Abstract
The US3 protein is a viral serine/threonine kinase that is conserved among all members of the Alphaherpesvirinae. The US3 protein of different alphaherpesviruses causes dramatic alterations in the actin cytoskeleton, such as the disassembly of actin stress fibers and formation of cell projections, which have been associated with increased intercellular virus spread. Here, we find that inhibiting group A p21-activated kinases (PAKs), which are key regulators in Cdc42/Rac1 Rho GTPase signaling pathways, impairs US3-mediated actin alterations. By using PAK1−/− and PAK2−/− mouse embryo fibroblasts (MEFs), we show that US3-mediated stress fiber disassembly requires PAK2, whereas US3-mediated cell projection formation mainly is mediated by PAK1, also indicating that PAK1 and PAK2 can have different biological effects on the organization of the actin cytoskeleton. In addition, US3 was found to bind and phosphorylate group A PAKs. Lack of group A PAKs in MEFs was correlated with inefficient virus spread. Thus, US3 induces its effect on the actin cytoskeleton via group A PAKs.
Keywords: herpesvirus, Rho GTPase signaling, stress fibers, projections, viral kinase
Alphaherpesviruses constitute the largest subfamily of the herpesviruses and contain different, closely related pathogens of humans and animals. In humans, herpes simplex virus (HSV) causes cold sores and genital lesions but may also cause keratitis, blindness, and encephalitis, and varicella-zoster virus (VZV) causes chickenpox and shingles. The closely related porcine alphaherpesvirus pseudorabies virus (PRV) is often used as a model organism to study general aspects of alphaherpesvirus biology (1).
Recently, we and others have found that the US3 protein of PRV is able to reorganize the actin cytoskeleton of an infected host cell (2–6). This actin reorganization consists of the disassembly of actin stress fibers and the formation of actin-containing cell projections and is associated with an increase in the efficiency of intercellular virus spread (3). US3 is a serine/threonine kinase that is conserved among all alphaherpesviruses, and US3 orthologs of HSV-2 and Marek's disease virus, a devastating alphaherpesvirus in poultry, have also been shown to induce disassembly of actin stress fibers (7, 8). In addition, HSV-1 and VZV have also been reported to induce cell projections that may be involved in intercellular virus spread (9–11).
These data on herpesviruses, as well as other data on other viruses, like retroviruses (e.g., HIV) and poxviruses (e.g., vaccina virus), have led to the recent concept that viral reorganizations of the cytoskeleton, including the formation of intercellular cell projections, present a novel and important route of viral transmission (12–17). Elucidating the mechanism of these virus-induced cytoskeletal rearrangements may lead to novel avenues in the development of antiviral strategies.
In the current report, we elucidate the mechanism of the US3-mediated effects on the actin cytoskeleton. We report that US3 induces the actin rearrangements via group A p21-activated kinases, key downstream effectors of Cdc42 and Rac1 Rho GTPase signaling pathways, with important consequences for intercellular virus spread.
Results
Inhibition of Rho GTPases Does Not Inhibit US3-Mediated Actin Rearrangements.
The kinase activity of PRV US3 is required for actin reorganization (6), which opens up the possibility that US3 phosphorylates and modulates specific components of the cellular actin-regulating small Rho GTPase signaling pathways (18). To determine whether US3 acts directly on small Rho GTPases, the effect of small Rho GTPase inhibition on US3-mediated actin rearrangements was analyzed in swine testicle (ST) cells. As described before, cell projections are most notable in sparsely seeded cells, whereas stress fibers are best visualized in monolayers of cells (3, 6). General inhibition of small Rho GTPase signaling (Clostridium difficile toxin B; 200 ng/mL), as well as specific inhibition of Cdc42 signaling (secramine A; 50 μM) and Rac1 signaling (Rac1 inhibitor NSC 23766; 100 μM), had no obvious effect on either actin stress fiber disassembly or projection formation (Fig. S1). The different inhibitors used were active on ST cells at the concentrations used, because they impaired cell spreading in control experiments that were performed as described previously (19) (Fig. S1). In line with this, dominant-negative Cdc42 and Rac1 constructs did not affect the ability of PRV to mediate actin rearrangements (Fig. S1). Experiments with inhibitors and dominant-negative constructs yielded similar results in mouse embryonic fibroblasts (MEFs; Fig. S2). Hence, it is possible that US3 acts downstream of the small Rho GTPases in the Rho GTPase signaling cascades.
Inhibition of Group A PAKs Interferes with Rearrangement of the Actin Cytoskeleton by the US3 Protein.
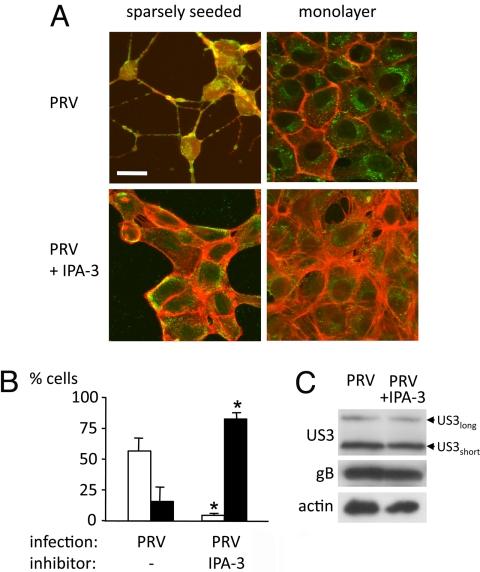
Group A PAKs are crucial downstream signaling molecules of Cdc42 and Rac1. Activation of group A PAKs has been shown to result in actin stress fiber disassembly and projection formation (20, 21), very reminiscent of the effects observed with PRV US3. The effect of a cell-permeable, specific inhibitor of group A PAK activity, IPA-3 (22), on the US3-mediated phenotype was analyzed. In the presence of IPA-3, group A PAKs adopt a conformation that did not allow activation (22). IPA-3 strongly suppressed PRV-induced actin stress fiber disassembly and cell projection formation without affecting expression of viral structural proteins gB and US3 (Fig. 1). IPA-3 similarly inhibited US3-mediated actin rearrangements in US3 transfection assays (Fig. S3).
Fig. 1.
Inhibition of group A PAKs impairs US3-induced actin rearrangements. (A) Pictures show actin architecture in PRV-infected ST in the presence or absence of the group A PAK inhibitor IPA-3. Viral antigens are in green, and actin is in red. (Scale bar: 10 μm.) (B) Graphs show percentages of cells with cell projections (white bars) or intact actin stress fibers (black bars). *, Significant differences compared with the control at the 0.05 level. (C) Western blots show expression of viral proteins US3 and gB. Arrowheads indicate position of US3 long and short isoforms. Actin served as a loading control.
In addition, cotransfection of US3 with PAK inhibitory domain (PID), another inhibitor of group A PAKs, also inhibited the US3-induced actin rearrangements (Fig. S3). PID specifically binds to the kinase domain of all group A PAKs, thereby inactivating them (21, 23).
Thus, group A PAKs are of critical importance in cytoskeletal rearrangements induced by US3.
US3 Phosphorylates and Activates Group A PAKs.
Activation of group A PAKs invariably is associated with serine/threonine phosphorylation of PAKs (24). Therefore, we investigated whether US3 interacts with and phosphorylates group A PAKs.
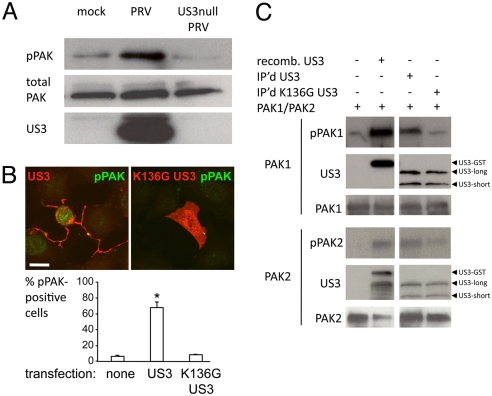
Phosphorylation of a critical, conserved threonine residue in the activation loop of group A PAKs is strongly correlated with activation (24). By using an antibody that specifically recognizes this phosphorylated threonine in group A PAKs, we found that infection with wild-type PRV, but not with US3 null PRV, led to a strong increase in threonine-phosphorylated PAK (Fig. 2A). In addition, transfection of US3 also resulted in a strong increase in threonine-phosphorylated PAK, which was not the case upon transfection of kinase-dead US3 (Fig. 2B).
Fig. 2.
US3 phosphorylates group A PAKs. (A) MEFs were either mock-infected or infected with PRV or isogenic US3 null PRV. At 12 hpi, cell lysates were analyzed by Western blotting for threonine-phosphorylated PAK versus total PAK and actin. (B) MEFs transfected with US3 or kinase-dead (K136G) US3 were analyzed by immunofluorescence for threonine-phosphorylated PAK. Pictures (Upper) show US3 and threonine-phosphorylated PAK. (Scale bar: 10 μm.) Graph (Lower) shows percentage of cells showing threonine-phosphorylated PAK. *, Significant differences compared with the mock-transfected control at the 0.05 level. (C) Kinase assays using recombinant GST-US3 or immunoprecipitated WT US3 or kinase-dead (K136G) US3 and PAK1 (Upper) or PAK2 (Lower). Top blots show phosphorylated PAK1 or PAK2; middle blots show US3; and lower blots show total PAK1 or PAK2.
PAK1 and PAK2 are the most abundant and most widely expressed group A PAKs (25–27). Pull-down assays using recombinant GST-tagged US3 and recombinant PAK1 and PAK2 showed that US3 can bind both PAK1 and PAK2 (Fig. S4). Importantly, kinase assays using recombinant US3 and recombinant PAK1 or PAK2 showed that US3 is able to directly phosphorylate PAK1 as well as PAK2 (Fig. 2C). Kinase assays using immunoprecipitated US3 or kinase-dead US3 further confirmed that US3 is able to phosphorylate PAK1 and PAK2. PAK2 phosphorylation appeared less efficient than PAK1 phosphorylation in these assays. Hence, PRV infection and US3 transfection lead to phosphorylation and activation of group A PAKs, and US3 is able to bind and phosphorylate PAK1 and PAK2.
PAK2 Is Crucial for US3-Mediated Stress Fiber Disassembly.
To further analyze a role for PAK1 and PAK2 in US3-mediated actin rearrangements, MEFs derived from PAK1−/− or PAK2−/− knockout mice were used. Western blot analysis confirmed the absence of the respective PAKs in the knockout cell lines, whereas both PAKs were expressed in control MEFs (Fig. S5A). Western blot analysis of infected cells showed similar expression of the structural viral proteins gB, gE, and US3 in all cell lines (Fig. S5B). In line with this, intracellular PRV titers were similar in all 3 cell lines (Fig. S5C). These data indicate that potential differences in actin dynamics in the 3 cell lines during PRV infection are not due to differences in basic virus replication efficiency. Interestingly, extracellular PRV titers were reduced in PAK2−/− cells at 12 and 24 hours after infection (hpi), indicating a reduced efficiency in virus egress in these cells (Fig. S5C).
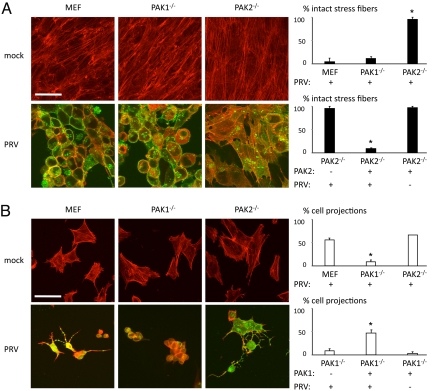
To determine a possible involvement of PAK1 and/or PAK2 in US3-mediated stress fiber disassembly, monolayers of wild-type, PAK1−/−, and PAK2−/− MEFs were infected with PRV. At 12 hpi, virtually all MEFs and PAK1−/− MEFs had lost their actin stress fibers. However, in PAK2−/− MEFs, PRV-induced stress fiber disassembly was almost entirely abolished (Fig. 3A). Transfection of PAK2 in PAK2−/− MEFs rescued the ability of PRV to disassemble stress fibers (Fig. 3A), indicating that the inability of PRV to disassemble actin stress fibers in PAK2−/− MEFs is specifically due to the lack of PAK2.
Fig. 3.
Group A PAKs PAK1 and PAK2 play nonredundant roles in PRV-induced actin rearrangements. (A) To analyze PRV-induced actin stress fiber disassembly, MEF, PAK1−/− MEF or PAK2−/− MEF cell monolayers were either mock-infected or infected with PRV and analyzed at 12 h after inoculation. (Left) Filamentous actin is shown in red, and viral antigens are shown in green. (Scale bar: 20 μm.) (Right Upper) Graph shows the corresponding percentage of PRV-infected cells that display intact actin stress fibers. *, Significant differences compared with the MEF control at the 0.05 level. (Right Lower) Graph shows the effect of expressing PAK2 in PAK2−/− MEFs on the percentage of intact actin stress fibers. *, Significant differences compared with the WT PRV-infected PAK2−/− MEF control at the 0.05 level. (B) To analyze PRV-induced cell projection formation, sparsely seeded MEFs, PAK1−/− MEFs, or PAK2−/− MEFs were either mock-infected or infected with PRV and analyzed at 12 h after inoculation. (Left) Filamentous actin is shown in red, and viral antigens are shown in green. (Scale bar: 20 μm.) (Right Upper) Graph shows the corresponding percentage of PRV-infected cells that display cell projections. *, Significant differences compared with the MEF control at the 0.05 level. Lower graph shows the effect of expressing PAK1 in PAK1−/− MEFs on the percentage of cell projections. *, Significant differences compared with the WT PRV-infected PAK1−/− MEF control at the 0.05 level.
Taken together, these data indicate that US3 requires PAK2 to induce actin stress fiber disassembly.
PAK1 Is Crucial for US3-Mediated Projection Formation.
To analyze a role for PAK1 and PAK2 in the US3-mediated induction of cell projections, sparsely seeded wild-type, PAK1−/−, and PAK2−/− MEFs were infected with PRV and analyzed for the presence of projections at 12 h after inoculation. PRV-infected wild-type MEFs and PAK2−/− MEFs showed cell projection formation. However, in PAK1−/− MEFs, cell projection formation was almost entirely abolished (Fig. 3B). Transfection of PAK1 in PAK1−/− MEFs largely rescued the ability of PRV to induce cell projections in these cells, indicating that the lack of cell projections is specifically due to the lack of PAK1 (Fig. 3B). Analysis of single infected cells in experiments with low moi confirmed these and the previous findings (Fig. S6). Lack of PAK1 impaired the formation of cell projections but did not affect the ability of PRV to disassemble actin stress fibers. PRV-infected PAK2−/− MEFs, on the other hand, did not show disassembly of actin stress fibers but did show cell projection formation, although projections appeared to be shorter in this experimental setup.
Hence, PAK1 and PAK2 both are involved in PRV-mediated reorganization of the actin cytoskeleton: PAK1 is mainly involved in the development of PRV-induced cell projections, and PAK2 is crucial for PRV-induced disassembly of actin stress fibers.
Effect of PAK1 or PAK2 Knockout on Virus Spread.
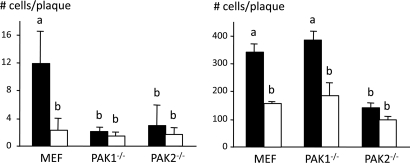
US3 has been implicated in efficient intercellular virus spread (3, 28). We therefore determined plaque sizes of wild-type PRV and US3 null PRV in wild-type, PAK1−/−, and PAK2−/− MEFs (Fig. 4 and Fig. S7). Plaque sizes were determined in sparsely seeded cells and monolayers of cells. Both in sparsely seeded MEFs and monolayers of MEFs, US3 null PRV plaques were smaller compared with wild-type PRV, which is in line with earlier reports (3, 28).
Fig. 4.
Lack of group A PAKs results in impaired intercellular spread of PRV. (Left) Graph showing the average number of cells per plaque in sparsely seeded MEFs, PAK1−/− MEFs, or PAK2−/− MEFs at 24 h after inoculation at a low moi with WT (black bars) or US3 null (white bars) PRV. Different letters indicate significant differences at the 0.05 level. (Right) Graph showing the average number of cells per plaque in cell monolayers of MEFs, PAK1−/− MEFs, or PAK2−/− MEFs at 48 h after inoculation at a low moi with WT (black bars) or US3 null (white bars) PRV. Different letters indicate significant differences at the 0.05 level.
In sparsely seeded cells, wild-type PRV plaques were smaller in both PAK1−/− and PAK2−/− MEFs compared with wild-type MEFs. In cell monolayers, on the other hand, wild-type PRV plaques were only reduced in PAK2−/− MEFs.
Hence, lack of PAK1 is associated with impaired intercellular spread of PRV in sparsely seeded cells, whereas lack of PAK2 is associated with impaired virus spread in sparsely seeded cells as well as in monolayers of cells.
Discussion
Earlier, we reported that the US3 kinase of alphaherpesviruses induces dramatic rearrangements of the actin cytoskeleton that consist of disassembly of actin stress fibers and the formation of actin-containing cell projections (2, 3). Here, we report that group A PAKs are critically involved in these US3-mediated changes in the actin cytoskeleton, and that US3 can bind and phosphorylate PAK1 and PAK2.
Interestingly, we observed phenotypic differences in the involvement of PAK1 and PAK2 in US3-mediated effects on the actin cytoskeleton. PAK2 was found to be crucial for US3-mediated actin stress fiber disassembly, whereas US3-induced projection formation mainly depended on the presence of PAK1. Only limited information is available at present about different properties and biological functions of PAK1 and PAK2 in mammals. First, PAK1 and PAK2 may be differently localized, with PAK1 distributed to cytoplasmic granules in unstimulated cells, and in focal complexes, plasma membrane, and nucleus in stimulated cells (29–32), whereas PAK2 is reported to reside at the Golgi complex (33). Second, although certain substrates are common to both these kinases, others appear to be unique targets of PAK1 (34). Third, PAK2 but not PAK1 is cleaved and activated by caspase-3 and plays a role in some of the morphological changes associated with apoptosis (35). Fourth, gene knockout studies in mice show that loss of PAK1 leads to relatively mild defects in the CNS and immune system, whereas loss of PAK2 leads to early embryonic death (36, 37). Finally, of special importance in the current context, PAK1 and PAK2 display isoform-specific roles in the organization of the actin cytoskeleton (38). For example, PAK1 but not PAK2 was found to be involved in the heregulin-induced formation of lamellipodia (38). Although the PRV-induced cell protrusions are different from lamellipodia, this may point to a preferential role for PAK1 in the regulation of actin-based cell protrusions. In addition, Coniglio et al. (38) found that PAK2 also differs from PAK1 by suppressing RhoA signaling. Because suppression of RhoA signaling is associated with actin stress fiber disassembly (39), this appears to be in line with our current findings that mainly PAK2 but not PAK1 is involved in PRV-induced actin stress fiber disassembly. Thus, our findings are consistent with a growing body of literature that points to distinct biological roles for PAK1 and PAK2.
What are the potential benefits of these actin rearrangements for virus infection and spread? We have shown before that the US3-induced actin rearrangements are associated with enhanced intercellular virus spread (3). In line with this, we found that the absence of group A PAKs hampered intercellular virus spread in MEFs. Presence of PAK2, which is crucial for US3-mediated actin stress fiber disassembly, was required for efficient virus spread in monolayers as well as in sparsely seeded cells. In this context, we have found that extracellular virus titers were reduced in PAK2−/− cells at 12 and 24 h after inoculation, indicating reduced virus egress in this cell type. Future research will show whether this may correlate with the defect in intercellular virus spread in this cell type. Possibly in line with this, the poxvirus vaccinia virus (VV) has been reported to induce similar cytoskeletal rearrangements, and these rearrangements have been implicated in promoting viral egress from infected cells by increasing microtubule dynamics and changes in the dynamics of cortical actin (40, 41). Actin stress fiber disassembly is generally also associated with loss in focal adhesions and cell–cell contacts (39). Therefore, virus-mediated stress fiber disassembly, loss in focal adhesions, loss in cell–cell contacts, and/or other effects on the cytoskeleton may also be implicated in PAK2-mediated efficient PRV spread. Absence of PAK1 did not affect PRV spread in monolayers of cells, although it did hamper spread of PRV in sparsely seeded cells. Although speculative at this point, it may be that the importance of PAK1 in virus spread in sparsely seeded cells lies in the PAK1-US3-mediated induction of cell projections, which may bridge relatively distant, otherwise unconnected cells. In line with this hypothesis, increasing recent evidence is supporting the concept of viral spread between relatively distant cells via intercellular nanotube bridges (12, 14). Another important question is how spread in monolayers and sparsely seeded cells relates to spread in vivo. Monolayers may be more closely related to tissue, although in vivo experiments will be required to fully address the importance of group A PAKs and US3-mediated actin rearrangements in virus spread.
HIV, a retrovirus, and VV, a poxvirus, have both been shown to induce actin rearrangements that are reminiscent of those induced by PRV US3: actin stress fiber disassembly and formation of actin-containing cell projections (16, 17, 42, 43). For these viruses, the responsible viral proteins do not display kinase activity. For VV, the F11L protein induces the cytoskeletal rearrangements by interacting with and inactivating RhoA (17). For HIV, the Nef protein induces the actin reorganization by binding and indirectly activating group A PAKs via the Rho GTPases Cdc42 and Rac1, and possibly the guanine nucleotide exchange factor Vav (42, 44–46).
The current data unravel a previously undescribed—catalytic—mechanism of virus-induced actin stress fiber disassembly and projection formation, and thereby show that different viruses have developed different mechanisms to induce very similar actin rearrangements, underscoring the potential importance of such actin rearrangements in the viral life cycle.
The finding that US3-mediated actin stress fiber disassembly and cell projection formation are mediated by group A PAKs is in line with what is currently known about the biology of group A PAKs. Several years ago, it was shown that activation of group A PAKs may lead to actin stress fiber disassembly, loss of focal adhesions, cell retraction, and formation of filopodia and other cell projections (21). There are several substrates and/or interacting proteins of the group A PAK kinases that can account for the effects of these kinases on the actin cytoskeleton and cell morphology, including LIM kinases and myosin light chain kinase (47). Phosphorylated and activated LIM kinases are able to phosphorylate cofilin, and phosphorylated cofilin and phosphorylated myosin light chain kinase are key intermediates in the formation and stabilization of cell projections, such as filopodia and lamellipodia. Regarding US3- and PAK2-mediated actin stress fiber disassembly, it is of interest that depletion of PAK2 but not of PAK1 has been associated with suppression in RhoA-mediated signaling (38) which, in turn, is associated with actin stress fiber disassembly (39). This appears to be in line with our current finding that PAK2 is crucial in US3-mediated actin stress fiber disassembly. In low-moi infections in cell monolayers, PRV-induced cell projections observed in PAK2−/− MEFs appeared shorter than in wild-type MEFs. Although speculative at this point, it is possible that in cell monolayers, which contain more abundant actin stress fibers than sparsely seeded cells, PAK2-mediated, PRV-induced stress fiber disassembly is a prerequisite for efficient PAK1-mediated formation of long cell projections.
Activation of group A PAKs can occur by association, via their CRIB domain, with small Rho GTPases Cdc42 and Rac1. However, group A PAK activation has also been reported to occur via phoshorylation (47). One cellular kinase that is able to directly phosphorylate and activate PAK is PDK1, which phosphorylates PAK on the activation-associated threonine residue (Thr-423) (48). Our current data indicate that a viral kinase, such as US3, may also be able to directly phosphorylate group A PAKs. Although our data show that US3 results in phosphorylation at the critical Thr-423 site in the activation loop, we cannot rule out at this moment that US3 may phosphorylate additional or other phosphorylation sites in PAK.
In conclusion, our results demonstrate that p21-activated kinases PAK1 and PAK2 play a hitherto unknown and crucial role in alphaherpesvirus US3-induced actin rearrangements; that US3 is able to phosphorylate both PAK1 and PAK2; and that PAK1 and PAK2 may have different downstream effects on the actin cytoskeleton. The indications that group A PAKs are involved in efficient virus egress and spread may open new avenues in the design of antiviral strategies.
Materials and Methods
ST cells and MEFs were used and cultured as decribed previously (6, 49). WT MEFs and PAK1−/− MEFs were described previously (50), and Pak2−/− MEF cells were established from 7.5- to 8.5-days postcoitum mouse embryos and immortalized and cultured as described previously for MEF cells (50).
Immunofluorescence assays, Western blotting assays, and plaque assays were performed, essentially as described previously (3, 6, 51).
Details about cells, viruses, antibodies, inhibitors, plasmids, transfections, immunofluorescence, Western blotting, plaque assays, and the US3-PAK-binding assay procedure are described in SI Text.
In Vitro Kinase Assay.
Recombinant WT PAK1 (200 ng) was dephosphorylated with 100 units of λ-phosphatase (New England Biolabs) at 30 °C for 1 h. λ-Phosphatase was inactivated with 2 mM Na3Vo4 and 1 M β-glycerophosphate at room temperature for 30 min. Alternatively, 200 ng of the PAK2-M322G gatekeeper mutant was used in the presence of 2.5 nmol of the specific inhibitor NMPP1 (Calbiochem). Compared with recombinant Pak1, which needs Cdc42 for activation, recombinant Pak2 is highly active by autophosphorylation in bacteria. To distinguish autophosphorylation of Pak2 from phosphorylation by US3, the kinase assay using Pak2 as a substrate was done using the Pak2-M322G mutant (22, 52). The gatekeeper mutation renders the Pak2 mutant sensitive to NMPP1. Other endogenous kinases are not inhibited by NMPP1. Recombinant Pak2 was dephosphorylated similarly to Pak1. To prevent Pak2 autophosphorylation, NMPP1 was added to the kinase assay.
Recombinant or immunoprecipitated US3 was added to 200 ng of PAK1 or PAK2 in phosphobuffer with 5-μCi P32-ATP. As a negative control, no US3 or kinase-dead US3 was added. Reactions were incubated at 30 °C for 45 min. The gel was run, transferred to the membrane, and the membrane was exposed for 1 h. Equal loading for US3 and PAK1 and PAK2 was checked.
Statistics.
Three independent replicates of each experiment were performed, data shown are means and standard deviations, and statistical analysis was performed by using the SPSS software. Means were compared with an analysis of variance and a least significant difference post hoc test for a multiple comparison of means (α = 0.05).
Supplementary Material
Acknowledgments.
We thank N. De Corte, C. Boonen, L. Sys, C. Vanmaercke, and N. Dennequin for excellent technical assistance, as well as E. Manser (Institute of Molecular and Cell Biology, Singapore), L. Olsen and L. Enquist (Princeton University, Princeton, NJ), the Kirchhausen lab (Harvard Medical School, Boston, MA) and the Hammond lab (University of Louisville, Louisville, KY), B. Klupp and T. Mettenleiter (Friedrich-Loeffler-Institut, Greifswald, Germany), the Institute for Animal Science and Health (Lelystad, The Netherlands), and B. Verhasselt (Ghent University, Ghent, Belgium) for reagents. We would also like to thank G. Van Minnebruggen for initial work on PRV–actin interactions. This research was supported by a cooperative research action fund of the Research Council of Ghent University and a research grant from the Research Foundation-Flanders (FWO-Vlaanderen), Grant G.0196.06. J.C. was supported by National Institutes of Health Grant R01-CA117884 and United States Department of Defense Grant W81XWH-06-1-0213.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900436106/DCSupplemental.
References
- 1.Pomeranz LE, Reynolds AE, Hengartner CJ. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev. 2005;69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Minnebruggen G, Favoreel HW, Jacobs L, Nauwynck HJ. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J Virol. 2003;77:9074–9080. doi: 10.1128/JVI.77.16.9074-9080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc Natl Acad Sci USA. 2005;102:8990–8995. doi: 10.1073/pnas.0409099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favoreel HW, Enquist LW, Feierbach B. Actin and Rho GTPases in herpesvirus biology. Trends Microbiol. 2007;15:426–433. doi: 10.1016/j.tim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Calton CM, Randall JA, Adkins MW, Banfield BW. The pseudorabies virus serine/threonine kinase Us3 contains mitochondrial, nuclear and membrane localization signals. Virus Genes. 2004;29:131–145. doi: 10.1023/B:VIRU.0000032796.27878.7f. [DOI] [PubMed] [Google Scholar]
- 6.Van den Broeke C, et al. The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology. 2009;385:155–160. doi: 10.1016/j.virol.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Murata T, Goshima F, Daikoku T, Takakuwa H, Nishiyama Y. Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells. 2000;5:1017–1027. doi: 10.1046/j.1365-2443.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher D, Tischer BK, Trapp S, Osterrieder N. The protein encoded by the US3 orthologue of Marek's disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J Virol. 2005;79:3987–3997. doi: 10.1128/JVI.79.7.3987-3997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zapata HJ, Nakatsugawa M, Moffat JF. Varicella-zoster virus infection of human fibroblast cells activates the c-Jun N-terminal kinase pathway. J Virol. 2007;81:977–990. doi: 10.1128/JVI.01470-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter JE, Hutchinson JA, Jackson W, Grose C. Egress of light particles among filopodia on the surface of Varicella-Zoster virus-infected cells. J Virol. 2008;82:2821–2835. doi: 10.1128/JVI.01821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Boissiere S, Izeta A, Malcomber S, O'Hare P. Compartmentalization of VP16 in cells infected with recombinant herpes simplex virus expressing VP16-green fluorescent protein fusion proteins. J Virol. 2004;78:8002–8014. doi: 10.1128/JVI.78.15.8002-8014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis DM, Sowinski S. Membrane nanotubes: Dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 13.Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol. 2008;129:539–550. doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattentau Q. Avoiding the void: Cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 15.Sowinski S, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 16.Greber UF, Way M. A superhighway to virus infection. Cell. 2006;124:741–754. doi: 10.1016/j.cell.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Valderrama F, Cordeiro JV, Schleich S, Frischknecht F, Way M. Vaccinia virus-induced cell motility requires F11L-mediated inhibition of RhoA signaling. Science. 2006;311:377–381. doi: 10.1126/science.1122411. [DOI] [PubMed] [Google Scholar]
- 18.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 19.Xu B, Pelish H, Kirchhausen T, Hammond GB. Large scale synthesis of the Cdc42 inhibitor secramine A and its inhibition of cell spreading. Org Biomol Chem. 2006;4:4149–4157. doi: 10.1039/b609143a. [DOI] [PubMed] [Google Scholar]
- 20.Sells MA, et al. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhao ZS, et al. A conserved negative regulatory region in alphaPAK: Inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deacon SW, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beeser A, Chernoff J. Production and use of a cell permeable inhibitor of group A Paks (TAT-PID) to analyze signal transduction. Methods. 2005;37:203–207. doi: 10.1016/j.ymeth.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Eswaran J, Soundararajan M, Kumar R, Knapp S. UnPAKing the class differences among p21-activated kinases. Trends Biochem Sci. 2008;33:394–403. doi: 10.1016/j.tibs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 26.Teo M, Manser E, Lim L. Identification and molecular cloning of a p21cdc42/rac1-activated serine/threonine kinase that is rapidly activated by thrombin in platelets. J Biol Chem. 1995;270:26690–26697. doi: 10.1074/jbc.270.44.26690. [DOI] [PubMed] [Google Scholar]
- 27.Sells MA, Chernoff J. Emerging from the Pak: The p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- 28.Demmin GL, Clase AC, Randall JA, Enquist LW, Banfield BW. Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection. J Virol. 2001;75:10856–10869. doi: 10.1128/JVI.75.22.10856-10869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh RR, Song C, Yang Z, Kumar R. Nuclear localization and chromatin targets of p21-activated kinase 1. J Biol Chem. 2005;280:18130–18137. doi: 10.1074/jbc.M412607200. [DOI] [PubMed] [Google Scholar]
- 30.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manser E, et al. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Z, Ling J, Traugh JA. Localization of p21-activated protein kinase gamma-PAK/Pak2 in the endoplasmic reticulum is required for induction of cytostasis. J Biol Chem. 2003;278:13101–13109. doi: 10.1074/jbc.M212557200. [DOI] [PubMed] [Google Scholar]
- 34.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 35.Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 36.Allen JD, et al. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood. 2009;113:2695–2705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J Cell Sci. 2004;117:4343–4354. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- 38.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 40.Arakawa Y, Cordeiro JV, Schleich S, Newsome TP, Way M. The release of vaccinia virus from infected cells requires RhoA-mDia modulation of cortical actin. Cell Host Microbe. 2007;1:227–240. doi: 10.1016/j.chom.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Arakawa Y, Cordeiro JV, Way M. F11L-mediated inhibition of RhoA-mDia signaling stimulates microtubule dynamics during vaccinia virus infection. Cell Host Microbe. 2007;1:213–226. doi: 10.1016/j.chom.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Fackler OT, Luo W, Geyer M, Alberts AS, Peterlin BM. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol Cell. 1999;3:729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 43.Fackler OT, Krausslich HG. Interactions of human retroviruses with the host cell cytoskeleton. Curr Opin Microbiol. 2006;9:409–415. doi: 10.1016/j.mib.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Renkema GH, Manninen A, Mann DA, Harris M, Saksela K. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr Biol. 1999;9:1407–1410. doi: 10.1016/s0960-9822(00)80086-x. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen DG, Wolff KC, Yin H, Caldwell JS, Kuhen KL. “UnPAKing” human immunodeficiency virus (HIV) replication: Using small interfering RNA screening to identify novel cofactors and elucidate the role of group I PAKs in HIV infection. J Virol. 2006;80:130–137. doi: 10.1128/JVI.80.1.130-137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu X, et al. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr Biol. 1996;6:1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 47.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 48.King CC, et al. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1) J Biol Chem. 2000;275:41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- 49.Geenen K, Favoreel HW, Olsen L, Enquist LW, Nauwynck HJ. The pseudorabies virus US3 protein kinase possesses anti-apoptotic activity that protects cells from apoptosis during infection and after treatment with sorbitol or staurosporine. Virology. 2005;331:144–150. doi: 10.1016/j.virol.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 50.ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deruelle M, Geenen K, Nauwynck HJ, Favoreel HW. A point mutation in the putative ATP binding site of the pseudorabies virus US3 protein kinase prevents Bad phosphorylation and cell survival following apoptosis induction. Virus Res. 2007;128:65–70. doi: 10.1016/j.virusres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Bishop AC, et al. Design of allele-specific inhibitors to probe protein kinase signaling. Curr Biol. 1998;8:257–266. doi: 10.1016/s0960-9822(98)70198-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.