Abstract
Recent studies have suggested a close relationship between CD4+FOXP3+ regulatory T cells (Tregs) and proinflammatory IL-17-producing T helper cells (TH17) expressing the lineage-specific transcription factor RORγt. We report here the unexpected finding that human memory Tregs secrete IL-17 ex vivo and constitutively express RORγt. IL-17-secreting Tregs share some phenotypic and functional features with conventional TH17 cells, expressing high levels of CCR4 and CCR6 and low levels of CXCR3. However, unlike conventional TH17 cells, they express low levels of CD161 and mostly fail to cosecrete IL-22 and TNF-α ex vivo. Ex vivo secretion of IL-17 and constitutive expression of RORγt by human memory Tregs suggest that, in addition to their well-known suppressive functions, these cells likely play additional, as yet undescribed, proinflammatory functions.
CD4+ T cells encompass different subsets that express lineage-specific transcription factors and play different roles, not only in initiating and supporting the development of immune responses, but also in orchestrating and regulating them. T helper type 1 (TH1) cells, which differentiate in the presence of IL-12 and IFN-γ, express the transcription factor T-bet and assure protection against intracellular pathogens and cancer (1). TH2 cells differentiate in the presence of and produce IL-4, express the transcription factor GATA-3, and are involved in allergic reactions and protection against extracellular parasites (1). Whereas the TH1 and TH2 subsets have been described since the late 1980s (2), 2 additional CD4+ T cell subsets, regulatory/suppressor T cells (Tregs) and T helper IL-17-producing cells (TH17), have been described more recently. Tregs are characterized by the expression of the lineage-specific transcription factor FOXP3, which is involved both in their development and in their suppressor functions (3, 4), and they express high levels of CD25 and low levels of CD127 (5–7). At variance with TH1 and TH2 cells that are memory cells, human FOXP3+ Tregs exist both at the naïve (NnTreg) and the memory (MTreg) stages (8). FOXP3+ Tregs are suppressive ex vivo, anergic, and fail to secrete IL-2 or IFN-γ (4, 9). They play a major role in the control of self-tolerance—their depletion or functional alteration resulting in the development of autoimmune diseases—and are involved in the regulation of T cell homeostasis as well as of immune responses to pathogens, alloantigens, and cancer (10). Finally, the most recently defined subset, TH17 cells, produce IL-17A and IL-17F, 2 members of the IL-17 family that have similar proinflammatory functions, including recruitment and activation of neutrophils, and trigger the production of other proinflammatory chemokines and cytokines by monocytes, endothelial cells, and epithelial cells (11, 12). Several studies have suggested that TH17 cells are involved in protection against extracellular bacteria and fungi but also are involved in the pathogenesis of certain autoimmune diseases, including experimental autoimmune encephalitis and collagen-induced arthritis (13, 14). The nuclear hormone receptor RA-related orphan receptor γt (RORγt), originally defined as a thymic specific isoform of RORγ (15), has been identified as the lineage-specific transcription factor for TH17 cells, required for their generation (16, 17).
Initial studies assessing the differentiation of Tregs and TH17 cells from murine naïve CD4+ T cells have reported reciprocally regulated and mutually exclusive differentiation programs for the 2 subsets (18, 19). Recently, however, several reports have suggested that the relationship between Tregs and TH17 cells could be more complex than anticipated, involving plasticity of the respective differentiation programs (20) and in vivo equilibrium of the 2 subsets (21).
In this study, by assessing ex vivo cytokine production by CD4+ T cells from healthy donors, we found, unexpectedly, that a significant proportion of circulating memory FOXP3+ Tregs secrete IL-17 and express high levels of RORγt ex vivo. Together, our findings shed light on the close relationship between human Tregs and TH17 cells and suggest that, in addition to their well-recognized immune suppressive functions, memory FOXP3+ Treg cells likely play additional, as yet undescribed proinflammatory functions.
Results
Human Memory FOXP3+ Tregs Secrete IL-17 ex Vivo.
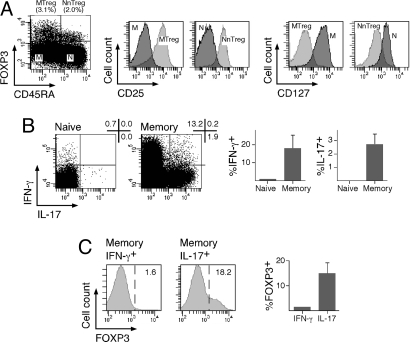
Human natural CD4+ Tregs are characterized by ex vivo expression of the specific transcription factor FOXP3. As assessed by intracellular staining using specific antibodies, about 5% of total circulating CD4+ T cells from healthy donors expressed FOXP3 ex vivo. CD4+FOXP3+ Tregs included a population of CD45RA+ cells corresponding to NnTregs (8), as well as CD45RA− MTregs (Fig. 1A). Most FOXP3+ Tregs, both naïve and memory, expressed CD25 and were CD127low. To assess ex vivo cytokine production with respect to differentiation stage and FOXP3 expression, we stimulated purified CD4+ T cells with phorbol 12-myristate 13-acetate (PMA)/ionomycin and then stained them with CD45RA-specific, FOXP3-specific, and cytokine-specific antibodies. As expected, significant proportions of memory but not naïve CD4+ T cells secreted IFN-γ or IL-17 ex vivo (Fig. 1B). Consistent with the reported inability of FOXP3+ Tregs to secrete IFN-γ, most memory cells secreting IFN-γ ex vivo were FOXP3− (Fig. 1C). Unexpectedly, however, a significant proportion (up to 20%) of memory CD4+ T cells secreting IL-17 ex vivo were FOXP3+ Tregs.
Fig. 1.
A fraction of CD4+ T cells producing IL-17 ex vivo express FOXP3. (A) CD4+ T cells isolated from PBMCs of healthy donors were stained with anti-FOXP3, anti-CD25, anti-CD45RA, and anti-CD127 antibodies and analyzed by flow cytometry. Expression of FOXP3 and CD45RA defines memory (M; FOXP3−CD45RA−) and naïve (N; FOXP3−CD45RA+) conventional CD4+ T cells and memory (MTreg; FOXP3+CD45RA−) and naïve (NnTreg; FOXP3+CD45RA+) Tregs. Histograms show the expression of CD25 and CD127 gated on the defined CD4+ T cell populations. Results are shown for 1 of 3 donors. (B and C) Enriched CD4+ T cells were stimulated for 6 h with PMA/ionomycin and stained with antibodies specific for CD45RA, FOXP3, IL-17, and IFN-γ. (B) The proportion of IL-17-secreting and IFN-γ-secreting cells is shown gated on total naïve (CD45RA+) and memory (CD45RA−) CD4+ T cells. Dot plots for 1 donor and data for 3 donors are shown (mean ± SD). (C) The proportion of FOXP3-expressing cells is shown gated on IL-17-secreting and IFN-γ-secreting cells as indicated. Histograms for 1 donor and data for 3 donors are shown (mean ± SD).
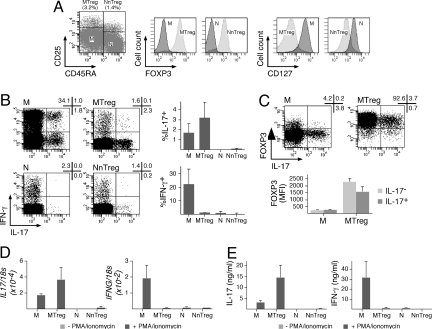
To confirm and quantify ex vivo secretion of IL-17 by MTregs, we isolated CD4+ conventional T cell and Treg subsets from circulating lymphocytes of healthy donors by cell sorting based on the expression of CD25 and CD45RA. The purified populations were assessed for purity (routinely >97%), FOXP3 and CD127 expression (Fig. 2A), and for cytokine production after stimulation with PMA/ionomycin. Isolated FOXP3+ MTregs secreted IL-17 ex vivo (Fig. 2 B and C). In addition, unlike IL-17-producing T cells in conventional memory CD4+ T cells, which contained a significant proportion of cells coproducing IFN-γ, the large majority of IL-17-producing cells in Tregs did not produce IFN-γ. The proportion of IL-17-secreting MTregs was higher compared with conventional memory CD4+ T cells. Accordingly, IL17 mRNA levels as well as the levels of secreted IL-17 were higher in stimulated MTregs than in conventional memory CD4+ T cells (Fig. 2 D and E). Because the intensity of FOXP3 expression in IL-17+ MTregs was slightly lower than in IL-17− MTregs (Fig. 2C), it was important to assess the suppressive function of the identified population. Direct ex vivo assessment of suppressive functions was unfeasible because of the low frequency of the identified population together with the lack of methods allowing the isolation of living IL-17-secreting cells. Therefore, to directly address the suppressive function of FOXP3+ IL-17-producing Tregs, we cloned ex vivo-sorted FOXP3+CD25+CD127lowCD4+ T cells, isolated clones that had maintained the ex vivo phenotype of the defined population (FOXP3 expression and IL-17 secretion), and assessed their suppressor function (see SI Materials and Methods). As shown in Fig. S1, FOXP3+ IL-17-producing clones derived from Tregs exhibited high suppressive activity.
Fig. 2.
Human FOXP3+ MTregs secrete IL-17 ex vivo. (A) Enriched CD4+ T cells were stained with anti-CD8, anti-CD25, and anti-CD45RA antibodies, and CD8− cells were separated by flow cytometry cell sorting into memory (M; CD25−CD45RA−) and naïve (N; CD25−CD45RA+) conventional CD4+ T cells and memory (MTreg; CD25+CD45RA−) and naïve (NnTreg; CD25+CD45RA+) Tregs (dot plot). A fraction of sorted populations was stained with anti-FOXP3 and anti-CD127 antibodies and analyzed by flow cytometry (histograms). Results are shown for 1 of 3 donors. (B) The fraction of IL-17-secreting and IFN-γ-secreting cells among sorted CD4+ T cell populations (M, MTreg, N, and NnTreg) was determined by intracellular cytokine staining and flow cytometry analysis after 6 h of stimulation with PMA/ionomycin. Dot plots for 1 donor and data for 3 donors are shown (mean ± SD). (C) Sorted M and MTreg cells were stimulated for 6 h with PMA/ionomycin and stained with anti-FOXP3 and anti-IL-17 antibodies and analyzed by flow cytometry. Dot plots for 1 of 3 donors are shown (Upper). Mean fluorescence intensity (MFI) of FOXP3 staining in IL-17+ and IL-17− cells is shown for 3 donors (mean ± SD; Lower). (D and E) Sorted CD4+ T cell populations were stimulated for 24 h in the absence or presence of PMA/ionomycin, and IL17 and IFNG mRNA levels were determined by quantitative PCR (D), and the quantity of IL-17 and IFN-γ secreted in the culture supernatant was measured by ELISA (E). Data are shown for 3 donors (mean ± SD).
Memory FOXP3+ Tregs Express RORγt ex Vivo.
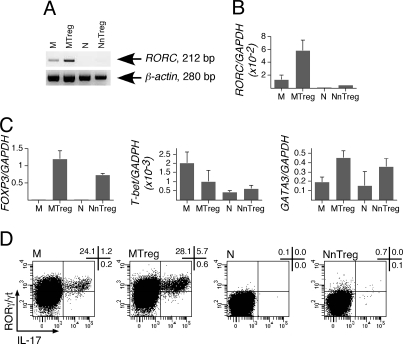
Because IL-17 secretion has been associated with expression of RORγt, ex vivo secretion of IL-17 by MTregs prompted us to assess the expression of RORγt in conventional CD4+ T cell and Treg isolated populations ex vivo. Remarkably, by using RT-PCR, we detected ex vivo RORC mRNA expression in unstimulated MTregs and, at lower levels, in conventional memory but not in naïve CD4+ T cells (Fig. 3A). To confirm and quantify the increased expression of RORC in MTregs, we performed quantitative PCR analysis (Fig. 3B). The results confirmed ex vivo expression of RORC in MTregs at levels about 100-fold higher than those found in conventional naïve CD4+ T cells and 4-fold higher than in conventional memory CD4+ T cells. For internal comparison, we also assessed expression of FOXP3, T-bet, and GATA3 in ex vivo isolated populations by quantitative PCR (Fig. 3C). As expected, both naïve and memory Tregs expressed highly increased levels of FOXP3 compared with conventional CD4+ T cells. Ex vivo expression of T-bet was slightly higher in conventional memory CD4+ T cells compared with all other populations, and ex vivo expression of GATA3 was slightly more elevated in Tregs (both NnTregs and MTregs) than in conventional CD4+ T cells. Finally, we assessed ex vivo RORγt expression with respect to IL-17 production in isolated populations by intracellular staining after stimulation with PMA/ionomycin, using RORγ/γt-specific antibodies that very recently became available. As illustrated in Fig. 3D, this analysis confirmed the presence, among MTregs, of RORγt-expressing/IL-17-producing cells, which were found in increased proportions compared with conventional memory CD4+ T cells. No significant levels of RORγt-expressing/IL-17-producing cells were detectable in naïve populations.
Fig. 3.
Ex vivo expression of RORγt by MTregs. (A and B) CD4+ T cells were separated by flow cytometry as in Fig. 2A, and RORC mRNA expression in sorted populations was assessed by conventional (A) and quantitative (B) PCR. (C) FOXP3, T-bet, and GATA3 mRNA expression was determined in sorted CD4+ T cell populations by quantitative PCR. (D) RORγ/γt expression and IL-17 production by sorted populations were assessed by intracellular staining using specific antibodies after 6 h of stimulation with PMA/ionomycin. Results are shown for 1 of 3 donors.
Memory FOXP3+ IL-17-Secreting Tregs Share some but Not all Phenotypic and Functional Features with Conventional TH17 Cells.
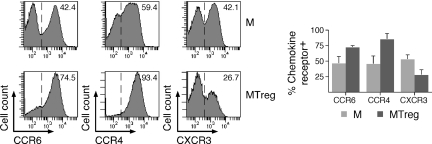
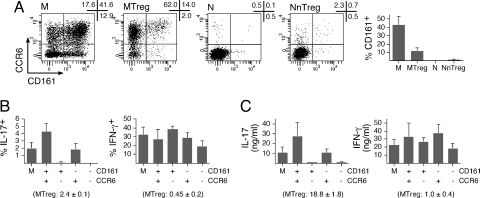
To further characterize IL-17-producing MTregs, we compared some of their phenotypic features to those recently described for conventional TH17 cells. Recent studies have assessed the phenotype of TH17 cells based on the expression of chemokine receptors. TH17 cells were exclusively found in the fraction expressing CCR6, a receptor that mediates homing to mucosal tissues and skin (17). Within the CCR6+ fraction, cells producing IL-17 but not IFN-γ were found in the population expressing CCR4, previously reported to be preferentially associated with TH2 cells, whereas those cosecreting IL-17 and IFN-γ (called TH17/TH1 cells) (22) were found in the population expressing CXCR3, associated with TH1 cells. As illustrated in Fig. 4, in line with the ex vivo secretion of IL-17 in the absence of IFN-γ, MTregs were enriched in CCR6+ and CCR4+ cells but contained lower levels of CXCR3+ cells compared with conventional memory CD4+ T cells. Cosmi et al. (23) have recently proposed that CD161, a lectin-like receptor expressed by a subset of human NK and T cells, is a marker of TH17 cells, particularly in association with CCR6. To assess expression of CD161 in MTregs, we costained purified CD4+ T cells with antibodies specific for CD45RA, CD25, CD161, and CCR6. A large proportion (>40%) of memory but only a few naïve CD4+ T cells expressed CD161 (Fig. 5). In contrast to CCR6, the proportion of CD161-expressing cells was lower among MTregs compared with conventional memory CD4+ T cells. To directly assess the correlation between expression of CCR6/CD161 and IL-17 secretion, we isolated 4 subpopulations of conventional memory CD4+ T cells by cell sorting according to the expression of the 2 markers, and we assessed IL-17 (and IFN-γ) production after stimulation with PMA/ionomycin. We found a strict association between ex vivo expression of CCR6 and IL-17 secretion. In contrast, IL-17 secretion did not strictly segregate with CD161 expression, because the proportion of IL-17-secreting cells within the CCR6+CD161+ fraction was, on average, only increased 2-fold compared with that found among CCR6+CD161− cells. Thus, lack of CD161 expression by Tregs was not at odds with their capacity to secrete IL-17 ex vivo.
Fig. 4.
CD4+ T cells were stained with antibodies specific for CD25 and CD45RA as well as for CCR4, CCR6, or CXCR3. Chemokine receptor expression was analyzed gated on CD25−CD45RA− (M) and CD25+CD45RA− (MTreg) cells. Histograms for 1 donor and data for 3 donors are shown (mean ± SD).
Fig. 5.
Low proportions of MTregs express CD161. (A) CD4+ T cells were stained with antibodies specific for CD25, CD45RA, CCR6, and CD161. Dot plots from 1 donor are shown gated on the indicated population defined as in Fig. 2A. Data for 3 donors are shown (mean ± SD). (B and C) CD4+ T cells were stained with anti-CD25, anti-CD45RA, anti-CCR6, and anti-CD161 antibodies, and conventional memory cells (CD25−CD45RA−) were sorted by flow cytometry either as 1 population or separated into 4 populations according to the expression of CCR6 and CD161. Sorted populations were stimulated, and cytokine production was assessed as in Fig. 2B (B) and Fig. 2E (C) (mean ± SD, n = 2).
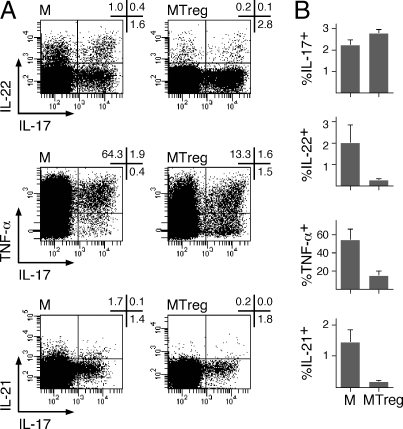
Finally, the ability of MTregs to secrete IL-17 ex vivo prompted us to assess other cytokines that have been reported to be coproduced by conventional TH17 cells. With this aim, we stimulated purified MTregs and conventional memory CD4+ T cells with PMA/ionomycin ex vivo and costained them with antibodies against IL-17 and IL-22, TNF-α, or IL-21. As illustrated in Fig. 6, whereas up to 20% of conventional CD4+ T cells secreting IL-17 ex vivo co-produced IL-22, only a minority (<5%) of IL-17-secreting MTregs cosecreted IL-22. Similarly, whereas the majority (80%) of conventional memory CD4+ T cells secreting IL-17 coproduced TNF-α, only half of IL-17-secreting MTregs cosecreted TNF-α. Finally, only a minority of conventional CD4+ T cells secreting IL-17 ex vivo coproduced IL-21, and no significant proportions of IL-21-producing cells could be detected among MTregs. Collectively, these results show that MTregs able to secrete IL-17 ex vivo share some but not all phenotypic and functional features with conventional TH17 cells, supporting the concept that these populations could at least partially colocalize in the same tissues and partially share effector functions.
Fig. 6.
Cytokine production by IL-17-producing MTregs. M and MTreg populations were sorted by flow cytometry as in Fig. 2A, and IL-17 and IL-22, TNF-α, or IL-21 production was determined by intracellular cytokine staining after 6 h of stimulation with PMA/ionomycin. Dot plots for 1 donor (A) and data for 3 donors (B; mean ± SD) are shown.
Discussion
We have reported here the unexpected finding that human MTregs secrete IL-17 and express high levels of RORγt ex vivo. Whereas previous studies have documented conversion of murine and human Tregs into TH17 cells after stimulation under various conditions (24–26), the ability of Tregs to secrete IL-17 ex vivo was not appreciated to date. Similarly, expression of RORγt in human Tregs was not known before the present report. This finding, however, is consistent with the recent observation by Lochner et al. (21) that up to 50% of murine RORγt+ Tαβ cells express FOXP3 and are functionally Treg cells. In addition, forced expression of RORγt in murine and human T cells has been shown to inhibit IL-2 expression by competing with NFAT for binding to DNA (15, 27), consistent with the inability of Tregs to secrete IL-2 in response to T cell receptor (TCR)-mediated stimulation (9).
Our findings raise several questions. First, what factors/mechanisms are responsible for RORγt expression in Tregs? A likely candidate is TGF-β, which promotes the generation of human and murine Tregs and has clearly been shown to mediate, in association with IL-6, the generation of murine TH17 cells (18), although its role in the generation of human TH17 cells is debated (28–31). In line with this hypothesis, stimulation of CD4+ T cells with TGF-β has been shown to induce expression of both FOXP3 and RORγt (32, 33). It is, however, noteworthy that although NnTregs and MTregs express similar levels of FOXP3, expression of RORγt in MTregs is significantly higher than in NnTregs, which fail to produce significant levels of IL-17 ex vivo. Thus, expression of RORγt in Treg cells may be more difficult to achieve compared with expression of FOXP3, and only becomes significant at late differentiation stages.
Our finding that FOXP3+ MTregs secrete IL-17 ex vivo was unexpected, also taking into account recent studies reporting that FOXP3 inhibits IL-17 secretion by antagonizing RORγt (33, 34). However, the findings confirm recent data from Osorio et al. (25) that some murine Tregs can produce IL-17 while retaining FOXP3 expression.
Other important questions relate to the biological significance of RORγt expression and IL-17 production in Tregs. Because human TH17 and IL-17-secreting Treg populations have partially common differentiation and migratory pathways, our findings support the concept that these 2 populations may not only coexist in the same tissues, but may share some proinflammatory functions. IL-17-secreting Tregs present at mucosal sites may participate in early responses to infection by attracting mediators of innate immunity. Additionally, it must be taken into account that nuclear hormone receptors, such as RORγt, perform diverse functions in development and homeostasis by regulating cell growth, differentiation, and apoptosis. In addition to TH17 cells, RORγt expression is found in double-positive thymocytes; in a subset of intestinal lamina propria T lymphocytes, most of which constitutively produce IL-17; in intestinal CD3− lymphoid tissue-inducer cells, also producing IL-17 and clustered in cryptopatches that are precursor structures of lymphoid follicles (16, 35); as well as in subpopulations of NK cells and γδ T cells (36–38). Thus, MTregs share RORγt expression and IL-17 production not only with proinflammatory TH17 cells but also with other populations involved in innate immunity and lymphogenesis, strongly suggesting additional as yet undescribed roles of Tregs in these processes. Finally, because Tregs are believed to be autoreactive, their ability to secrete IL-17 after TCR-mediated stimulation could indicate a physiologic role for these cells in tissue homeostasis. Future studies addressing these issues will undoubtedly contribute to further elucidate the mechanisms regulating the balance between inflammation and tolerance under physiological conditions as well as in a variety of pathological conditions.
Materials and Methods
Samples, Cell Purification, and Sorting.
Peripheral blood samples were obtained from the Etablissement Français du Sang Pays de la Loire (Nantes, France). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient sedimentation using LSM 1077 lymphocyte separation medium (PAA Laboratories). CD4+ T cells were enriched by positive selection from PBMCs by magnetic cell sorting (Miltenyi Biotec). For flow cytometry cell sorting, enriched CD4+ T cells were stained with fluorescent-conjugated antibodies specific for CD8 (BD Biosciences), CD45RA (BD Biosciences), and CD25 (Beckman Coulter). After gating on CD8− lymphocytes, cells were separated into memory (M; CD25−CD45RA−) and naïve (N; CD25−CD45RA+) conventional CD4+ T cells and memory (MTregs; CD25+CD45RA−) and naïve (NnTregs; CD25+CD45RA+) Tregs to high purity (>97%) using a FACSAria (BD Biosciences). A fraction of sorted populations was stained with antibodies specific for CD127 and FOXP3 (eBiosciences) and analyzed by flow cytometry. In some experiments, CD4+ T cells were stained with antibodies specific for CD8, CD45RA, CCR6 (BD Biosciences), CD161 (BD Biosciences), and CD25, and conventional memory CD4+ T cells were sorted into CD161+CCR6+, CD161+CCR6−, CD161−CCR6+ and CD161−CCR6− populations.
Phenotypic Analysis and Assessment of Cytokine Production.
Conventional CD4+ T cell and Treg, naïve and memory populations were assessed phenotypically by staining of total CD4+ T cells with antibodies specific for CD45RA, CD25, and CD127 together with antibodies specific for FOXP3, CCR4 (BD Biosciences), CXCR3 (BD Biosciences), or CCR6 and CD161, as indicated, and analysis by flow cytometry using FACSAria or an LSR II device (BD Biosciences). Cytokine production by enriched total CD4+ T cells was assessed ex vivo by intracellular staining after stimulation with PMA (100 ng/mL; Sigma–Aldrich) and ionomycin (1 μg/mL; Sigma–Aldrich) for 6 h. Brefeldin A (10 μg/mL; Sigma–Aldrich) was added 1 h after the beginning of the incubation. Cells were then fixed, permeabilized, and stained with antibodies specific for FOXP3, IL-17 (eBiosciences), and IFN-γ (BD Biosciences), and were analyzed by flow cytometry. Cytokine production by sorted CD4+ T cell populations was assessed ex vivo after stimulation with PMA and ionomycin in a 6-h intracellular cytokine staining assay using antibodies specific for IL-17, IFN-γ, and IL-22 (R & D Systems), TNF-α (BD Biosciences), IL-21 (BD Biosciences), or RORγ/γt (eBiosciences), as indicated. Cytokine production by CD4+ T cell populations was also assessed ex vivo in a secretion assay. Briefly, sorted CD4+ T cell populations (5 × 105 per well) were stimulated in the absence or presence of PMA and ionomycin in round-bottom 96-well plates. After 24 h, secreted IL-17 and IFN-γ were measured in the culture supernatant by ELISA (R & D Systems and Invitrogen, respectively), and IL-17 and IFN-γ mRNA levels in cells were determined by real-time quantitative RT-PCR as detailed below.
Conventional and Real-Time Quantitative RT-PCR.
RNA was prepared from ex vivo-sorted CD4+ T cell populations (5 to 10 × 105 cells) by using an RNeasy Mini Kit (Qiagen). cDNA synthesis was performed by using Promega Reverse Transcription System A3500 (Promega). Semiquantitative PCR was performed by using GoTaq Flexi DNA Polymerase (Promega). cDNA integrity was tested by amplification of β-actin mRNA in a 35-cycle PCR, and RORC mRNA expression was assessed by using the following primers: forward primer, 5′-TTTTCCGAGGATGAGATTGC-3′; reverse primer: 5′-CTTTCCACATGCTGGCTACA-3′. Quantitative real-time PCR was performed with a TaqMan assay on an ABI 7000 system (Applied Biosystems) using Assays-on-Demand Gene Expression probes for RORC (Hs01076112_m1), FOXP3 (Hs00203958_m1), T-bet (Tbx21; Hs00203436_m1), GATA3 (Hs00231122_m1), IL17 (IL17A; Hs99999082_m1), and IFNG (Hs99999041_m1) (Applied Biosystems). For control of input RNA, we used either Assays-on-Demand Gene Expression probes for 18s rRNA (Hs99999901_s1) or Taqman probe (FAM-5′-AAGGTGAAGGTCGGAGTCAACGGATTTG-3′-TAMRA) and primers (5′-CCACATCGCTCAGACACCAT-3′ and 5′-CCAGGCGCCCAATACG-3′) for GAPDH, as indicated. Relative mRNA expression was calculated as 2(Ct control RNA−Ct test RNA).
Supplementary Material
Acknowledgments.
The study was supported by the Ludwig Institute for Cancer Research, the Cancer Research Institute, Institut National de la Santé et de la Recherche Médicale, Institut National du Cancer, Conseil Régional des Pays de la Loire, and European Structural Funds (Fonds Européen de Développement Économique et Régional program).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900621106/DCSupplemental.
References
- 1.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 6.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 11.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 12.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calida DM, et al. Cutting edge: C3, a key component of complement activation, is not required for the development of myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis in mice. J Immunol. 2001;166:723–726. doi: 10.4049/jimmunol.166.2.723. [DOI] [PubMed] [Google Scholar]
- 14.Gran B, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: Evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 15.He YW, Deftos ML, Ojala EW, Bevan MJ. RORgamma t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9:797–806. doi: 10.1016/s1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 18.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 19.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lochner M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosmi L, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: Regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 25.Osorio F, et al. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 27.Littman DR, et al. Role of the nuclear hormone receptor ROR gamma in transcriptional regulation, thymocyte survival, and lymphoid organogenesis. Cold Spring Harb Symp Quant Biol. 1999;64:373–381. doi: 10.1101/sqb.1999.64.373. [DOI] [PubMed] [Google Scholar]
- 28.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 29.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci USA. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpe E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 32.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichiyama K, et al. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 35.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 36.Cupedo T, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 37.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 38.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: An important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.