Abstract
The 3-phosphoinositide-dependent kinase-1 (PDK1) plays an important role in the regulation of cellular responses in multiple organs by mediating the phosphoinositide 3-kinase (PI3-K) signaling pathway through activating AGC kinases. Here we defined the role of PDK1 in controlling cardiac homeostasis. Cardiac expression of PDK1 was significantly decreased in murine models of heart failure. Tamoxifen-inducible and heart-specific disruption of Pdk1 in adult mice caused severe and lethal heart failure, which was associated with apoptotic death of cardiomyocytes and β1-adrenergic receptor (AR) down-regulation. Overexpression of Bcl-2 protein prevented cardiomyocyte apoptosis and improved cardiac function. In addition, PDK1-deficient hearts showed enhanced activity of PI3-Kγ, leading to robust β1-AR internalization by forming complex with β-AR kinase 1 (βARK1). Interference of βARK1/PI3-Kγ complex formation by transgenic overexpression of phosphoinositide kinase domain normalized β1-AR trafficking and improved cardiac function. Taken together, these results suggest that PDK1 plays a critical role in cardiac homeostasis in vivo by serving as a dual effector for cell survival and β-adrenergic response.
Keywords: AGC kinase, apoptosis, heart failure, receptor internalization
Heart failure, a major cause of morbidity and mortality worldwide, is a clinical syndrome in which the heart is incapable of pumping blood at a rate commensurate with systemic demands (1). Injurious stresses from extrinsic or intrinsic origins trigger the complex intracellular signaling pathways in cardiomyocytes and thereby activate the compensatory mechanisms involving alterations in survival and growth signals, calcium handling, and energy production (2). Simultaneously, the sympathetic nervous, renin-angiotensin-aldosterone, and cytokine systems are activated to cope with a decline in cardiac performance. Although these compensatory systems initially maintain cardiac function within a physiological range, prolonged activation of these systems paradoxically leads to cardiac damage and worsens clinical prognosis (2). Therefore, for the elucidation of the pathophysiology of heart failure, it is very important to dissect the inherent complexity of intracellular signaling pathways that coordinate the cellular homeostasis and neurohumoral responses in cardiomyocytes.
The 3-phosphoinositide-dependent protein kinase-1 (PDK1) is a member of the AGC serine/threonine kinase family that functions downstream of phosphoinositide 3-kinase (PI3-K) and activates several AGC kinases, including Akt, p70 ribosomal S6 kinase (p70S6K), and serum- and glucocorticoid-induced protein kinase 1 (SGK1), by phosphorylating these enzymes at their activation loops (3). The physiological functions of PDK1 have been investigated by targeted disruption of Pdk1 gene. Mouse embryos systemically deficient for Pdk1 were lethal during early embryogenesis, displaying multiple abnormalities that included lack of somites, forebrain, and neural crest-derived tissues (4). Alessi et al. (5) recently generated striated muscle-specific PDK1 conditional knockout mice (PDK1-MCKCre) by crossing mice harboring a “floxed” Pdk1 allele with transgenic mice expressing Cre recombinase under the control of the muscle creatine kinase (MCK) promotor. PDK1-MCKCre mice died of heart failure by 11 weeks of age. Interestingly, PDK1-MCKCre mice showed attenuation of cardimyocyte cell growth and impairment of left ventricular (LV) contraction. It was reported that cardiomyocytes deficient for Pdk1 were sensitive to hypoxia (5), and that ischemic preconditioning failed to protect Pdk1-hypomorphic mutant mice against myocardial infarction (MI) (6). However, the mechanisms of how PDK1 deficiency induces these cardiac abnormalities remain to be resolved.
In this study, we found that the expression levels of PDK1 protein were significantly decreased in the failing hearts of murine models. We generated tamoxifen-inducible and heart-specific PDK1 conditional knockout mice (PDK1-MerCre) to elucidate the relevance of PDK1 to the pathogenesis of heart failure. We disrupted the Pdk1 gene in the adulthood and demonstrated that PDK1 plays a role in the regulation of normal cardiac function by preventing cardiomyocyte apoptosis and by preserving responsiveness to β-adrenergic stimulation.
Results
Generation of Tamoxifen-Inducible and Heart-Specific PDK1 Knockout Mice.
We examined alterations in the expression levels of PDK1 in failing hearts. Heart failure was induced in mice by producing myocardial infarction or administering doxorubicin i.p. Two weeks after operation of myocardial infarction or doxorubicin injection, expression levels of PDK1 were significantly decreased in the failing hearts, compared with control hearts (Fig. S1).
To assess the pathophysiological significance of PDK1 down-regulation, we created a model of temporally regulated inactivation of Pdk1 specifically in the adult hearts. We crossed Pdk1flox/flox mice (7, 8) with transgenic mice expressing tamoxifen-inducible Cre recombinase protein fused to two mutant estrogen-receptor ligand-binding domains (MerCreMer) under the control of the α-myosin heavy chain promoter (9). In the resulting Pdk1flox/flox/MerCreMer+ mice (PDK1-MerCre) at the age of 10 weeks, we administered tamoxifen successively for 5 days and confirmed by immunoblot analysis that functional PDK1 expression was almost undetectable specifically in the hearts on day 7 after the initiation of tamoxifen treatment (Fig. S2A).
Next, we examined whether the activation of kinases downstream of PDK1 were suppressed in the hearts of PDK1-MerCre. In mammalian cells, Akt is fully activated through PDK1-dependent phosphorylation of Thr-308 and PDK1-independent phosphorylation of Ser-473 (10). Insulin-induced phosphorylation of Akt at Thr-308 in PDK1-MerCre hearts was significantly attenuated, compared with control hearts, while phosphorylation level at Ser-473 was unchanged (Fig. S2B). As a consequence, Akt kinase activity was markedly reduced in PDK1-MerCre hearts (Fig. S2C). Consistently, insulin-induced phosphorylation levels of glycogen synthase kinase (GSK) 3β at Ser-9, mammalian target of rapamycin (mTOR) at Ser-2448, and p70S6K at Thr-389 (11) were attenuated in the PDK1-MerCre hearts (Fig. S2B). Collectively, these results indicate that Akt signaling is inhibited in PDK1-MerCre hearts.
Lethal Heart Failure in PDK1-MerCre Mice.
Without tamoxifen treatment, PDK1-MerCre mice survived normally and were indistinguishable in appearance from control littermates. Strikingly, all PDK1-MerCre mice died from 5 to 15 weeks after the initiation of tamoxifen treatment (Fig. 1A).
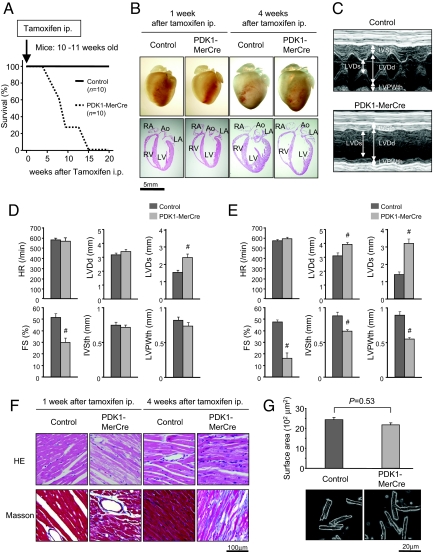
Fig. 1.
Severe heart failure observed in PDK1-MerCre mice. (A) Kaplan-Meier survival curves of PDK1-MerCre mice (n = 10) and control mice (n = 10). Mice were injected with tamoxifen at the age of 10–11 weeks. (B) Macroscopic findings and 4-chamber sections of the hearts from PDK1-MerCre and control mice 1 and 4 weeks after the initiation of tamoxifen treatment. Ao, aorta; LA, left atrium; LV, left ventricule; RA, right atrium; RV, right ventricule. (C) Representative M-mode echocardiograms of mice 1 week after tamoxifen treatment. (D) Echocardiographic measurements of PDK1-MerCre and control mice 1 week after tamoxifen treatment. HR, heart rate; LVDd, LV dimension in diastole; LVDs, LV dimension in systole; FS, fractional shortening; IVSth, interventricular septum thickness; LVPWth, LV posterior wall thickness. Values represent the mean ± SEM of data from 10 mice in each group. #, P < 0.01 versus control group. (E) Echocardiographic measurements of PDK1-MerCre and control mice 4 weeks after tamoxifen treatment. Values represent the mean ± SEM of data from 6 mice in each group. #, P < 0.01 versus control group. (F) Histological sections with hematoxylin and eosin (HE) staining and Masson's trichrome (Masson) staining of PDK1-MerCre and control mice 1 and 4 weeks after tamoxifen treatment. (G) Surface areas of isolated cardiomyocytes (57 individual cardiomyocytes in each group) and sample pictures of isolated cardiomyocytes from PDK1-MerCre and control mice 1 week after tamoxifen treatment. Values represent the mean ± SEM.
One week after tamoxifen treatment, cardiac sizes were not significantly different between PDK1-MerCre mice and control mice (Fig. 1B). Echocardiographic examination revealed a significant decrease in the percent of fractional shortening (%FS), a parameter for contractile function, as early as 1 week after tamoxifen treatment in PDK1-MerCre mice (Fig. 1 C and D). During this period, there was no increase in LV dimension or thinning of LV wall, which was consistent with the macroscopic findings (Fig. 1 B and D). However, 4 weeks after tamoxifen treatment, progression of contractile dysfunction together with global chamber dilatation and wall thinning was observed in PDK1-MerCre mice (Fig. 1 B and E). Histologically, interstitial fibrosis was increased at 1 week in PDK1-MerCre hearts and further enhanced at 4 weeks after tamoxifen treatment (Fig. 1F). These results suggest that PDK1-MerCre mice exhibited cardiac dysfunction as early as 1 week after tamoxifen treatment and LV remodeling at 4 weeks.
It was reported that PDK1-MCKCre showed marked reduction both in the heart size and in cardiac contractility (5). Since the MCK promoter directs expression of Cre recombinase before birth (5, 12), retardation of heart growth that was not proportional to somatic growth after birth might lead to cardiac dysfunction. However, the surface areas of caridomyocytes were not significantly different between PDK1-MerCre mice and control mice 1 week after tamoxifen treatment (Fig. 1G). Given that LV dysfunction was already observed as early as 1 week after tamoxifen treatment (Fig. 1 C and D), we suppose that reduction of cardiomyocyte size is not critically involved in the impairment of LV contraction observed in PDK1-MerCre hearts.
Increased Cardiomyocyte Apoptosis in PDK1-MerCre Mice.
We next examined whether cardiomyocyte apoptosis was involved in the pathogenesis of heart failure in PDK1-MerCre mice. TUNEL staining revealed that the number of apoptotic cells was dramatically increased in PDK1-MerCre hearts 1 week after tamoxifen treatment (Fig. 2A). TUNEL-positive cells were cardiomyocytes, because these cells were positively stained with anti-sarcomeric α-actinin antibody (Fig. 2B). In addition, immunostaining revealed an increase in cardiomyocytes positively stained for cleaved caspase-3 in PDK1-MerCre hearts (Fig. 2C). The prevalence of TUNEL-positive cardiomyocytes was 1.14 ± 0.05% of total cardiomyocytes (Fig. 2D). Therefore, cardiomyocyte loss through apoptotic cell death may play an important role in the pathogenesis of heart failure in PDK1-MerCre mice.
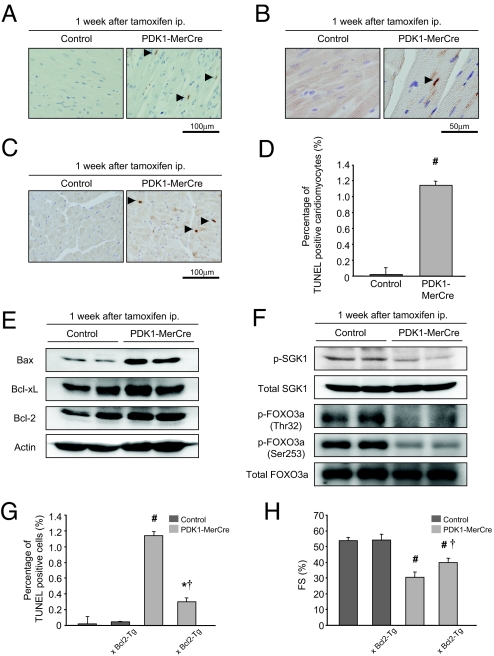
Fig. 2.
Cardiomyocyte apoptosis in the pathogenesis of heart failure in PDK1-MerCre mice. (A) TUNEL staining. Arrowheads indicate TUNEL-positive cardiomyocytes. (B) Double staining for TUNEL staining (brown) and sarcomeric α-actinin (red). Arrowheads indicate TUNEL-positive cardiomyocytes. (C) Immunostaining for cleaved caspase-3. Arrowheads indicate cardiomyocytes positively stained for cleaved caspase-3. (D) Percentage of TUNEL-positive caridomyocytes. Values represent the mean ± SEM (3,000 cardiomyocytes in each group). #, P < 0.01 versus control group. (E) Immunoblot analysis of Bcl-2 family proteins in the hearts. (F) Immunoblot analysis of phosphorylated-SGK1 at Ser-78, total SGK1, phosphorylated-FOXO3a at Thr-32 or at Ser-253, and total FOXO3a in the hearts. (G) Percentage of TUNEL-positive caridomyocytes in control, Bcl2-Tg, PDK1-MerCre, and PDK1-MerCre × Bcl2-Tg mice. Values represent the mean ± SEM (3,000 cardiomyocytes in each group). #, P < 0.01 versus control group; *, P < 0.05, versus control group; †, P < 0.01 versus PDK1-MerCre group. (H) Measurement of fractional shortening of control, Bcl2-Tg, PDK1-MerCre, and PDK1-MerCre × Bcl2-Tg mice by echocardiography. Values represent the mean ± SEM of data from control mice (n = 10), control × Bcl2-Tg mice (n = 6), PDK1-MerCre mice (n = 10), and PDK1-MerCre × Bcl2-Tg mice (n = 6). #, P < 0.01 versus control mice. †, P < 0.01 versus PDK1-MerCre mice. FS, % of fractional shortening.
In the hearts of PDK1-MerCre, the expression level of proapoptotic Bax was increased, whereas those of anti-apoptotic molecules such as Bcl-2 and Bcl-xL were unchanged (Fig. 2E). SGK1 has been reported to be functionally anti-apoptotic in the hearts (13). The basal level of phosphorylated SGK1 was reduced in PDK1-MerCre hearts (Fig. 2F). It has been reported that SGK1, in concert with Akt, mediates cell survival by phosphorylating and inactivating the Forkhead transcription factor FOXO3a (13, 14). FOXO3a is phosphorylated at Thr-32 and Ser-315 by SGK1, and Akt favors the phosphorylation of Thr-32 and Ser-253 (14). In PDK1-MerCre hearts, phosphorylation levels of FOXO3a at Thr-32 and Ser-253 were significantly decreased (Fig. 2F). Collectively, these results suggest that up-regulation of Bax protein and reduction of Akt and SGK1 activity were potentially involved in enhancing susceptibility of cardiomyocytes to apoptosis in PDK1-MerCre mice.
Overexpression of Bcl-2 Protein Prevented Cardiomyocyte Apoptosis and Partially Rescued Cardiac Dysfunction in PDK1-MerCre Mice.
To examine whether cardiomyocyte apoptosis plays a causative role in the pathogenesis of heart failure in PDK1-MerCre mice, we crossed PDK1-MerCre with transgenic mice with cardiac-specific overexpression of Bcl-2 (Bcl2-Tg mice) (15). In PDK1-MerCre × Bcl2-Tg hearts, the number of TUNEL-positive cardiomyocytes was significantly decreased in comparison with PDK1-MerCre hearts (Fig. 2G), and the %FS showed partial but significant improvement (Fig. 2H). These results suggest that cardiac dysfunction is caused in part by cardiomyocyte loss through apoptosis in PDK1-MerCre mice.
Impairment of β-adrenergic Responsiveness in PDK1-MerCre Hearts.
Incomplete restoration of cardiac function by prevention of cardiomyocyte apoptosis implies that some functional abnormalities persist in viable cardiomyocytes in PDK1-MerCre mice. To determine whether β-adrenergic responsiveness was changed in PDK1-MerCre hearts, we carried out Langendorff perfusion analysis in the hearts 1 week after tamoxifen treatment, and evaluated responsiveness to isoproterenol, a β-AR agonist, and forskolin, an activator of adenylate cyclase that increases cAMP independently of β-AR. As shown in Fig. 3A, the baseline parameters of +dp/dt and −dp/dt were significantly lower in PDK1-MerCre mice than in control mice. Both isoproterenol and forskolin induced positive chronotropic and inotropic responses in control mice (Fig. 3A). However, PDK1-MerCre mice showed a significant reduction in the maximal changes in HR, +dP/dt, and −dP/dt after the stimulation of isoproterenol (1 × 10−8 M), compared with control mice (Fig. 3B). In contrast, the maximal changes in these parameters after the stimulation of forskolin (1 × 10−7 M) did not differ significantly between PDK1-MerCre and control mice (Fig. 3B). These results suggest that the responsiveness of β-AR is impaired in PDK1-MerCre mice.
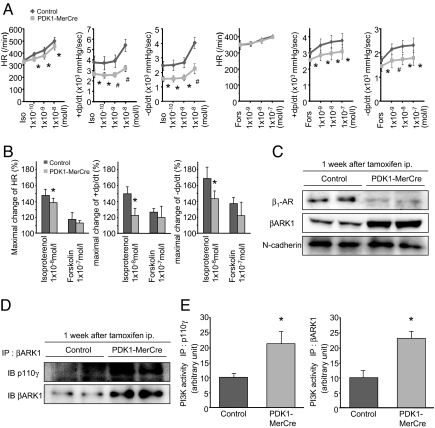
Fig. 3.
Impaired β-adrenergic responsiveness in PDK1-MerCre mice. (A) Effects of isoproterenol and forskolin on heart rate, contraction, and relaxation in Langendorff-perfused mouse hearts 1 week after tamoxifen treatment. +dp/dt, maximum rate of LV pressure development; −dp/dt, maximum rate of LV pressure decline; HR, heart rate. Values represent the mean ± SEM. *, P < 0.05 versus control group, #, P < 0.01 versus control group. (B) The % changes in HR, +dP/dt, and −dP/dt before and after treatment with isoproterenol (1 × 10−8 M) or forskolin- (1 × 10−7 M) were calculated. Values represent the mean ± SEM. *, P < 0.05 versus control group. (C) Immunoblot analysis of β1-AR and βARK1 in membrane fraction of the hearts. N-cadherin was used as an internal control for the amount of membrane protein. (D) Immunoblot analysis of βARK1-associated p110γ protein in the hearts. (E) Kinase assays for PI3-K activity. The hearts were subjected to immunoprecipitaion with antibody to p110γ, or βARK1, and the resulting precipitates were assayed for the kinase assay. PI3-K activity of control mice was adjusted to 10 arbitrary units.
Next, we measured the amount of β1-AR in the membrane fraction by immunoblot analysis. In PDK1-MerCre hearts, the expression levels of β1-AR in membrane fraction were markedly down-regulated (Fig. 3C). Inversely, the amount of β1-AR in cytosolic fraction was increased in PDK1-MerCre hearts, compared with control hearts, while the total amount of β1-AR was unchanged (Fig. S3 A and B), suggesting that receptor internalization underlies β1-AR down-regulation in membrane fraction of PDK1-MerCre hearts. In response to β-AR simulation, increased cAMP activates protein kinase A (PKA), which directly phosphorylates phospholamban (PLN) at Ser-16. PDK1-MerCre hearts showed a significant decrease in cAMP concentrations (Fig. S3C) and phosphorylation level of PLN at Ser-16 (Fig. S3D), compared with control hearts. Phosphorylated PLN dissociates from sarcoplasmic reticulum Ca2+-ATPase2 (SERCA2) and thereby enhances Ca2+ uptake by SERCA2, which leads to enhancement of cardiac contractility (2). These results suggest that, in PDK1-MerCre hearts, robust β1-AR internalization leads to contractile dysfunction.
It has been reported that phosphorylation of β-AR by β-AR kinase 1 (βARK1, commonly known as G protein-coupled receptor kinase 2) regulates receptor internalization (16). In the hearts of PDK1-MerCre mice 1 week after tamoxifen treatment, the expression levels of βARK1 (Fig. 3C) and βARK1-associated p110γ, a catalytic subunit of PI3-Kγ, were increased (Fig. 3D). Notably, PI3-K activity immunoprecipitated with antibodies to either p110γ or βARK1 was enhanced (Fig. 3E) in PDK1-MerCre hearts. βARK1 forms complex with PI3-Kγ through the phosphoinositide kinase (PIK) domain, and protein kinase activity of PI3-Kγ in this complex is required for receptor internalization (17). Therefore, these results suggest that enhanced PI3-Kγ activity in PDK1-MerCre hearts increases βARK1/PI3-Kγ complex formation, and that βARK1 phosphorylates β-AR to cause robust receptor internalization.
Disruption of βARK1/PI3-Kγ Complex Restored β-AR Internalization and Partially Rescued Cardiac Dysfunction in PDK1-MerCre Mice.
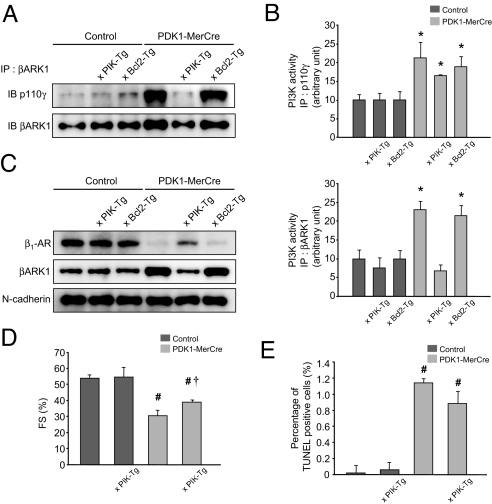
To corroborate that enhanced PI3-Kγ activity promotes β-AR internalization by forming complex with βARK1 and that robust β-AR internalization causes cardiac dysfunction, we examined whether disruption of the βARK1/PI3-Kγ complex normalizes β-AR trafficking and improves cardiac function in PDK1-MerCre mice. For that purpose, we crossed PDK1-MerCre mice with transgenic mice harboring cardiac-specific overexpression of PIK domain (PIK-Tg mice) (16), which competitively inhibits the association between βARK1 and PI3-Kγ. The amount of βARK1-associated p110γ protein was significantly decreased in PDK1-MerCre × PIK-Tg mice, compared with PDK1-MerCre mice (Fig. 4A). Importantly, βARK1-associated PI3-K activity was markedly decreased in PDK1-MerCre × PIK-Tg mice, compared with PDK1-MerCre mice (Fig. 4B, Lower), although total PI3-Kγ activity remained elevated (Fig. 4B, Upper). As a consequence, in PDK1-MerCre × PIK-Tg mice 1week after tamoxifen treatment, the expression levels of β1-AR in membrane fraction were restored (Fig. 4C). The %FS in echocardiographic examination showed partial but significant improvement (Fig. 4D). Overexpression of PIK domain did not influence cardiomyocyte apoptosis, because the prevalence of TUNEL-positive cardiomyocytes (Fig. 4E), as well as the amount of cleaved poly(ADP-ribose) polymerase, Bax, and phosphrylated FOXO3a (Fig. S4), was unchanged in PDK1-MerCre hearts. In addition, overexpression of Bcl-2 protein did not influence β-adrenergic response, because the amount of βARK1-associated p110γ protein (Fig. 4A), βARK1-associated PI3-K activity (Fig. 4B), the expression levels of membranous β1-AR (Fig. 4C), as well as cAMP concentration and phosphorylation levels of PLN at Ser-16 (Fig. S5), were unchanged in PDK1-MerCre hearts. These results suggest that enhancement of βARK1-associated PI3-Kγ activity induces robust β-AR internalization, and thereby contributes to cardiac dysfunction, independently of cardiomyocyte apoptosis, in PDK1-MerCre mice.
Fig. 4.
Alleviated cardiac dysfunction in PDK1-MerCre mice by overexpression of PIK domain or Bcl-2 protein. (A) Immunoblot analysis of βARK1-associated p110γ protein in the hearts. (B) Kinase assays for PI3-K activity in the hearts. The hearts were subjected to immunoprecipitaion with antibody to p110γ (Upper) or βARK1 (Lower), and the resulting precipitates were assayed for the kinase assay. PI3-K activity of control mice was adjusted to 10 arbitrary units. (C) Immunoblot analysis of β1-AR and βARK1 in membrane fraction in the hearts. N-cadherin was used as an internal control for the amount of membrane protein. (D) Fractional shortening measured by echocardiography. Values represent the mean ± SEM of data from control mice (n = 10), control × PIK-Tg mice (n = 6), PDK1-MerCre mice (n = 10), and PDK1-MerCre × PIK-Tg mice (n = 6). #, P < 0.01 versus control mice. †, P < 0.01 versus PDK1-MerCre mice. FS, % of fractional shortening. (E) Percentage of TUNEL-positive caridomyocytes. Values represent the mean ± SEM (3,000 cardiomyocytes in each group). #, P < 0.01 versus control group. †, P < 0.01 versus PDK1-MerCre group.
Discussion
Our present study revealed that PDK1 plays an integrative role in normal cardiac function by coordinating survival signals and β-adrenergic response (Fig. S6). Besides the fundamental role in promoting cell growth and survival observed in many tissues in common (18–21), PDK1 uniquely accommodates β-adrenergic response to prevent cardiac decompensation. In addition, decreased expression of PDK1 protein in experimental models of heart failure raises a possibility that functional alterations of PDK1 may be implicated in the pathogenesis of heart failure, although it remains unclear how PDK1 expression is regulated in stressed hearts.
β-AR signaling plays a pivotal role in the chronotropic and inotropic functions in the hearts (22). In PDK1-MerCre hearts, the activity of βARK1-associated PI3-Kγ was enhanced, which enforced robust β1-AR down-regulation. PDK1 is a direct downstream effector of PI3-K and may participate in the negative feedback regulation of PI3-K signaling pathway (20). Importantly, overexpression of PIK-domain prevented β1-AR down-regulation by interfering βARK1/PI3-Kγ complex formation, and alleviated cardiac dysfunction in PDK1-MerCre mice. A recent report demonstrated that PI3-Kγ negatively modulates cardiac contractility by promoting phosphodiesterase 3B-mediated destruction of cAMP in a kinase-independent manner (23), but we did not observe significant change in the activity of phosphodiesterase 3B in PDK1-MerCre hearts despite enhanced PI3-Kγ activity (Fig. S7). Therefore, we suppose that impairment of β-adrenergic responsiveness results from intense β-AR down-regulation in PDK1-MerCre hearts.
It remains controversial whether down-regulation and desensitization of β-AR function is beneficial or detrimental in failing hearts. Indeed, clinical trials have indicated that the use of β-AR antagonists improves morbidity and mortality in patients of heart failure (1). Sustained β-AR overstimulation promotes energy consumption and apoptosis in cardiomyocytes (1, 24). But, accumulating evidence has suggested that normalization of β-adrenergic signaling by interfering βARK1 function rescued numerous genetic and experimental models of heart failure in mice (16, 25–28). A possible explanation for this discrepancy is that the therapeutic window for optimal level of β-AR signaling may be narrow in failing hearts (22, 28). It has been reported that the proapoptotic effect of β1-AR stimulation is dependent on Ca2+/calmodulin-dependent kinase II (CaMKII) (24). The phosphorylation level of CaMKII was decreased in PDK1-MerCre hearts, and restored to a subnormal level by overexpression of PIK domain (Fig. S8). Importantly, normalization of β1-AR did not induce excessive activation of CaMKII and cardiomyocyte apoptosis (Fig. 4E and Fig. S4). Thus, the β1-AR normalization may improve contractile function without evoking a ‘fight or flight’ reaction, unlike the simple β1-AR activation. Alternatively, robust β-AR internalization may activate adverse intracellular signaling pathways through β-arrestins (29) and abrogate the cardioprotective effects mediated by transactivation of epidermal growth factor receptor (30). Further investigations will be required to clarify the entire mechanisms of how normalization of β-AR signaling confers therapeutic benefits on failing hearts.
A growing body of evidence has suggested that cardiomyocyte apoptosis plays an important role in the pathogenesis of heart failure (31). In PDK1-MerCre hearts, the phosphorylation levels of Akt, SGK1 and FOXO3a were reduced, which may give rise to marked increase in cardiomyocyte apoptosis. In addition, PDK1-MerCre hearts showed an increase in expression level of Bax protein, a key molecule that translocates to the mitochondrial membrane and triggers the release of cytochrome c into the cytoplasm (31). Overexpression of Bcl-2 attenuated apoptotic loss of cardiomyocytes and alleviated cardiac dysfunction in PDK1-MerCre mice, suggesting that cardiomyocyte apoptosis contributes to the development of heart failure.
The previous paper demonstrated that PDK1-MCKCre mice showed growth retardation and contractile dysfunction of cardimyocytes (5). In our study, PDK1-MerCre mice showed severe heart failure without alterations in cardiomyocyte size. Besides regulation of cell growth, PDK1 controls cardiac homeostasis by promoting cell survival and preserving β-AR response. The phenotypic difference between PDK1-MerCre mice and PDK1-MCKCre mice resulted from the timing of gene disruption. The Pdk1 gene was deleted within a week in tamoxifen-treated PDK1-MerCre hearts of adult mice, but in contrast, Pdk1 disruption commenced before birth in PDK1-MCKCre mice. The number of apoptotic cardiomyocytes was pronouncedly increased in PDK1-MerCre hearts, but was unchanged in PDK1-MCKCre hearts (5). Some compensation mechanisms may prevent proapoptotic effects of Pdk1 disruption in PDK1-MCKCre mice.
In conclusion, PDK1 is a pivotal effector with dual functions to promote survival of cardiomyocytes and to preserve β-AR response in vivo (Fig. S6). In this regard, up-regulation of PDK1 in the hearts may emerge as a potential therapeutic strategy for heart failure.
Methods
Generation of PDK1-MerCre Mice.
Mice harboring a Pdk1flox allele were previously described (7, 8). Mice expressing MerCreMer under the control of α-myosin heavy chain promoter were previously described (9). Details are in SI Methods. Bcl2-Tg mice and PIK-Tg mice were kindly gifted by Dr. Michael D. Schneider (Imperial College, London, U.K.) (15) and Dr. Howard A. Rockman (Duke University Medical Center, Durham, NC) (16). All of the experimental protocols were approved by the Institutional Animal Care and Use Committee of Chiba University.
Echocardiography and Isolated Heart Preparation.
Transthoracic echocardiography was performed on conscious mice with Vevo 660 Imaging System using a 25-MHz linear probe (Visual Sonics Inc.). For analyses of hemodynamic parameters, hearts were excised rapidly and mounted on a Langendorff perfusion system, and a balloon was inserted into the cavity of the left ventricle (32). Isolated hearts were stabilized for 30 min by perfusion of Krebs-Henseleit buffer followed by perfusion of isoproterenol (NIKKEN Chemical Laboratory) or forskolin (Sigma). For measurement of surface areas of cardiomyocytes, hearts were enzymatically dissociated as described previously (33).
Histological Analysis and Immunohistochemistry.
Hearts were excised and immediately fixed in 10% neutralized formalin, embedded in paraffin. Serial sections at 5 μm were stained with hematoxylin and eosin for morphological analysis, and with Masson's trichrome for detection of fibrosis. For immunohistochemistry, Vectastain ABC kit (Vector Laboratories) was used to detect the primary antibodies. TUNEL assay was performed on paraffin sections, using an in situ apoptosis detection kit (Takara Bio Inc.).
Western Blot Analysis and Subcellular Fractionation.
Protein samples were fractionated by SDS/PAGE, and immunoblot analysis was performed as described previously (34). The membrane and cytosol fractions were isolated from lysate of the hearts as previously described (35).
Assay for PI3-K Activities.
PI3-K acitivity was measured as previously described (36). We determined Akt activity using a Akt Kinase Assay Kit according to the manufacturer's protocol (Cell Signaling Technology).
Antibodies.
The following antibodies were used: p110γ, phosphorylated-SGK, and cleaved caspase-3 (Cell Signaling Technology), βARK1, Bax, Bcl-xL, Bcl-2 (Santa Cruz Biotechnology), β1-AR (Affinity BioReagents), N-cadherin (Zymed Laboratories Inc.), SGK1, FOXO3a, phosphorylated-FOXO3a (Thr-32), phosphorylated-FOXO3a (Ser-253) (Upstate) and actin (Sigma).
Statistical Analysis.
All data are presented as means ± SEM. All data were analyzed by one-way ANOVA followed by the Fisher procedure for comparison of means. A probability value of P < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments.
We thank Drs. M. D. Schneider and H. A. Rockman for generously providing Bcl2-Tg and PIK-Tg mice, respectively. We thank M. Akao and Y. Oike for technical advice, and A. Furuyama, M. Ikeda, Y. Ohtsuki, and I. Sakamoto for their excellent technical assistance. This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports, and Culture, and Health and Labor Sciences Research Grants (to IK and HA); grants from Japan Intractable Diseases Research Foundation, Kowa Life Science Foundation, and Takeda Science Foundation (to HA).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900064106/DCSupplemental.
References
- 1.Katz AM. The “modern” view of heart failure: How did we get here? Circ Heart Fail. 2008;1:63–71. doi: 10.1161/CIRCHEARTFAILURE.108.772756. [DOI] [PubMed] [Google Scholar]
- 2.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 3.Toker A, Newton AC. Cellular signaling: Pivoting around PDK-1. Cell. 2000;103:185–188. doi: 10.1016/s0092-8674(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 4.Lawlor MA, et al. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora A, et al. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budas GR, Sukhodub A, Alessi DR, Jovanovic A. 3′Phosphoinositide-dependent kinase-1 is essential for ischemic preconditioning of the myocardium. FASEB J. 2006;20:2556–2558. doi: 10.1096/fj.06-6252fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaue H, et al. Requirement for 3-phosphoinositide-kependent dinase-1 (PDK-1) in insulin-induced glucose uptake in immortalized brown adipocytes. J Biol Chem. 2003;278:38870–38874. doi: 10.1074/jbc.M306151200. [DOI] [PubMed] [Google Scholar]
- 8.Inoue H, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Sohal DS, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 10.Williams MR, et al. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 11.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruning JC, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama T, et al. Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation. 2005;111:1652–1659. doi: 10.1161/01.CIR.0000160352.58142.06. [DOI] [PubMed] [Google Scholar]
- 14.Brunet A, et al. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 16.Perrino C, et al. Restoration of beta-adrenergic receptor signaling and contractile function in heart failure by disruption of the betaARK1/phosphoinositide 3-kinase complex. Circulation. 2005;111:2579–2587. doi: 10.1161/CIRCULATIONAHA.104.508796. [DOI] [PubMed] [Google Scholar]
- 17.Naga Prasad SV, Jayatilleke A, Madamanchi A, Rockman HA. Protein kinase activity of phosphoinositide 3-kinase regulates beta-adrenergic receptor endocytosis. Nat Cell Biol. 2005;7:785–796. doi: 10.1038/ncb1278. [DOI] [PubMed] [Google Scholar]
- 18.Mora A, Lipina C, Tronche F, Sutherland C, Alessi DR. Deficiency of PDK1 in liver results in glucose intolerance, impairment of insulin-regulated gene expression and liver failure. Biochem J. 2005;385:639–648. doi: 10.1042/BJ20041782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Hashimoto N, et al. Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat Genet. 2006;38:589–593. doi: 10.1038/ng1774. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto Y, et al. Restoration of glucokinase expression in the liver normalizes postprandial glucose disposal in mice with hepatic deficiency of PDK1. Diabetes. 2007;56:1000–1009. doi: 10.2337/db06-1322. [DOI] [PubMed] [Google Scholar]
- 21.Belgardt BF, et al. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 23.Patrucco E, et al. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Zhu WZ, et al. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ARK1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci USA. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AS, et al. In vivo ventricular gene delivery of a beta-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103:1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 27.Nienaber JJ, et al. Inhibition of receptor-localized PI3K preserves cardiac beta-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest. 2003;112:1067–1079. doi: 10.1172/JCI18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raake PW, et al. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 30.Noma T, et al. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki M, et al. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 33.Sambrano GR, et al. Navigating the signalling network in mouse cardiac myocytes. Nature. 2002;420:712–714. doi: 10.1038/nature01306. [DOI] [PubMed] [Google Scholar]
- 34.Akazawa H, et al. Diphtheria toxin-induced autophagic cardiomyocyte death plays a pathogenic role in mouse model of heart failure. J Biol Chem. 2004;279:41095–41103. doi: 10.1074/jbc.M313084200. [DOI] [PubMed] [Google Scholar]
- 35.Takeishi Y, Jalili T, Ball NA, Walsh RA. Responses of cardiac protein kinase C isoforms to distinct pathological stimuli are differentially regulated. Circ Res. 1999;85:264–271. doi: 10.1161/01.res.85.3.264. [DOI] [PubMed] [Google Scholar]
- 36.Sakaue H, et al. Phosphoinositide 3-kinase is required for insulin-induced but not for growth hormone- or hyperosmolarity-induced glucose uptake in 3T3–L1 adipocytes. Mol Endocrinol. 1997;11:1552–1562. doi: 10.1210/mend.11.10.9986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.