Abstract
Mesenteric fat is known to undergo inflammatory changes after 2,4,6,-trinitrobenzensulphonic acid (TNBS)–induced colitis. Neurotensin (NT) and neurotensin receptor 1 (NTR1) have been shown to play a major role in the pathogenesis of intestinal inflammation. This led us to explore whether NT and NTR1 are expressed in the mesenteric fat depots during TNBS-induced colitis and whether NT participates in the increased interleukin (IL)–6 secretion in this inflammatory response. TNBS-induced inflammation in the colon increases NT and NTR1 expression in mesenteric adipose tissues, including mesenteric preadipocytes. Compared with wild-type mice, NT knockout (KO) mice have reduced TNBS-induced colitis accompanied by diminished inflammatory responses in mesenteric adipose tissue. Specifically, IL-6 and p65 phosphorylation levels in mesenteric fat of NT KO mice are also reduced compared with wild-type mice. Mouse 3T3-L1 preadipocytes express NTR1 and its expression is increased after stimulation of preadipocytes with proinflammatory cytokines. NT stimulation of 3T3-L1 preadipocytes overexpressing NTR1 causes PKCδ phosphorylation and IL-6 secretion in a time- and dose-dependent fashion. Moreover, NT-mediated IL-6 expression is nuclear factor–κB and PKCδ dependent. We also found that supernatants from NT-exposed 3T3-L1-NTR1 preadipocytes and mesenteric fat obtained from wild-type mice 2 days after TNBS administration stimulate an IL-6–dependent macrophage migration measured by a macrophage migration assay, whereas this response is reduced when mesenteric fat from NT KO mice is used. These results demonstrate an important role for NT in acute colitis and adipose tissue inflammation associated with experimental colitis that involves direct NT proinflammatory responses in preadipocytes.
Keywords: cytokine, intestinal inflammation, macrophages, neuropeptide
Adipose tissue is an important source of several hormones and cytokines (1). In certain inflammatory conditions the adipose tissue is infiltrated by immune cells, contributing to cytokine production (2, 3), whereas several hormones and cytokines, including leptin, interleukin (IL)–8, and tumor necrosis factor–α (TNFα), also called adipokines, are released by adipocytes within white adipose tissue (WAT) (1). Patients with Crohn's disease (CD) accumulate adipokine-releasing intra-abdominal fat from the onset of the disease (4, 5), indicating that expansion of mesenteric fat depots may be an important feature of inflammatory bowel disease (IBD). Along these lines, we have recently reported that acute 2,4,6,-trinitrobenzensulphonic acid (TNBS)–induced colitis in mice is associated with major inflammatory changes in the proximal mesenteric fat, such as infiltration with neutrophils and possibly macrophages and increased expression of tumor necrosis factor–α (TNFα), interleukin (IL)–6, monocyte chemotactic protein–1 (MCP-1), and chemokine keratinocyte chemoattractant (KC) as well as receptors for the neuropeptide substance P (6).
Neurotensin (NT) is a 13 aa peptide highly expressed in the brain and gastrointestinal tract (7). NT is produced and secreted by specific endocrine cells in the gastrointestinal tract, where it mediates motility and secretory responses in the small and large intestines (8) and chloride secretion in human colon (9) via its high affinity G-protein–coupled receptor NTR1 (8, 9). Evidence also indicates that NT and NTR1 mediate important colonic responses related to inflammation (10–12). However, whether adipose tissue or adipocytes express NT or NTR1 is not known and the potential modulation of these molecules during intestinal inflammation has never been investigated.
Here we examined whether induction of acute colitis has an effect on the inflammatory state as well as on the expression of NT and NTR1 in mesenteric fat tissues in vivo and preadipocytes in vitro. We recently showed that IL-6, a cytokine secreted by several cell types, including adipocytes that have been associated with obesity and insulin resistance (1), is an important mediator in TNBS-induced colitis (13). Because a possible association between NT and IL-6 has never been studied, we also assessed the importance of NT in TNBS-induced mesenteric fat–derived IL-6 and compared this response in wild-type and in NT KO mice. The importance of NT in the development of experimental colitis and colitis-associated responses in mesenteric fat was also compared between wild-type and NT KO mice. Inflammatory responses in fat tissue induced by intestinal inflammation can be mediated by newly acquired properties of mesenteric adipocytes as well as by invading macrophages. Thus, an assay for macrophage migration was also used to measure changes in migration of these cells following preadipocyte exposure to NT.
Results
Elevated NT and NTR1 Expression in Preadipocytes of Mesenteric Fat of Mice During TNBS-Induced Colitis.
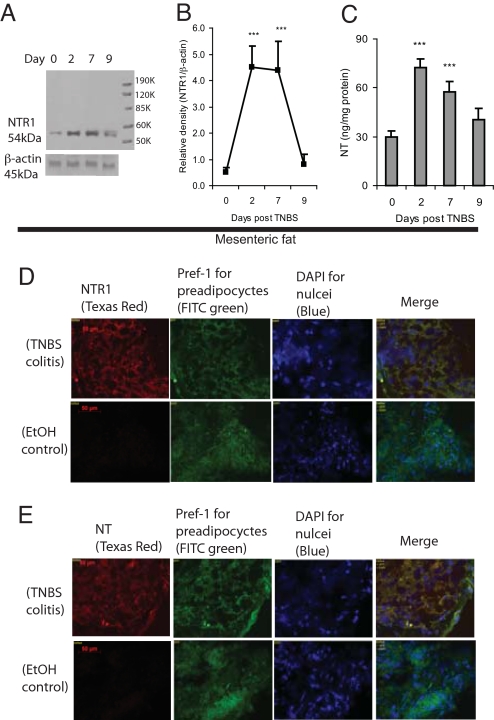
We induced colitis in mice with TNBS and found that mesenteric fat expresses NTR1 (Fig. 1 A and B) as well as NT (Fig. 1C) after induction of colitis. Two days after TNBS administration, NTR1 (54 kDa) protein expression in mesenteric fat was increased by 9-fold, remained at a high level on day 7, and declined to control levels on day 9 (Figs. 1A, 1B). NTR1 mRNA expression (Relative density of (NTR1/β actin) was also significantly changed in colon of mice after TNBS exposure in a similar time-dependent manner (day 2, 3.5 ± 0.14 fold, P < 0.01; day 7, 2.4 ± 0.14 fold, P < 0.05; day 9, 1.1 ± 0.16 fold, compared with day 0, mean ± SEM, n = 6 mice per group). In TNBS-exposed mice, NT peptide expression in mesenteric fat was significantly increased at day 2 and then declined by day 9, although it remained at higher levels compared with control values (Fig. 1C). Immunofluorescence staining demonstrated a very low level of NTR1 and NT expression in adipose tissues of ethanol-treated control mice, but increased expression of both molecules in mesenteric fat of TNBS-exposed mice (Fig. 1 D and E), consistent with the results shown in Fig. 1 A–C. Dual immunofluorescence staining of mesenteric fat with an antibody against Pref-1, a marker for preadipocytes, but not mature adipocytes (14), and antibodies against NT and NTR1 indicated that most NTR1 and NT were expressed in preadipocytes (Fig. 1 D and E).
Fig. 1.
Increased expression of NT and NTR1 in mesenteric fat of mice during TNBS-induced colitis: Eight-week-old male C57BL/6 mice (six mice per group) were treated with either TNBS or 30% ethanol (vehicle) for 0, 2, 7, and 9 days. Protein levels of NTR1 in mesenteric fat were measured by Western blot (A). (Right lane) Migration of prestained molecular weight protein standards (k = 1000 daltons) as described in Materials and Methods. (B) Densitometric measurements of NTR1 Western blots were normalized by β-actin. (C) Peptide levels of NT in mesenteric fat were measured by enzyme-linked immunosorbent assay (ELISA) (***P < 0.001 vs. day 0). Immunofluorescence staining for NTR1 (D), NT (E) as well as Pref-1 (a preadipocyte marker), and nuclear stain DAPI (D, E) of mesenteric fat from mice with TNBS colitis (day 2) and ethanol-treated control mice. Overlapping expression of NTR1/NT (Texas Red) with Pref-1 (FITC green) indicates preadipocytes expressing NTR1 or NT (yellow); magnification ×200.
NT-Deficient/-Knockout (KO) Mice Have Reduced Inflammatory Changes and IL-6 Levels in Their Mesenteric Fat During TNBS-Induced Colitis.
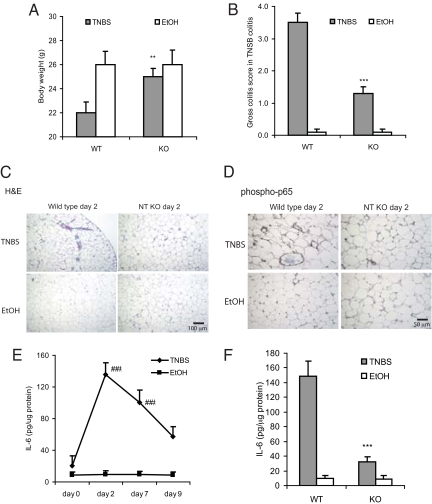
When wild-type and NT KO mice (15, 16) were examined after exposure to intracolonic TNBS for 2 days, TNBS-exposed wild-type mice had increased colitis clinical scores, as defined by the criteria listed in our previous report (17), with substantial weight loss compared with vehicle-exposed littermates (Fig. 2 A and B). However, NT KO mice had significantly less colitis and less weight loss compared with TNBS-administered wild-type mice (Fig. 2 A and B). Histologic examination showed that TNBS-associated inflammatory changes in the mesenteric fat were dramatically reduced in NT KO mice compared with wild-type mice (Fig. 2C). Immunostaining with an antibody against the phosphorylated p65 subunit of nuclear factor–κB (NF-κB) indicated that p65 phosphorylation was decreased in mesenteric fat of NT KO compared with wild-type mice (Fig. 2D). In wild-type mice, TNBS administration resulted in increased mesenteric fat IL-6 protein levels after 2, 7, and 9 days, with the highest levels 2 days post-TNBS (Fig. 2E), whereas no increase in fat-associated IL-6 levels were evident in vehicle-treated mice. IL-6 content, however, was significantly lower in mesenteric fat from NT KO mice compared with wild-type mice after 2 days of TNBS administration (Fig. 2F). In contrast, no difference in IL-6 levels were evident between NT KO and wild-type mice in response to vehicle (ethanol) (Fig. 2F).
Fig. 2.
NT KO mice are protected from colonic tissue damage, adipose inflammation, and NF-κB activation caused by TNBS-induced colitis. Homozygous NT KO and wild-type mice were treated with TNBS for 2 days and body weight changes (A) and gross colitis score (B) were measured. ***P < 0.001, **P < 0.01 vs. wild-type, TNBS-treated mice. Mesenteric fat tissues from the same mouse groups were either stained with hematoxylin and eosin (C) or an antibody against phosphorylated p65 (D). (E) Mesenteric fat IL-6 levels of wild-type mice throughout the course of TNBS treatment were measured. ###P < 0.001 vs. day 0 of TNBS treated mice. (F) Mesenteric fat IL-6 levels on day 2 post-TNBS from wild-type and NT KO mice were measured. All experiments are representative of six mice per group. *** P < 0.001 vs. wild-type mice with TNBS treatment. Magnification ×100 (C) or ×200 (D).
Cytokine Stimulation Increases NTR1 and NT Levels in Preadipocytes.
To determine whether preadipocytes express NT and NTR1, we used well-characterized mouse 3T3-L1 cells (18). Treating these cells with a cytokine mixture for various time points stimulate persistent expression of NTR1 at 4–24 hours [supporting information (SI) Fig. S1 A and B] and transient expression of NT at ≈4 hours (Fig. S1C). To mimic in vivo conditions of increased expression of NTR1 (Fig. 1), we retrovirally transfected 3T3-L1 cells overexpressing NTR1 and used these cells for all in vitro experiments described below.
NT Stimulates PKCδ and p65 Phosphorylation and IL-6 Transcription in Mouse Preadipocytes.
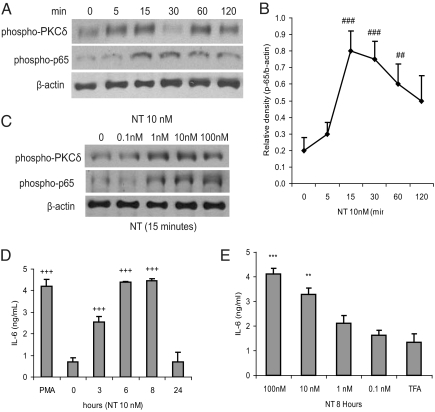
Incubation of 3T3-L1-NTR1 preadipocytes with NT stimulated a biphasic time-dependent phosphorylation of PKCδ starting at 5 minutes, with an initial peak at 15 minutes, a transient decline at 30 minutes, and then peaked again at 60 minutes with another decline at 120 minutes (Fig. 3A). NT also induced a time-dependent phosphorylation of NF-κB p65 subunit peaking at 15–30 minutes and then declining at 60 minutes (Fig. 3A and densitometric quantification in Fig. 3B). NT also stimulated a dose-dependent phosphorylation of PKCδ and p65 (Fig. 3C). However, similar NT concentrations failed to phosphorylate PKC θ or ε (data not shown). NT also stimulated IL-6 secretion in a time- and dose-dependent manner (Fig. 3 D and E). The highest levels of NT-dependent IL-6 secretion were evident at 8 hours with 100 nM of NT (Fig. 3 D and E). NT exposure also results in a time- and dose-dependent increase in IL-6 promoter activity, indicating that NT induces transcriptional activation of IL-6 in these cells (Fig. S2 A and B).
Fig. 3.
NT induces IL-6 secretion in 3T3-L1-NTR1 preadipocytes: (A, C) Cultured 3T3-L1-NTR1 3T3-L1 preadipocytes were exposed to different concentrations of NT at various time intervals. Cells were lysed and equal amounts of proteins were blotted for detection of phosphorylated PKCδ and p65, or β-actin. (B) Densitometric analyses of phospho-p65 signal normalized to β-actin signal. ###P < 0.001, ##P < 0.01 vs. 0 minute. Cultured 3T3-L1-NTR1 preadipocytes were exposed to NT (10 nM) or vehicle trifluoroacetic acid 0.1% (TFA) for 0–24 hours (D), and at various doses (0–100 nM) for 8 hours (E). Treatment with 1 μM proinflammatory phorbol ester phorbol-12-myristate-13-acetate (PMA) for 8 hours served as a positive control. Conditioned media were collected for mouse IL-6 ELISA. +++P < 0.001 vs. 0 h. ***P < 0.001, **P < 0.01 vs. TFA control group. Results are representative of three independent experiments.
NT-Induced IL-6 Secretion in 3T3-L1-NTR1 Mouse Preadipocytes Involves PKCδ- and NF-κB–Dependent Pathways.
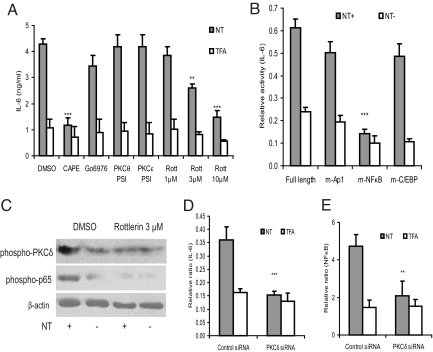
The NF-κB inhibitor caffeic acid phenethyl ester (CAPE) and PKCδ inhibitor Rottlerin (mallotoxin), respectively, significantly inhibited IL-6 secretion in response to NT, whereas inhibitors of PKCθ (PKCθ pseudosubstrate inhibitor), PKCε (PKCε pseudosubstrate inhibitor) or Ca2+ dependent PKC (Go6976) had no effect (Fig. 4A). Rottlerin alone did not affect basal IL-6 secretion without NT stimulation (Fig. 4A). However, 3 and 10 μM of Rottlerin significantly inhibited IL-6 secretion in response to NT by ≈45% and 70%, respectively, whereas 1 μM had no inhibitory effect (Fig. 4A).
Fig. 4.
NT-induced IL-6 secretion in 3T3-L1-NTR1 mouse preadipocytes involves PKCδ- and NF-κB–dependent pathways: (A) 3T3-L1-NTR1 preadipocytes were pretreated with dimethyl sulfoxide (DMSO; vehicle), CAPE (10 μM), Ca2+ dependent PKC inhibitor Go6976 (10 μM), Rottlerin (1–10 μM), PKCθ pseudosubstrate inhibitor (PKCθ PSI; 10 μM) or PKCε pseudosubstrate inhibitor (PKCε PSI; 10 μM) for 30 minutes, followed by NT (10 nM) or TFA (vehicle) for 8 hours. Conditioned media were then collected for mouse IL-6 ELISA. Results were representative of three independent experiments. (B) 3T3-L1-NTR1 preadipocytes were transfected with either full-length IL-6 promoter construct or IL-6 promoter constructs containing mutations in the NF-κB (m-NF-κB), AP1 (m3/m5-Ap1), or C/EBP (mC/EBP) transcription binding sites, followed by NT (10 nM) treatment for 8 hours. Cell lysates were then used to perform luciferase reporter assays. ***P < 0.001 vs. full-length group. (C) 3T3-L1-NTR1 preadipocytes were pretreated with DMSO or Rottlerin (3 μM) for 30 minutes, followed by NT exposure (10 nM) for 30 minutes. Cells were lysed, and equal amounts of protein were used to detect phospho-PKCδ, phospho-p65, and β-actin. (D, E) 3T3-L1-NTR1 preadipocytes were co-transfected with control siRNA or PKCδ siRNA together with a IL-6 promoter (D) and NF-κB luciferase construct (E), followed by exposure to NT (10 nM) for 8 hours. Cell lysates were used to perform IL-6/NF-κB luciferase reporter assay. ***P < 0.001 vs. control siRNA transfected NT-treated group. Results are representative of three independent experiments.
To confirm the importance of NF-κB in NT-induced IL-6 transcription, we transfected 3T3-L1-NTR1 preadipocytes with either a wild-type IL-6 promoter construct or IL-6 promoter constructs with mutated NF-κB, activator protein 1 (AP-1) or CCAAT/enhancer binding protein (C/EBP) binding sites. NT-induced IL-6 promoter activity was dramatically reduced in cells transfected with an IL-6 promoter construct containing a mutated NF-κB binding site, whereas mutation of either AP-1 or C/EBP sites had no significant effect (Fig. 4B). Pharmacological inhibition of NF-κB by CAPE, but not the calcium dependent PKC inhibitor Go6976, reduced NT-induced IL-6 promoter activity (Fig. S2C). Moreover, Rottlerin, but not pseudosubstrate inhibitors of PKCθ or PKCε, diminished NT-induced IL-6 promoter activity (Fig. S2C). Together, these results show that IL-6 transcription in response to NT in preadipocytes involves PKCδ- and NF-κB-dependent pathways.
Rottlerin at 3 μM also reduced phosphorylation of PKCδ and p65 to basal levels after stimulation of 3T3-L1-NTR1 preadipocytes with NT (Fig. 4C). Moreover, PKCδ siRNA, but not control siRNA, completely blocked NT-stimulated IL-6 promoter activity and NF-κB luciferase activity, indicating that NT-induced NF-κB activation and IL-6 transcription in preadipocytes is regulated by PKCδ (Fig. 4 D and E). The effectiveness of PKCδ siRNA in reducing PKCδ protein expression compared with control siRNA is shown in Fig. S2D.
NT Stimulates Preadipocyte-Dependent Macrophage Migration via IL-6 Secretion.
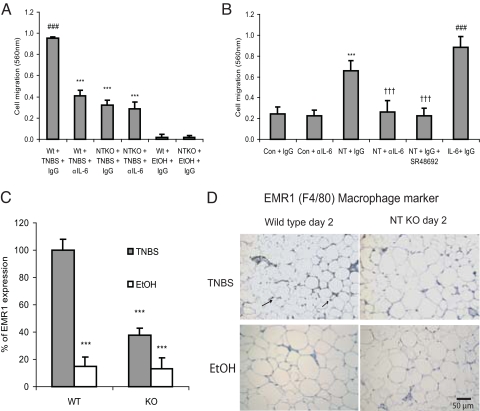
Our results indicated that NT KO mice have diminished IL-6 expression and reduced inflammatory changes in adipose tissue during colitis (Fig. 2). One possibility is that there is a NT-associated migration of macrophages into the adipose tissues during colitis. To explore this we removed mesenteric fat depots 2 days after TNBS or vehicle administration and performed a migration assay in Boyden chambers using Raw264.7 macrophages. We found that mesenteric fat extracts from wild-type mice with TNBS colitis strongly stimulate macrophage migration, an effect that was inhibited by anti-IL-6 antibody (Fig. 5A). Compared with the wild-type mice, there was a significant reduction in macrophage migration when mesenteric fat extracts from NT KO mice were used, and the residual migration was not significantly reduced by addition of a neutralizing antibody against IL-6 (Fig. 5A). These results suggest that NT induced IL-6 secretion in mesenteric fat promotes macrophage infiltration during TNBS-induced colitis.
Fig. 5.
NT stimulates preadipocyte-dependent macrophage migration via IL-6 secretion. (A) Mesenteric fat extracts from wild-type and NT KO mice were was placed in the lower compartment of modified Boyden chambers together with either anti-mouse IL-6–neutralizing IgG or control IgG. Murine macrophage Raw264.7 cells were then seeded into the upper compartment; after 8 hours, cells that had migrated to the membrane were stained and lysed, and cell migration was determined by absorbance measurements at 560 nm. ###P < 0.001 vs. Wt+EtOH+IgG; ***P < 0.001 vs. Wt+TNBS+IgG. (B) 3T3-L1-NTR1 preadipocytes, placed onto the lower compartment of modified Boyden chambers, were treated with IgG, anti-IL-6 antibody or NTR1 receptor antagonist SR48692 (2 μM) followed by exposure to NT (10 nM) or TFA (1 μl) for 8 hours and measurement of macrophage cell migration as described in (A). ***P < 0.01 vs. Con+IgG; †††P < 0.01 vs. NT+IgG; ###P < 0.001 vs. Con+IgG. Results are representative of six independent experiments. (C) Homozygous NT KO and wild-type mice were treated with TNBS for 2 days. RNA was isolated from mesenteric fat and macrophage-specific EMR1 (F4/80) mRNA was quantitated by real-time RT-PCR. (D) Mesenteric fat tissues from the same mouse groups were also stained with an antibody against EMR1. All experiments are representative of six mice per group. ***P < 0.01 vs. wild-type mice. Magnification ×200.
To identify the role of preadipocytes in NT-associated adipose inflammation, we used conditioned medium from NT-stimulated 3T3-L1-NTR1 preadipocytes to determine migration of Raw264.7 macrophages. We found that conditioned medium from NT-treated preadipocytes induced macrophage migration that was significantly inhibited by an anti-IL-6 antibody or a NTR1 receptor antagonist (SR48692), suggesting an important role for NTR1 in this response (Fig. 5B). Recombinant mouse IL-6 added to the preadipocytes-containing lower chamber also induced macrophage migration (Fig. 5B). Because NT does not directly induce Raw264.7 macrophage migration (19), our findings suggest that IL-6 secreted in response to NT induces macrophage migration.
Macrophage infiltration is a cardinal feature of colitis (20). To assess macrophage infiltration of mesenteric fat depots during TNBS-induced colitis, we quantified mRNA expression of the macrophage marker EMR1 (F4/80) (21). We found increased EMR1 (F4/80) mRNA expression in mesenteric fat after TNBS induction, but significantly less EMR1 (F4/80) mRNA expression in mesenteric fat (≈50%) of NT KO mice compared with wild-type controls (Fig. 5C). Furthermore, immunohistochemical analyses of these tissues with a specific EMR1 antibody showed significant macrophage infiltration in the mesenteric fat depots of wild-type mice after TNBS administration (Fig. 5D). In contrast, NT KO mice had less EMR1-positive cells in mesenteric fat (Fig. 5D), suggesting an important role for NT in this response.
Discussion
We report here that inflammatory changes in the proximal mesenteric fat depots in the Crohn's disease-like TNBS model of colitis (22, 23) were associated with increased expression of mRNAs for the neuropeptide NT and its high affinity receptor NTR1. In this model, activation of the NF-κB system and release of IL-6 were also evident. We also found that animals genetically deficient in NT had reduced inflammatory responses in both colonic and mesenteric fat tissues in response to TNBS, providing direct evidence for an important role for NT in colitis and colitis-associated changes in fat depots. The increase in NT can result from activated preadipocytes or macrophages within the fat depots as well as from macrophages migrating into fat tissue during colonic inflammation. However, this report demonstrates that adipocytes have the ability to produce these molecules and potentially influence the development of intestinal inflammation. Our findings that mesenteric fat tissue inflammatory changes during colitis were associated with macrophage infiltration, and that this response was diminished in NT-deficient mice (Fig. 5), indicates that NT plays an important role in macrophage function in response to colonic inflammation.
Activated macrophages within abdominal fat are a cardinal feature in Crohn's disease patients (4, 24). Our evidence that NT stimulates a preadipocyte-dependent macrophage migration that involves IL-6 secretion (Fig. 5), together with the ability of white adipose tissue macrophages to secrete IL-6 during inflammation (25), indicates a potentially important role for NT–adipose tissue–macrophage responses in the pathophysiology of IBD via release of IL-6 and possibly other soluble inflammatory factors. Our results are consistent with a recent report demonstrating direct IL-6 chemoattraction of monocytic cells (26). However, indirect effects of IL-6 on macrophage migration have also been demonstrated (27, 28). Several reports indicate the importance of IL-6 in the pathophysiology of experimental colitis and human IBD (13, 29), and humanized anti–IL-6 monoclonal antibodies are clinically tested as therapeutic agents for Crohn's disease (30).
Our in vitro studies with human 3T3-L1 preadipocytes show that NT stimulates phosphorylation of PKCδ, a signaling response that was not previously recognized. Few studies indicate that PKCδ is involved in obesity and insulin resistance at the adipocyte level. For example, increased reactive oxygen species production in adipocytes from obese and insulin-resistant mice involves activation of PKCδ (31), whereas the PKCδ antagonist Rottlerin inhibits insulin-stimulated glucose transport in 3T3-L1 preadipocytes (32). Our results indicate that antagonism of PKCδ by either the specific inhibitor Rottlerin or by gene-silencing approaches reduces NF-κB activation and IL-6 transcription in 3T3-L1-NTR1 preadipocytes treated with NT (Fig. 4). These results suggest that this PKC isoform may be involved in proinflammatory signaling mechanisms in response to NT in adipocytes.
Our results may be relevant to the pathophysiology of IBD, especially Crohn's disease, characterized by accumulation of intra-abdominal fat from the onset of this disease and increased expression of peroxisome proliferator-activated receptor–γ and TNFα in this tissue (4). Preadipocytes may be involved in colonic inflammation by production of proinflammatory cytokines in response to NT, such as IL-6, and recruitment of macrophages or other immune cells into adipose tissues. In addition, our data demonstrating the ability of NT to stimulate preadipocyte-derived IL-6 release may reflect a potential role for this neuropeptide in the regulation of energy balance due to the involvement of fat tissue in processes such as fatty acid storage and insulin resistance. Indeed, dysregulated inflammatory responses are associated with the development of insulin resistance and type 2 diabetes, whereas TNFα and IL-6 play a predominant role in these disease states (33). Moreover IL-6 inhibits adipogenesis and decreases secretion of the anti-inflammatory adiponectin (33), suggesting a role for IL-6 in obesity and insulin resistance.
Our finding indicating that NT-induced IL-6 transcription in preadipocytes involves NF-κB pathways (Fig. 4) is consistent with our previous results showing that NT activates this transcription factor thereby stimulating expression of NF-κB–related cytokines (34). Together, these results provide direct evidence for an important role of NT in the development of colitis and associated inflammatory changes in the adjacent fat depots, and point toward a new direction in the search for therapeutic targets for Crohn's disease.
Materials and Methods
3T3-L1-NTR1 Cell Line.
Mouse 3T3-L1 preadipocytes obtained from ATCC were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). Because the NTR1 expression in 3T3-L1 preadipocytes and adipose tissues under normal condition was relatively low, we transfected NTR1 into 3T3-L1 by retroviral approach to reflect increased NTR1 expression during inflammation. Full-length mouse NTR1 was cloned and ligated into the retroviral vector pCMBP (34). Retroviruses were prepared using a previously described procedure (35). Infected 3T3-L1 preadipocytes were used for NT (Calbiochem) or 0.1% trifluoroacetic acid (TFA, vehicle control) treatment.
TNBS-Induced Colitis in Mice.
Male, 10–12-week-old, homozygous NT knockout and control littermates (15) were maintained at the animal research facility of Beth Israel Deaconess Medical Center. Animal studies were approved by the institutional animal care and use committee of the Beth Israel Deaconess Medical Center. Mice received standard pelleted chow and tap water ad libitum. TNBS colitis was induced as previously reported (17). Control groups were injected with 100 μl of 30% ethanol intracolonically. Mice were then returned to their cages and killed on days 0, 2, 7, and 9 by carbon dioxide euthanasia. Colon tissues and mesenteric fat were dissected for further investigations.
Pharmacological Inhibition.
The PKCδ inhibitor Rottlerin (mallotoxin 0.1–1 μM), PKCθ pseudosubstrate inhibitor (Myr-LHQRRGAIKQAKVHHVKC-NH2 10 μM), PKCε pseudosubstrate inhibitor (EAVSLKPT 10 μM), Ca2+ dependent PKC inhibitor (Go6976 10 μM), and NF-κB inhibitor (caffeic acid phenethyl ester/CAPE 10 μM) were obtained from Calbiochem.
Macrophage Cell Migration Assay.
Murine macrophage Raw264.7 cells obtained from ATCC were maintained in DMEM with 10% FBS. Cell migration assays were performed using a modified Boyden chamber approach (Billerica, MA). Ex vivo experiments were as follows. Mesenteric fat from wild-type or homozygous NT KO mice was removed 2 days post-TNBS, homogenized in DMEM, and centrifuged at 14,000 g for 15 min. Aliqoots (50 μl) aliquots of the supernatants (≈40 μg protein/ml) or equal volume of DMEM were added in the lower chamber, and murine Raw264.7 macrophages (2.5 × 104 cells) were seeded in the upper chamber. Both chambers were treated with anti-mouse IL-6 neutralizing antibody (10 μg/ml, R&D Systems) at the same time and incubated for 8 hours at 37 °C. In vitro experiments were as follows. 3T3-L1-NTR1 preadipocytes (500 μl/0.5 × 106 cells) were placed in the lower chamber of Boyden chambers, and murine Raw264.7 macrophages (2.5 × 104 cells) were seeded in the upper chamber. At the same time, anti-mouse IL-6 neutralizing antibody (10 μg/ml, R&D Systems), control IgG, or NTR1 receptor antagonist SR48692 (2 μM) were added to both chambers and incubated for 30 minutes at 37 °C. NT (10 nM) 8, TFA (vehicle, 1 μl) or recombinant mouse IL-6 (5 ng/ml, R&D Systems) were then added in the lower chamber and incubated for 8 hours at 37 °C. Macrophages that had migrated through the membrane were stained and lysed according to the manufacturer's protocol and the quantity of migrated cells was measured by absorbance at 650 nm.
Statistical Analysis.
Results were analyzed by using Prism professional statistics software program (GraphPad Software, San Diego, CA). Analysis of variance was used for intergroup comparisons.
Supplementary Material
Acknowledgments.
This work was supported by National Institute of Health grant DK060729 to C.P. and by the Crohn's and Colitis Foundation of America Research Fellowship Awards (to H.W.K and I.K.). We also thank Sanofi-Aventis for generously providing the NTR1 antagonist SR48692.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903499106/DCSupplemental.
References
- 1.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desreumaux P, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 5.Schaffler A, Scholmerich J, Buchler C. Mechanisms of disease: Adipocytokines and visceral adipose tissue—emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:273–280. doi: 10.1038/ncpgasthep0186. [DOI] [PubMed] [Google Scholar]
- 6.Karagiannides I, et al. Induction of colitis causes inflammatory responses in fat depots: Evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci USA. 2006;103:5207–5212. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carraway R, Leeman SE. Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem. 1976;251:7045–7052. [PubMed] [Google Scholar]
- 8.Zhao D, Pothoulakis C. Effects of NT on gastrointestinal motility and secretion, and role in intestinal inflammation. Peptides. 2006;27:2434–2444. doi: 10.1016/j.peptides.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Riegler M, Castagliuolo I, Wang C, Wlk M, Sogukoglu T, et al. Neurotensin stimulates Cl(–) secretion in human colonic mucosa in vitro: Role of adenosine. Gastroenterology. 2000;119:348–357. doi: 10.1053/gast.2000.9310. [DOI] [PubMed] [Google Scholar]
- 10.Brun P, et al. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G621–G629. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 11.Castagliuolo I, et al. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. J Clin Invest. 1999;103:843–849. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–932. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 13.Gay J, Kokkotou E, O'Brien M, Pothoulakis C, Karalis KP. Interleukin-6 genetic ablation protects from trinitrobenzene sulfonic acid-induced colitis in mice. Putative effect of antiinflammatory cytokines. Neuroimmunomodulation. 2006;13:114–121. doi: 10.1159/000096656. [DOI] [PubMed] [Google Scholar]
- 14.Kim KA, Kim JH, Wang Y, Sul HS. Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol Cell Biol. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobner PR, Fadel J, Deitemeyer N, Carraway RE, Deutch AY. Neurotensin-deficient mice show altered responses to antipsychotic drugs. Proc Natl Acad Sci USA. 2001;98:8048–8053. doi: 10.1073/pnas.141042198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinkead B, et al. Neurotensin-deficient mice have deficits in prepulse inhibition: Restoration by clozapine but not haloperidol, olanzapine, or quetiapine. J Pharmacol Exp Ther. 2005;315:256–264. doi: 10.1124/jpet.105.087437. [DOI] [PubMed] [Google Scholar]
- 17.Castagliuolo I, et al. Protective effects of neurokinin-1 receptor during colitis in mice: Role of the epidermal growth factor receptor. Br J Pharmacol. 2002;136:271–279. doi: 10.1038/sj.bjp.0704697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafson B, Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3–L1 preadipocytes. J Biol Chem. 2006;281:9507–9516. doi: 10.1074/jbc.M512077200. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, et al. Neurotensin enhances nitric oxide generation via the JAK2-STAT1 pathway in murine macrophage Raw264.7 cells during costimulation with LPS and IFNgamma. Neuropeptides. 2006;40:221–229. doi: 10.1016/j.npep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Kanai T, et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn's disease. Gastroenterology. 2001;121:875–888. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- 21.Weng M, et al. Alternatively activated macrophages in intestinal helminth infection: Effects on concurrent bacterial colitis. J Immunol. 2007;179:4721–4731. doi: 10.4049/jimmunol.179.7.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath M, Fuss I, Strober W. TNBS colitis. Int Rev Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- 23.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Delivery Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Schaffler A, Herfarth H. Creeping fat in Crohn's disease: Travelling in a creeper lane of research? Gut. 2005;54:742–744. doi: 10.1136/gut.2004.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 26.Clahsen T, Schaper F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J Leukoc Biol. 2008;84:1521–1529. doi: 10.1189/jlb.0308178. [DOI] [PubMed] [Google Scholar]
- 27.Kaplanski G, Marin V, Montero–Julian F, Mantovani A, Farnarier C. IL-6: A regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 28.Romano M, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 29.Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016–1023. doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto N, Kishimoto T. Interleukin 6: From bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 31.Talior I, Tennenbaum T, Kuroki T, Eldar-Finkelman H. PKC-delta-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: Role for NADPH oxidase. Am J Physiol Endocrinol Metab. 2005;288:E405–E411. doi: 10.1152/ajpendo.00378.2004. [DOI] [PubMed] [Google Scholar]
- 32.Kayali AG, Austin DA, Webster NJ. Rottlerin inhibits insulin-stimulated glucose transport in 3T3–L1 adipocytes by uncoupling mitochondrial oxidative phosphorylation. Endocrinology. 2002;143:3884–3896. doi: 10.1210/en.2002-220259. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- 34.Zhao D, et al. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J Biol Chem. 2001;276:44464–44471. doi: 10.1074/jbc.M104942200. [DOI] [PubMed] [Google Scholar]
- 35.Zhao D, et al. Neurotensin stimulates IL-8 expression in human colonic epithelial cells through Rho GTPase-mediated NF-kappa B pathways. Am J Physiol Cell Physiol. 2003;284:C1397–C1404. doi: 10.1152/ajpcell.00328.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.