Abstract
Although higher levels of physical activity are inversely associated with risk of colon cancer, few prospective studies have evaluated overall digestive system cancer mortality in relation to cardiorespiratory fitness (CRF). The authors examined this association among 38,801 men aged 20−88 years and who performed a maximal treadmill exercise test at baseline in the Aerobics Center Longitudinal Study (Dallas, Texas) during 1974−2003. Mortality was assessed over 29 years of follow-up (1974−2003). 283 digestive system cancer deaths occurred during a mean 17-year of observation. Age-adjusted mortality rates per 10,000 person-yrs according to low, moderate, and high CRF groups were 6.8, 4.0, and 3.3 for digestive system cancer (trend p < 0.001). After adjustment for age, examination year, body mass index, smoking, drinking, family history of cancer, personal history of diabetes, hazard ratios for overall digestive cancer deaths (95% confidence interval) for those in the middle and upper 40% of the distribution of CRF relative to those in the lowest 20% were 0.66 (0.49, 0.88) and 0.56 (0.40, 0.80), respectively. Being fit (the upper 80% of CRF) was associated with a lower risk of mortality from colon (0.61 [0.37, 1.00]), colorectal (0.58 [0.37, 0.92]), and liver cancer (0.28 [0.11, 0.72]), compared with being unfit (the lowest 20% of CRF). These findings support a protective role of CRF against total digestive tract, colorectal, and liver cancer deaths in men.
Keywords: exercise, primary prevention, cohort study, digestive cancer mortality, men
Introduction
Digestive system cancers include those of the alimentary canal below the neck (e.g., esophagus, stomach, small and large intestines) and key digestive organs (i.e., pancreas, liver, gallbladder). Considering all digestive cancers together, these constitute the second leading cause of cancer-related mortality of men in the United States (1). Of all digestive tract sites, colon and pancreas account for the majority of deaths (colon because it is so common and pancreas because it has such poor prognosis). The etiology of various digestive cancers is not fully understood. Several potential risk factors including genetic components, diet, smoking, and physical inactivity have been identified for colon cancer (2). However, other than smoking and diabetes (3, 4), few lifestyle factors have been linked to pancreatic cancer. Recent evidence suggests insulin resistance and abnormal glucose metabolism, without diagnosis of diabetes also may be risk factor for pancreatic cancer (5, 6).
Although higher levels of physical activity are inversely associated with risk of colon cancer (7, 8), the association between pancreatic cancer and physical activity remains inconclusive. Several studies have found an inverse relationship (9, 10) while other studies have reported no association (11-13). Very few cohort studies have reported on physical activity and other sites of digestive system cancer, and the findings are inconsistent (14, 15). No studies have been conducted to assess the association between physical activity and cancers from liver or small intestine. There is some indication that greater amounts of activity are associated with higher risk of stomach (14) and bladder (15) cancer and lower risk of oral/oesophagus cancer (15). It may be that measurement errors inherent in self-reported physical activity are partly responsible for these discrepant findings. Cardiorespiratory fitness (CRF), an objective and more reproducible measure, reflects the functional consequences of physical activity habits of the individual, and therefore may provide a better exposure with which to evaluate associations with relevant health outcomes.
To the best of our knowledge, only one study (16) has been conducted on CRF and mortality from cancers of the gastrointestinal system among men. However, this study only examined men with pre-diabetes and diabetes. There is lack of data in the general population. Because the five-year survival rate for digestive cancers as a group is very low (about 45%; and <10% for some sites such as pancreas and esophagus), identification of modifiable risk factors for these deadly cancers may provide important opportunities for reducing overall cancer mortality (17). We therefore examined the association between the CRF, objectively measured by maximal exercise test on a treadmill, and overall and site-specific digestive cancer mortality in men from the Aerobic Center Longitudinal Study (ACLS). We tested the hypothesis that CRF is associated with cancers of colorectum, pancreas, esophagus, stomach or gall bladder for which there is prior evidence of an association (7-10, 14, 15), and generated hypotheses on the association between cancers of the liver or small intestine and either CRF or other measures of physical activity for which there are no prior data.
Materials and Methods
Study population. The ACLS is an ongoing cohort study of patients who were each examined during a preventive medical examination in Dallas, Texas, between 1974 and 2003. This study was reviewed and approved by the Cooper Institute Institutional Review Board on an annual basis. The sample for the current analysis was 38,801 primarily white, well-educated, middle-to-upper socioeconomic status men aged 20−88 years. The inclusion criteria required men had no prior history of cancer, ulcer disease, gallbladder trouble, jaundice, hepatitis, cirrhosis, or colon polyps. At baseline, all participants completed a symptom limited treadmill test. The men in the analyses reported here are very similar to the overall ACLS cohort, with only minor differences in some clinical variables. The death rate for the subgroup of men in this analysis is not significantly different from the age, risk factor, health status, and family history-adjusted rates for the overall cohort.
Baseline examination. Participants arrived for the clinical examination after an overnight fast of at least 12 hours and gave their written informed consent to participate in the examination and the follow-up study. Information was collected pertaining to personal and family health histories, fasting blood chemistry analyses, anthropometry, resting blood pressure and electrocardiogram, and a maximal graded exercise test. Examination methods and procedures followed a standard manual of operations, as described previously (18). Briefly, body mass index (BMI) was computed from measured height and weight (kg/m2). Resting blood pressure was recorded as the first and fifth Korotkoff sounds by ausculatatory methods. Serum samples were analyzed for lipids and glucose using standardized automated bioassays by a laboratory that participates in the CDC Lipid Standardization Program and meets its quality control standards. Information on smoking habits (never, past, and current smoker), alcohol intake (number of drinks per week), personal history of diabetes, and family (from parents and siblings; i.e., first-degree relatives) history of cancer from all-cause was obtained from a standardized questionnaire. One unit of alcohol is defined as 12 ounces (3.41 dl) of beer, 5 ounces (1.421 dl) of wine, or 1.5 ounces (0.4262 dl) of hard liquor.
We determined CRF at the baseline examination using a maximal exercise test on a treadmill. CRF was assessed as the duration of the exercise test using a modified Balke protocol (18, 19). The treadmill speed was 88m • min−1 for the first 25 min. During this time the grade was 0% for the first minute, 2% the second minute and increased 1% for each minute. After 25 min, the grade remained constant while the speed increased 5.4 m • min−1 each minute until test termination. Patients were encouraged to give a maximal effort during the test. Men included in the present analyses reached at least 85% of their age-predicted maximal heart rate (220-age [years] beats per minute) on the test. The duration of the maximal exercise treadmill test on this protocol is highly (and positively) correlated with directly measured maximal oxygen uptake in men (20) (r = 0.92), an accepted measure of CRF. Maximal metabolic equivalents (METs, 1 MET = 3.5 ml O2 uptake • kg−1 • min−1) were estimated from the final treadmill speed and grade (21). We used our previously published age-specific distribution of treadmill duration from the overall ACLS population to define fitness groups as low (lowest 20%), moderate (middle 40%), and high (upper 40%) to maintain consistency in the study methods, and because we have found that a low level of fitness, defined in this way, is an independent predictor of mortality (18, 22) and morbidity (23). The respective cut points for total treadmill time and METs in the low, moderate, and high fitness groups were described in detail in a recent report (23).
Ascertainment of digestive cancer death. All participants were followed from the date of their baseline examination until their date of death or December 31, 2003. The National Death Index (NDI) was the primary data source for mortality surveillance. The NDI has been shown to be an accurate method of ascertaining deaths in observational studies, with high sensitivity (96%) and specificity (100%) (24). The underlying cause of death was determined from the NDI report or by a nosologist's review of official death certificates obtained from the department of vital records in the decedent's state of residence. Causes of cancer death were identified using International Classification of Diseases, Ninth Revision (ICD-9) codes for deaths occurring before 1999 and Ten Revision (ICD-10) codes (in parentheses) for deaths during 1999−2003. Our primary outcome for this analysis was death from digestive cancers, 150−159 (C15-C26); and our secondary mortality outcomes were: esophagus, 150 (C15); stomach, 151 (C16); small intestine, 152 (C17); colon, 153 (C18); rectum, 154 (C19-C21); liver, 155 (C22); gall bladder and intrahepatic bile ducts, 156 (C23-C24); pancreas, 157 (C25); and other and ill-defined digestive organs, 158−159 (C26).
Statistical analysis. Baseline characteristics of the population were calculated for the entire study group and by CRF categories. Differences in covariates were assessed using F-tests. Kaplan-Meier plots were used to compare survival curves. The crude and multivariate adjusted log-rank tests were used to determine significance. Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs), associated 95% confidence intervals (CIs), mortality rates (deaths/10,000 person-years of follow-up), and linear trends of mortality for levels of each fitness category. When calculating HRs, the low fitness group was used as the reference category. Multivariable-adjusted models controlled for the potential confounding effects of baseline age (years), examination year, smoking (never, past, or current smoker), alcohol intake (drinks per week), and family history of cancer (whether present). Examination year was included as a covariate to control for variation in the length of follow-up in this on-going study. We conducted additional analyses that further adjusted for baseline differences in two factors that could plausibly mediate the association between CRF and digestive cancer mortality: BMI (<25 vs. ≥25 kg/m2), and diabetes (whether diagnosed prior to, or at the examination). Unfortunately, we have no information on nonsteroidal anti-inflammatory drug (NSAIDs) usage to include in the model. Cumulative hazard plots grouped by exposure had no appreciable violations of the proportional hazards assumption.
Next, we conducted Cox-regression analyses of CRF stratified by categories of BMI (< 25 vs. ≥ 25 kg/m2). We also examined the risk of total digestive system cancer across increments of METs to assess the shape of the fitness-mortality curve. Finally, we explored the site-specific cancer deaths across fitness levels. Statistical analyses were performed using SAS (version 9.1, SAS Institute, Cary, NC) software. All p values were calculated based on two-sided hypothesis tests, and CIs were calculated at the 95% level.
Results
At baseline, the mean (SD) age of the study participants was 43.8 (9.7) years, the mean treadmill test duration was 17.9 (5.2) minutes, and the mean CRF measure was 11.6 (2.5) METs. The distribution of participant characteristics for several digestive cancer risk factors is given in Table 1 across categories of CRF. Men in the high-fitness group were more likely to have a lower BMI, to have more favorable lipid and blood pressure profiles, to be non-smokers, and to have less diabetes, compared to men with low CRF. The Kaplan-Meier plot depicts the total digestive cancer death rates by fitness group (Fig. 1). After adjusting for all the risk factors, the resulting log-rank test did not change materially (Chi-square=14.9, p <0.001).
Table 1.
Baseline characteristics according to cardiorespiratory fitness (CRF), Aerobics Center Longitudinal Study, Dallas, Texas, 1974−2003
| Cardiorespiratory Fitness |
|||||
|---|---|---|---|---|---|
| Characteristic | All (n=38,801) | Low CRF (n=6,665) | Moderate CRF (n=15,315) | High CRF (n=16,821) | P for trend |
| Mean age (years) | 43.8 (9.7)* | 43.6 (9.4) | 44.0 (9.6) | 43.6 (9.9) | <0.001 |
| Mean height (cm) | 178.9 (6.9) | 178.0 (8.6) | 179.0 (6.6) | 179.3 (6.5) | <0.001 |
| Mean body mass index (kg/m2) | 26.3 (3.4) | 28.6 (4.2) | 26.7 (3.2) | 24.9 (2.5) | <0.001 |
| Mean maximal METs | 11.6 (2.5) | 8.5 (1.3) | 10.7 (1.2) | 13.7 (1.9) | <0.001 |
| Mean treadmill time duration (minutes) | 17.9 (5.2) | 11.1 (2.7) | 16.0 (2.5) | 22.3 (3.5) | <0.001 |
| Mean lipids (mmol/L) | |||||
| Total cholesterol | 5.5 (1.1) | 5.7 (1.1) | 5.5 (1.0) | 5.3 (1.2) | <0.001 |
| HDL-C | 1.2 (0.3) | 1.0 (0.3) | 1.1 (0.3) | 1.3 (0.3) | <0.001 |
| Triglycerides | 1.6 (1.4) | 2.2 (2.1) | 1.7 (1.2) | 1.2 (1.0) | <0.001 |
| Mean fasting blood glucose (mmol/L) | 5.6 (2.8) | 5.8 (1.5) | 5.6 (1.0) | 5.5 (4.1) | <0.001 |
| Mean blood pressure (mmHg) | |||||
| Systolic | 122 (14) | 124 (14) | 122 (13) | 120 (13) | <0.001 |
| Diastolic | 81 (10) | 84 (10) | 82 (10) | 79 (9) | <0.001 |
| Cigarette smoking (%) | |||||
| Never | 70.8 | 59.0 | 68.9 | 77.2 | |
| Past | 10.8 | 7.4 | 10.2 | 12.7 | <0.001 |
| Current | 18.4 | 33.6 | 20.9 | 10.1 | |
| Mean Alcohol drinking (drinks/week) | 7.9 (11.4) | 8.3 (11.5) | 8.1 (11.6) | 7.5 (11.2) | <0.001 |
| Diabetes† (%) | 5.4 | 10.1 | 5.7 | 3.3 | <0.001 |
| Family history of cancer (%) | 1.0 | 0.8 | 1.2 | 1.1 | 0.04 |
Numbers in parentheses, standard deviation.
Diabetes was defined as glucose≥126 mg/dL or history of physician-diagnosed diabetes
Abbreviations: METs, maximal metabolic equivalents achieved during the treadmill test; HDL-C, high density lipoprotein cholesterol.
Figure 1.
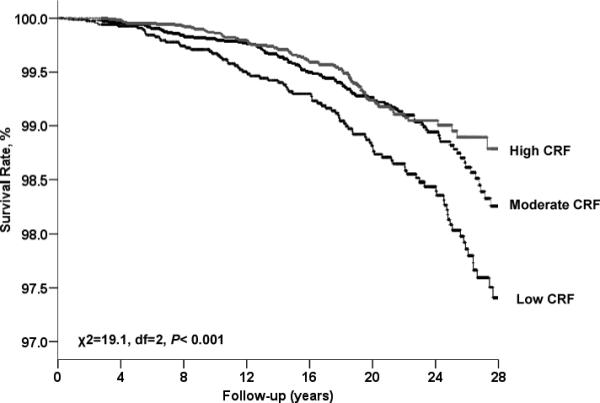
Kaplan-Meier plots for mortality due to total digestive system cancer, Aerobics Center Longitudinal Study, Dallas, Texas, 1974−2003. CRF, cardiorespiratory fitness.
In a mean length of 17 years follow-up and 661,169 person-years of observation, 283 total digestive cancer deaths were identified. A steep inverse gradient (p trend <0.001) of total digestive cancer mortality rates was observed across CRF groups (Table 2). After adjusting for potential confounders (age, examination year, smoking status, alcohol intake, family history of cancer), men with moderate and high CRF had 37 and 49 % lower risk of death from digestive cancers, respectively, than did men with low CRF (p trend < 0.001). Additional adjustment for BMI and personal history of diabetes did not materially change the magnitude or the pattern of the association.
Table 2.
Rates and hazard ratios for digestive system cancer mortality by cardiorespiratory fitness (CRF) groups, Aerobics Center Longitudinal Study, Dallas, Texas, 1974−2003
| Deaths from digestive system cancer |
Mortality rate* |
HR† |
95% CI† |
HR‡ |
95% CI‡ |
|
|---|---|---|---|---|---|---|
| All men (n=38,801) | ||||||
| Low CRF | 90 | 6.8 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 110 | 4.0 | 0.63 | 0.47, 0.85 | 0.66 | 0.49, 0.88 |
| High CRF | 83 | 3.3 | 0.51 | 0.37, 0.70 | 0.56 | 0.40, 0.80 |
| P linear trend | <0.001 | <0.001 | 0.001 | |||
| Men with BMI < 25 kg/m2 (n=15,422) | ||||||
| Low CRF | 18 | 5.7 | 1.00 | Referent | ||
| Moderate CRF | 37 | 3.8 | 0.75 | 0.42, 1.37 | ||
| High CRF | 40 | 2.7 | 0.51 | 0.28, 0.94 | ||
| P linear trend | 0.009 | 0.02 | ||||
| Men with BMI ≥ 25 kg/m2 (n=23,379) | ||||||
| Low CRF | 72 | 7.2 | 1.00 | Referent | ||
| Moderate CRF | 73 | 4.1 | 0.60 | 0.42, 0.85 | ||
| High CRF | 43 | 4.2 | 0.62 | 0.418, 0.94 | ||
| P linear trend | 0.003 | 0.01 | ||||
Rate is expressed as per 10,000 person-years and adjusted for age.
Model 1: adjusted for age, examination year, smoking status (never, past, or current), alcohol intake (drinks per week), and family history of cancer (present or not).
Model 2: adjusted for all variables in Model 1 plus BMI (<25 vs. ≥ 25 kg/m2) and personal history of diabetes (present or not).
Abbreviations: HR, hazard ratio; CI, conference interval; CRF, cardiorespiratory fitness; BMI, body mass index.
To explore possible effect modification of the association between CRF and total digestive cancer by BMI, we stratified the analysis according to BMI category (<25 and ≥25 kg/m2) (Table 2). The age-adjusted death rate was inversely related to CRF within the normal weight (18.5<BMI<25kg/m2) (p trend = 0.009) and overweight/obese (BMI≥25 kg/m2) (p trend = 0.003, because of the small number of deaths (only 1 death) in obese (BMI≥30 kg/m2) men with high CRF, we combined the overweight and obese groups). Similar patterns of association were noted after adjusting for confounders.
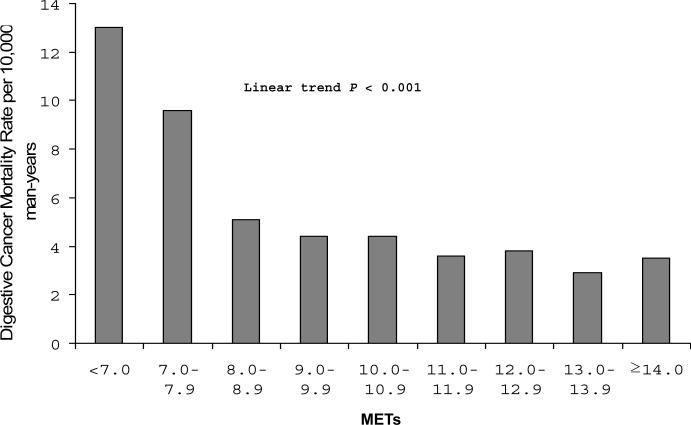
To examine the dose-response characteristics between CRF levels and total digestive cancer mortality in our population of men, we computed the age-adjusted death rates (per 10,000 person-yrs) for categories of CRF defined by increments of 1 MET across the range of 7 to 14 METs (Fig. 2). An exercise capacity of less than 8 METs was associated with a more than 3 fold higher risk of total digestive cancer mortality compared with men having a capacity of 11 METs and greater (p trend <0.001). Across incremental MET levels (from <7.0 to ≥14.0 METs), the covariates (including BMI and diabetes)-adjusted HRs of mortality were 1.0, 0.75 (0.43−1.30), 0.48 (0.30−0.76), 0.39 (0.23−0.66), 0.43 (0.26−0.71), 0.36 (0.21−0.63), 0.38 (0.21−0.69), 0.28 (0.15−0.53), and 0.38 (0.19−0.76), p trend <0.001. Excluding the first five-year of follow-up did not materially change the magnitude and the pattern of the association (p trend <0.001).
Figure 2.
Age- adjusted mortality rates (per 10,000 man-years) of total digestive system cancer by cardiorespiratory fitness levels quantified in 1-MET increments obtained during a maximal treadmill test in men, Aerobics Center Longitudinal Study, Dallas, Texas, 1974−2003. Number at risk (and number of cases) in <7.0, 7.0−7.9, 8.0−8.9, 9.0−9.9, 10.0−10.9, 11.0−11.9, 12.0−12.9, 13.0−13.9, and ≥14.0 was 859 (33), 1,096 (27), 4,465 (53), 4,135 (33), 6,014 (44), 5,827 (30), 5,180 (25), 5,872 (20), and 5,353 (18).
Because of the small number of site-specific cancer deaths and the similar trends in total digestive cancer mortality across fitness levels, the moderate- and high-fit groups were combined into one group (fit) and the low-fit group (unfit) was used as the referent (Fig. 3). For all digestive system cancers combined, the adjusted mortality risk associated with being fit was 0.62 (95% CI: 0.47, 0.82). Being fit was associated with a lower risk of mortality from colon cancer (0.61 [0.37, 1.00]), colorectal cancer (0.58 [0.37, 0.92]), and liver cancer (0.28 [0.11, 0.72]). The associations between fitness and small intestine, gall bladder, and pancreatic cancer were suggestive of a reduced risk, but the hazard ratios did not reach statistical significance (0.36 [0.02, 6.61]; 0.83 [0.09, 7.74], and 0.75 [0.45, 1.24], respectively).
Figure 3.
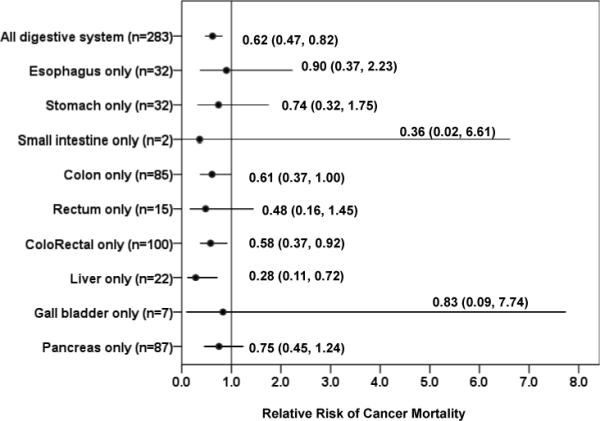
Risk of site-specific digestive cancer mortality associated with being fit (the upper 80% of the distribution of CRF) as defined by achieving at least a moderate level of fitness during maximal exercise testing, Aerobics Center Longitudinal Study, Dallas, Texas, 1974−2003. The reference group was the unfit group (the lowest 20% of the distribution of CRF). We used Cox proportional hazard models to estimate the hazard ratio, which include age, examination year, smoking, alcohol intake, personal history of diabetes, family history of cancer, and body mass index as covariates. The error bars represent the 95% confidence intervals.
Because baseline age may influence results, we conducted additional sensitivity analyses by repeating the above analysis in men with baseline age 35−74 years (N=32,137). The patterns of the association between fitness and digestive cancer mortality across different baseline age ranges were similar (data not shown).
Discussion
In this study, we observed an inverse association between CRF and the risk of total digestive cancer mortality, with men in the moderate and high CRF groups demonstrating a 34% and 44% lower risk, respectively, of dying of digestive cancers after adjustment of confounding by age, smoking, drinking, and family history of cancer. Excluding men with pre-diabetes and diabetes did not materially change the results. Men with an exercise capacity less than 8 METs had a more than 3-fold higher risk of dying of digestive cancer as compared with those with higher METs level (≥11). These data suggest that an exercise capacity of at least 8 METs may be needed to provide substantially protective benefits.
To the best of our knowledge, only one previous study has assessed the association of CRF to risk of dying of digestive cancer (16). However, that paper examined the role of CRF and risk of fatal digestive cancer events in men with pre-diabetes or diabetes, whereas our paper examined a much broader male population. Our results were similar to the previous paper's findings and show that higher levels of CRF are associated with substantially lower risk of dying from digestive cancers (including colorectal and liver cancers). In that study, Thompson and colleagues (16) found that being fit, as defined by achieving at least a moderate level of fitness during a maximal exercise test, had a 45% lower risk of digestive cancer mortality. In our study, we found that men with at least a moderate fitness level had a 34% lower digestive cancer risk than did men with low CRF. In our study, it appeared that beyond a CRF level of 8 METs (Fig. 2) there were no substantial decreases in risk of digestive cancer death. This finding of an apparent CRF threshold adds insight into the association between CRF and digestive cancer death. Although CRF has a genetic component (25−40%) (25, 26), it is clear that usual physical activity habits are the primary determinant of fitness. CRF can be enhanced in most individuals through participation in moderate and vigorous physical activities, such as brisk walking, bicycling, and jogging, for 30 minutes or more on most days of the week (about 8-kcal/kg per week) (27). This consensus public health recommendation will produce a maximal capacity of at least 8 METs in most individuals.
Our finding of an inverse association between physical activity and colorectal risk is consistent with evidence from previous studies (28-30). A previous meta-analysis estimated an approximately 20−40% lower risk of colon cancer for high versus low leisure-time physical activity (29). In our study, we found men with at least a moderate fitness level had a 42% lower risk of death from colorectal cancer than did men with low CRF.
We did not observe a significant inverse association between CRF and mortality from pancreatic cancer, a finding which is consistent with many studies (12, 13, 16, 31-35), but discrepant from others (9, 10, 36). However, we did observe a 25% reduction in mortality at this site among more fit men, but the small number of deaths limited the precision of our estimates. Given that the strength of the CRF-pancreatic cancer association was somewhat weaker than the risk estimates we observed for colorectal and liver cancer mortality (HR=0.28 to 0.58), it may be that the association between pancreatic cancer and activity-related exposure is weaker. Since physical activity is a complex behavior and often imprecisely measured in epidemiologic studies, the combination of exposure measurement error and a weaker association may account for the heterogeneity in previous reports using self-reported physical activity as the exposure. We speculate that these two factors may be contributing to the inconsistency in previous findings. Future studies will be warranted to further explore this issue and confirm the present findings.
Little information is available on the association between physical activity or CRF and other types of digestive cancer. In this study, higher fitness was shown to be associated with significantly lower risk of liver cancer. This is consistent with the findings among men with pre-diabetes and diabetes (16). The findings with regard to stomach cancer have not been consistent. We found an inverse trend on stomach cancer as well as in men with diabetes (16), though the trend was not statistically significant, possibly due to the small number of deaths. The British Regional Heart Study (BRHS) found the same nonsignificant inverse trend between physical activity and stomach cancer (15). In contrast, the Japanese Hawaiian Cancer Study (JHCS) found increased activity to be associated with higher risk of stomach cancer but the results were preliminary (14). Only one previous study reported a lower risk of oral/oesophagus cancer with moderate vigorous activity (15). We observed a similar trend. Regarding bladder cancer, neither JHCS (14) nor the current study found any association between activity and urinary bladder cancer, however, BRHS (15) showed significant increase in risk of bladder cancer among men who were vigorously active. Finally, we observed a non-statistically significant lower risk of small intestine cancer among men with high fitness. Despite the absence of a prior hypothesis for the sites shown in Fig. 3, fitness appeared to be protective overall. These findings may provide clues for future research, in studies having larger sample sizes and employing rigorous methods of measuring fitness (such as we had available to us).
Several biological mechanisms have been proposed to explain how higher levels of physical activity may protect against cancer in general and cancers of the digestive tract in particular. Physical activity is known to effect cancer development through immune system function, insulin sensitivity, and growth factor levels (37-39). It is unclear which mechanisms are important for different sites of digestive cancer. Any or all of these mechanisms may influence general susceptibility to cancer (38). There are links between colorectal cancer and central obesity (40, 41) and insulin and the insulin-like growth factor (e.g., IGF-1) axis (42). Biologically, it appears that insulin resistance and abnormal glucose metabolism may be related to increased risk of pancreatic cancer. We specifically examined two potential obesity-related mediators of the association (BMI and diabetes) in our sequential models, and found that adjustment for these factors had relatively little influence on the strength of associations observed. This finding suggests adiposity and diabetes, as measured in our study, are not strong mediators of the associations of interest. Evidence suggests that higher plasma glucose levels after an oral glucose load is predictive of pancreatic cancer mortality (5) as is a diagnosis of diabetes (4). However, little is known about the specific mechanisms between physical activity and stomach, small intestine, liver, bladder and other digestive tract cancers. Potential mechanisms, specific to gastrointestinal health, include decreased fecal transit time, reduced bile secretion, altered prostaglandin synthesis, and gut flora (43). Additional research is needed to clarify the complicated association between activity and digestive tract cancers.
This large prospective study with a long follow-up interval has a number of strengths that should be considered. First, it is rare to have a measure of fitness in a prospective study of digestive cancer mortality. Second, our extensive baseline examination to evaluate health status (such as cancer and diabetes), careful measurement of body-size, and other lifestyle factors addresses the potential for confounding by these factors to influence our results. Our study also has limitations that should be considered. First, we are unable to adjust for dietary factors such as fiber and saturated fat intake in the current study. Second, while we had a hard endpoint of digestive cancer mortality, it is not possible to determine completely whether higher levels of CRF protected men against developing cancer, or whether it aided their survival after their diagnosis. However, the low 5-year survival rates for many of these cancers (especially, pancreatic and liver cancer) make incidence and mortality essentially interchangeable (as virtually everyone diagnosed with the cancer dies of the cancer) (44). Fitness also appeared to be protective against esophagus, stomach, small intestine, and gall bladder cancer mortality, even though statistical significance was not achieved because of the small number of deaths associated with these sites. Third, few studies have examined the relationship between physical activity to cancer risk in anatomic segments of the colon with conflicting results (45). Unfortunately we do not have data regarding specific subsite colon cancer risk. Another limitation to the current findings is that the study population consists mainly of white men in the middle and upper socioeconomic strata; thus results may not be generalizable to other adult populations, but should not affect the internal validity of our findings. In terms of exposure assessment, we classified men at study enrollment, but in the present analysis we were unable to evaluate the impact of changes in fitness over time on our outcomes. It is possible, but not very likely, that many low-fit men increased their fitness levels at some point in the follow-up interval. Additionally, others may have experienced decreases in this component. Therefore, we can not examine whether changes in fitness and other exposures occurred during follow-up. However, such misclassification of exposure would likely lead to and underestimate of the magnitude of the association observed in the present study. We had insufficient information in order to assess the effect of aspirin and other NSAIDs on outcome. Future studies should include such information whenever possible.
In summary, the findings from this study provide evidence supporting a protective role of CRF on risk of digestive cancer mortality and that a relatively low, threshold of CRF may be needed. The consensus public health guideline to obtain 150 min/week of moderate-intensity physical activity will improve their fitness levels and produce this threshold in most individuals. Given the public health burden of digestive cancer, future research needs to determine the specific biological characteristics of exercise related to digestive cancer risk, and if a dose-response relationship exists.
Acknowledgments
Grants support: Supported by National Institutes of Health grants AG06945 and HL62508.
The authors thank Dr. Kenneth H. Cooper for establishing the Aerobics Center Longitudinal Study, the Cooper Clinic physicians and technicians for collecting the baseline data and staff at the Cooper Institute for data management.
Footnotes
Conflict of interest: none declared.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev. 1993;15:499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 3.Coughlin SS, Calle EE, Patel AV, Thun MJ. Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control. 2000;11:915–23. doi: 10.1023/a:1026580131793. [DOI] [PubMed] [Google Scholar]
- 4.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605–9. [PubMed] [Google Scholar]
- 5.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–8. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 6.McCarty MF. Insulin secretion as a determinant of pancreatic cancer risk. Med Hypotheses. 2001;57:146–50. doi: 10.1054/mehy.2001.1316. [DOI] [PubMed] [Google Scholar]
- 7.Colditz GA, Cannuscio CC, Frazier AL. Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Causes Control. 1997;8:649–67. doi: 10.1023/a:1018458700185. [DOI] [PubMed] [Google Scholar]
- 8.Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev. 2001;10:287–301. [PubMed] [Google Scholar]
- 9.Isaksson B, Jonsson F, Pedersen NL, Larsson J, Feychting M, Permert J. Lifestyle factors and pancreatic cancer risk: a cohort study from the Swedish Twin Registry. Int J Cancer. 2002;98:480–2. doi: 10.1002/ijc.10256. [DOI] [PubMed] [Google Scholar]
- 10.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–9. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 11.Lund Nilsen TI, Johnsen R, Vatten LJ. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br J Cancer. 2000;82:1358–63. doi: 10.1054/bjoc.1999.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee IM. Physical activity and cancer prevention--data from epidemiologic studies. Med Sci Sports Exerc. 2003;35:1823–7. doi: 10.1249/01.MSS.0000093620.27893.23. [DOI] [PubMed] [Google Scholar]
- 13.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:459–66. doi: 10.1158/1055-9965.EPI-04-0583. [DOI] [PubMed] [Google Scholar]
- 14.Severson Rk, Nomura AM, Grove JS, Stemmermann GN. A prospective analysis of physical activity and cancer. Am J Epidemiol. 1989;130:522–9. doi: 10.1093/oxfordjournals.aje.a115366. [DOI] [PubMed] [Google Scholar]
- 15.Wannamethee SG, Shaper AG, Walker M. Physical activity and risk of cancer in middle-aged men. Br J Cancer. 2001;85:1311–6. doi: 10.1054/bjoc.2001.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson AM, Church TS, Janssen I, Katzmarzyk PT, Earnest CP, Blair SN. Cardiorespiratory fitness as a predictor of cancer mortality among men with pre-diabetes and diabetes. Diabetes Care. 2008;31:764–9. doi: 10.2337/dc07-1648. [DOI] [PubMed] [Google Scholar]
- 17.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975−2005. 2008 http://seer.cancer.gov/csr/1975_2005/
- 18.Blair SN, Kohl HW, III, Paffenbarger RS, Jr., Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 19.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Med J. 1959;10:675–88. [PubMed] [Google Scholar]
- 20.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 21.American College of Sports Medicine . ACSM's Guidelines For Exercise Testing And Prescription. 7th ed. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 291–4. [Google Scholar]
- 22.Sui X, Laditka JN, Hardin JW, Blair SN. Estimated functional capacity predicts mortality in older adults. J Am Geriatr Soc. 2007;55:1940–7. doi: 10.1111/j.1532-5415.2007.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–23. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 25.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30:252–8. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Bouchard C, An P, Rice T, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol. 1999;87:1003–8. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity). Arterioscler Thromb Vasc Biol. 2003;23:E42–9. doi: 10.1161/01.ATV.0000087143.33998.F2. [DOI] [PubMed] [Google Scholar]
- 28.Slattery ML. Physical activity and colorectal cancer. Sports Med. 2004;34:239–52. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- 29.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7:204–13. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 30.Harriss DJ, Cable NT, George K, Reilly T, Renehan AG, Haboubi N. Physical activity before and after diagnosis of colorectal cancer: disease risk, clinical outcomes, response pathways and biomarkers. Sports Med. 2007;37:947–60. doi: 10.2165/00007256-200737110-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr. Physical activity, body weight, and pancreatic cancer mortality. Br J Cancer. 2003;88:679–83. doi: 10.1038/sj.bjc.6600782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, Kikuchi S, Tamakoshi A, et al. Obesity, physical activity and the risk of pancreatic cancer in a large Japanese cohort. Int J Cancer. 2007;120:2665–71. doi: 10.1002/ijc.22614. [DOI] [PubMed] [Google Scholar]
- 33.Nothlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control. 2007;18:165–75. doi: 10.1007/s10552-006-0100-0. [DOI] [PubMed] [Google Scholar]
- 34.Sinner PJ, Schmitz KH, Anderson KE, Folsom AR. Lack of association of physical activity and obesity with incident pancreatic cancer in elderly women. Cancer Epidemiol Biomarkers Prev. 2005;14:1571–3. doi: 10.1158/1055-9965.EPI-05-0036. [DOI] [PubMed] [Google Scholar]
- 35.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008;167:586–97. doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 36.Hanley AJ, Johnson KC, Villeneuve PJ, Mao Y. Physical activity, anthropometric factors and risk of pancreatic cancer: results from the Canadian enhanced cancer surveillance system. Int J Cancer. 2001;94:140–7. doi: 10.1002/ijc.1446. [DOI] [PubMed] [Google Scholar]
- 37.Lee IM. Exercise and physical health: cancer and immune function. Res Q Exerc Sport. 1995;66:286–91. doi: 10.1080/02701367.1995.10607913. [DOI] [PubMed] [Google Scholar]
- 38.Shephard RJ, Shek PN. Associations between physical activity and susceptibility to cancer: possible mechanisms. Sports Med. 1998;26:293–315. doi: 10.2165/00007256-199826050-00002. [DOI] [PubMed] [Google Scholar]
- 39.McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9:487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 40.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:920–31. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 42.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 43.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132:3456S–64S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 44.Hebert JR, Daguise VG, Hurley DM, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and gender disparities in a high-risk population. Cancer. 2009:00–000. doi: 10.1002/cncr.24270. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nilsen TI, Romundstad PR, Petersen H, Gunnell D, Vatten LJ. Recreational physical activity and cancer risk in subsites of the colon (the Nord-Trondelag Health Study). Cancer Epidemiol Biomarkers Prev. 2008;17:183–8. doi: 10.1158/1055-9965.EPI-07-0746. [DOI] [PubMed] [Google Scholar]