deafened children is yet unknown.
Conclusion
Prior to implantation, each of the subjects participating in the study had different auditory experiences, from virtually none (S1) to relatively consistent (S3). Each had 7 or greater years of pre-lingual deafness prior to implantation. After the CI was turned on, subjects demonstrated variable success in improving communication skills, which was fairly consistent with the amount of pre-implant auditory experience each of our three subjects had. Variability in late-implanted children is commonly reported [9], however, the impact of pre-implant auditory experience on central auditory processing skills and on AEPs has not been well characterized. The results of the current study seem to relate the three subjects’ early post-implant behavioral discrimination skills with their previous auditory experience. The subject with the least experience showed the least improvement in behavioral and electrophysiological measures, despite high motivation to communicate orally. This may be partly due to differences regarding the amount of cortical reorganization that occurs with reduced or lack of auditory input. It is likely that S3, our best performer, had auditory pathways not completely disrupted. Interestingly, all three children showed some ability to identify duration differences before they could identify frequency or intensity differences. This suggests that temporal characteristics of sound input may be easier to detect with CI use in general than spectral or intensity features.
Restrictions on the availability of some higher auditory processing pathways due to decoupling from primary auditory cortex [25, 26] may always exist in pre-lingually deafened late-implanted cochlear implant users. However, evidence from AEPs in the first few months of implant use indicates some plasticity of the auditory system remains in some late-implanted adolescents, which may precede improvement in performance measures. Children that show electrophysiologic evidence for auditory cortical responses to sound features important for speech understanding may be ideal candidates for enhanced therapeutic strategies to improve their auditory modality for communication. Further studies with larger groups will help to better characterize the patterns of central auditory pathways and plasticity in late-implanted pre-lingually deafened children.
Acknowledgments
We gratefully acknowledge our cochlear implant patients, their parents, and Dr. Sanjay Parikh, who performed the implant procedures. This research was supported by a grant from the National Institutes of Health (DC R01 006003) and the Institute of Human Communication at the Montefiore Medical Center, Bronx, New York.
Footnotes
Conflict of interest statement
There are no conflicts of interest for any of the authors of this report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: correlations with changes in structure and speech perception. Acta Otolaryngol. 2003;123(2):249–52. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- 2.Ponton CW, Moore JK, Eggermont JJ. Prolonged deafness limits auditory system developmental plasticity: evidence from an evoked potentials study in children with cochlear implants. Scand Audiol Suppl. 1999;51:13–22. [PubMed] [Google Scholar]
- 3.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23(6):532–9. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Dorman MF. Central auditory development in children with cochlear implants: clinical implications. Adv Otorhinolaryngol. 2006;64:66–88. doi: 10.1159/000094646. [DOI] [PubMed] [Google Scholar]
- 5.Hensch TK. Critical period regulation. Ann Rev Neurosci. 2004;27:549–79. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 6.Ponton CW, Don M, Eggermont JJ, Waring MD, Kwong B, Masuda A. Auditory system plasticity in children after long periods of complete deafness. Neuroreport. 1996;8(1):61–5. doi: 10.1097/00001756-199612200-00013. [DOI] [PubMed] [Google Scholar]
- 7.Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol. 2005;46(3):252–61. doi: 10.1002/dev.20052. [DOI] [PubMed] [Google Scholar]
- 8.Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear. 2008;29(4):492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schramm D, Fitzpatrick E, Séguin C. Cochlear implantation for adolescents and adults with prelinguistic deafness. Otol Neurotol. 2002;23(5):698–703. doi: 10.1097/00129492-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Osberger MJ, Zimmerman-Phillips S, Koch DB. Cochlear implant candidacy and performance trends in children. Ann Otol Rhinol Laryngol. 2002;189 (Suppl):62–5. doi: 10.1177/00034894021110s513. [DOI] [PubMed] [Google Scholar]
- 11.Waltzman SB, Roland JT, Jr, Cohen NL. Delayed implantation in congenitally deaf children and adults. Otol Neurotol. 2002;23(3):333–40. doi: 10.1097/00129492-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Manrique M, Cervera-Paz FJ, Huarte A, Perez N, Molina M, García-Tapia R. Cerebral auditory plasticity and cochlear implants. Int J Pediatr Otorhinolaryngol. 1999;49(Suppl 1):S193–7. doi: 10.1016/s0165-5876(99)00159-7. [DOI] [PubMed] [Google Scholar]
- 13.Teoh SW, Pisoni DB, Miyamoto RT. Cochlear implantation in adults with prelingual deafness. Part I. Clinical results. Laryngoscope. 2004;114(9):1536–40. doi: 10.1097/00005537-200409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4(12):1171–3. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- 15.Catalán-Ahumada M, Deggouj N, De Volder A, Melin J, Michel C, Veraart C. High metabolic activity demonstrated by positron emission tomography in human auditory cortex in case of deafness of early onset. Brain Res. 1993;623(2):287–92. doi: 10.1016/0006-8993(93)91439-y. [DOI] [PubMed] [Google Scholar]
- 16.Levänen S, Hamdorf D. Feeling vibrations: enhanced tactile sensitivity in congenitally deaf humans. Neurosci Lett. 2001;301(1):75–7. doi: 10.1016/s0304-3940(01)01597-x. [DOI] [PubMed] [Google Scholar]
- 17.Teoh SW, Pisoni DB, Miyamoto RT. Cochlear implantation in adults with prelingual deafness. Part II. Underlying constraints that affect audiological outcomes. Laryngoscope. 2004;114(10):1714–9. doi: 10.1097/00005537-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt DL, Yamoaha EN, Krubitzera L. Multisensory plasticity in congenitally deaf mice: how are cortical areas functionally specified? Neuroscience. 2006;139:1507–1524. doi: 10.1016/j.neuroscience.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Gilley PM, Sharma A, Dorman MF. Cortical reorganization in children with cochlear implants. Brain Res. 2008;1239:56–65. doi: 10.1016/j.brainres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann R, Shepherd RK, Heid S, Klinke R. Response of the primary auditory cortex to electrical stimulation of the auditory nerve in the congenitally deaf white cat. Hear Res. 1997;112(1–2):115–33. doi: 10.1016/s0378-5955(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 21.Klinke R, Kral A, Heid S, Tillein J, Hartmann R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science. 1999;285(5434):1729–33. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- 22.Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb Cortex. 2002;12(8):797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- 23.Kang E, Lee DS, Lee JS, Kang H, Hwang CH, Oh SH, Kim CS, Chung JK, Lee MC, Jang MJ, Lee YJ, Morosan P, Zilles K. Developmental hemispheric asymmetry of interregional metabolic correlation of the auditory cortex in deaf subjects. Neuroimage. 2003;19(3):777–83. doi: 10.1016/s1053-8119(03)00118-6. [DOI] [PubMed] [Google Scholar]
- 24.Kral A, Tillein J, Heid S, Klinke R, Hartmann R. Cochlear implants: cortical plasticity in congenital deprivation. Prog Brain Res. 2006;157:283–313. doi: 10.1016/s0079-6123(06)57018-9. [DOI] [PubMed] [Google Scholar]
- 25.Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15(5):552–62. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- 26.Kral A, Eggermont JJ. What’s to lose and what’s to learn: development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Res Rev. 2007;56(1):259–69. doi: 10.1016/j.brainresrev.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Thai-Van H, Micheyl C, Norena A, Veuillet E, Gabriel D, Collet L. Enhanced frequency discrimination in hearing-impaired individuals: a review of perceptual correlates of central neural plasticity induced by cochlear damage. Hear Res. 2007;233(1–2):14–22. doi: 10.1016/j.heares.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282(3):456–71. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 29.Harrison RV, Nagasawa A, Smith DW, Stanton S, Mount RJ. Reorganization of auditory cortex after neonatal high frequency cochlear hearing loss. Hear Res. 1991;54(1):11–9. doi: 10.1016/0378-5955(91)90131-r. [DOI] [PubMed] [Google Scholar]
- 30.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talwar SK, Gerstein GL. Reorganization in awake rat auditory cortex by local microstimulation and its effect on frequency-discrimination behavior. J Neurophysiol. 2001;86(4):1555–72. doi: 10.1152/jn.2001.86.4.1555. [DOI] [PubMed] [Google Scholar]
- 32.Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6(11):1216–23. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- 33.Sussman E, Steinschneider M, Gumenyuk V, Grushko J, Lawson K. The maturation of human evoked brain potentials to sounds presented at different stimulus rates. Hear Res. 2008;236:61–79. doi: 10.1016/j.heares.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Näätänen R, Teder W, Alho K, Lavikainen J. Auditory attention and selective input modulation: a topographical ERP study. Neuroreport. 1992;3(6):493–6. doi: 10.1097/00001756-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Ponton CW, Eggermont JJ, Don M, Waring MD, Kwong B, Cunningham J, Trautwein P. Maturation of the mismatch negativity: effects of profound deafness and cochlear implant use. Audiol Neurootol. 2005;(3–4):167–85. doi: 10.1159/000013878. [DOI] [PubMed] [Google Scholar]
- 36.Escera C, Alho K, Winkler I, Näätänen R. Neural mechanisms of involuntary attention to acoustic novelty and change. J Cogn Neurosci. 1998;10(5):590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- 37.Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event- related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev. 2001;25(4):355–73. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 38.Green DM, Swets JA. Signal detection theory and psychophysics. New York; Wiley: 1966. [Google Scholar]
- 39.Macmillan NA, Creelman CD. Detection Theory. 2. New Jersey; Lawrence Erlbaum Associates, Inc: 2005. p. 8. [Google Scholar]