Abstract
Winged morphs of aphids are essential for their dispersal and survival. We discovered that the production of the winged morph in asexual clones of the rosy apple aphid, Dysaphis plantaginea, is dependent on their infection with a DNA virus, Dysaphis plantaginea densovirus (DplDNV). Virus-free clones of the rosy apple aphid, or clones infected singly with an RNA virus, rosy apple aphid virus (RAAV), did not produce the winged morph in response to crowding and poor plant quality. DplDNV infection results in a significant reduction in aphid reproduction rate, but such aphids can produce the winged morph, even at low insect density, which can fly and colonize neighboring plants. Aphids infected with DplDNV produce a proportion of virus-free aphids, which enables production of virus-free clonal lines after colonization of a new plant. Our data suggest that a mutualistic relationship exists between the rosy apple aphid and its viruses. Despite the negative impact of DplDNV on rosy apple aphid reproduction, this virus contributes to their survival by inducing wing development and promoting dispersal.
Keywords: development, parvovirus, pathogen, polyphenism, synergism
Polyphenism, the production of discrete phenotypes based on the same genome, plays a central role in biology. The life cycle of alternate, cyclically parthenogenetic aphid species includes both a sexual generation and a number of asexual generations (1). In asexually reproducing clones, genetically identical aphids are either wingless (apterae) or winged (alate). Apterae show maximum fecundity, allowing rapid colony growth during long-day, warm conditions when resources are plentiful. Alates have lower fecundity, but are essential for dispersal and long-distance colonization of new plants (2, 3). Alates are generally not produced during the asexual phase of reproduction unless there is stress resulting from crowding or poor nutritional resources. The wing development in asexual clones of aphids is influenced by interactions between environmental and intrinsic factors. Several cues are implicated, including temperature, population density (tactile stimulation), nutritional quality of the host plant, and interactions with natural enemies and ants, although these cues are not universal inducers for wing development in asexual clones of different lines of the same aphid species (4, 5, 6). Increased production of alates was observed in Sitobion avenae reared on oats infected with barley yellow dwarf virus (7), although infection of Vicia faca with pea enation mosaic virus, bean yellow mosaic virus, or broad bean mottle virus did not increase production of alates in A. pisum (8). In addition, plant viruses have been reported to change aphid behavior as a result of physiological changes in the infected plants (reviewed in ref. 9).
Several viruses of aphids have been characterized, including Myzus persicae densovirus (10); aphid lethal paralysis virus (11) and Rhopalosiphum padi virus (RhPV) (12), both members of the family Dicistroviridae; an iflavirus Brevicoryne brassicae virus (13); and the unclassified Acyrthosiphon pisum virus (APV) (14). Relatively little is known about the effect of these virus infections on aphid physiology; however, a recent study reported a change in olfactory behavior in response to RhPV infection (15). We discovered 2 viruses in the rosy apple aphid, Dysaphis plantaginea, which occur singly or as mixed infections. These are Dysaphis plantaginea densovirus (DplDNV) and rosy apple aphid virus (RAAV). Here we report that the densovirus DplDNV plays a central role in induction of wing development and dispersal of asexual clones of the rosy apple aphid, which suggests that a mutualistic relationship exists between the rosy apple aphid and its viruses.
Results
Virus Diversity in the Rosy Apple Aphid.
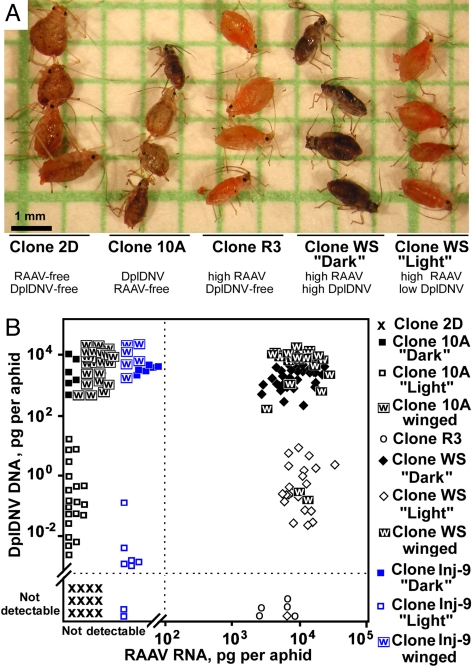
The original rosy apple aphid clones were established from single adults collected from apple trees at the end of summer 2002, in Warwickshire, U.K. Clones were maintained as asexual lineages on plantain, the summer host of the rosy apple aphid, under long-day conditions (16 h light/8 h dark), at constant temperature +20 °C ± 1 °C. Under these conditions the rosy apple aphid completes its life cycle (from newborn nymph to reproducing adult) in approximately 2 weeks. Two of the clones, WS and 2–11, contained a proportion of a different phenotype, which was smaller, darker, and had an ability to produce the winged morph. This was not observed in the other clones of rosy apple aphid.
Two approaches were used for virus discovery. First, aphids were screened for viruses with high similarity to previously reported aphid (insect) viruses, using PCR and RT-PCR with specific and degenerate primers. Second, we used the method for the amplification of encapsidated RNA and DNA (13, 16). Both strategies resulted in the identification of cDNA fragments from an RNA virus showing high sequence similarity with a virus from the pea aphid, APV (14). Amplification of DNA also resulted in the identification of DNA fragments encoding peptides having sequence similarity with proteins of densoviruses, an insect-infecting group of the Parvoviridae family (17). Two types of spherical virus particles, 22.0 ± 1.5 nm and 32.0 ± 1.5 nm (mean ± SD) with the buoyant densities of 1.35–1.45 g/cm3 in CsCl, were isolated from the aphids from clone WS [supporting information (SI) Fig. S1A]. The size of smaller particles was within the range reported for densoviruses (17), whereas the size of the larger particles was similar to that of APV (14). We determined the nucleotide sequences of the genomes of the previously undescribed viruses, which we named Dysaphis plantaginea densovirus, DplDNV (a DNA virus), and rosy apple aphid virus, RAAV (an RNA virus). The genome organization of DplDNV resembles that of the other members of the genus Densovirus (17) (Fig. S1B). The DplDNV ORF4 protein shows highest similarity with the coat protein of MpDNV (10) (37% aa identity). We identified densovirus sequences, MpDNV and putative A. pisum densovirus, in expressed sequence tags (ESTs) derived from aphid laboratory cultures of M. persicae and A. pisum (Table S1 and Table S2, and Fig. S2), suggesting that densoviruses may occur in a range of aphid species. The positive-strand RNA genome of RAAV has the same organization as the genome of APV (14) (Fig. S1C) and shows high similarity with it (87% aa identity).
Both RAAV and DplDNV infections were confirmed by RT-PCR in clones WS and 2–11 of rosy apple aphid (Fig. S1 D and E). RAAV was present in all tested adult aphids and fourth instars from clone WS, whereas results from PCR showed evidence that DplDNV is less abundant in light aphids without wing buds compared with dark aphids with wing buds and winged aphids (Fig. S2F). Indeed, qPCR showed that the levels of accumulation of DplDNV DNA in light aphids from the clone WS were significantly lower than those in the dark or winged aphids of clone WS (Table S3).
Transmission of Rosy Apple Aphid Viruses.
Both RAAV and DplDNV nucleic acids were detected by RT-PCR in plantain leaf tissue previously exposed to infected aphids. Replication of these viruses in plant cells is highly unlikely, as no increase in virus concentration was observed following the removal of the aphids from the plants. The majority of progeny nymphs by the aphids with high RAAV, reared on artificial diet, without exposure to plants, were RAAV free; in total, only 1 of 27 clones established from the individual progeny nymphs by the aphids with high RAAV levels (from clones WS and R3) was RAAV infected; the remaining 26 clones were RAAV free (Table S4). When virus-free aphids were exposed to leaves previously contaminated by direct exposure to infected aphids, they became RAAV positive. Aphids placed on leaves from distant unexposed parts of the same plant also became RAAV positive. It is likely that horizontal transmission of RAAV involves the plant vascular system, as in the case of the transmission of another aphid virus, RhPV (18, 19), and leafhopper A virus (20). Plant-mediated horizontal transmission of DplDNV also takes place, but only from leaves that have been in direct contact with the DplDNV-infected aphids. Thus, both DplDNV and RAAV associated with plant tissue are the source of inocula for their horizontal transmission. We observed also that there is efficient vertical transmission of DplDNV from infected adults to nymphs, with the majority of nymphs produced by DplDNV-infected aphids reared on an artificial diet being DplDNV infected; 9 of 10 clones established from individual progeny nymphs from aphids with high DplDNV levels (from clone WS) were DplDNV positive (Table S4). Nevertheless, a proportion of the progeny from DplDNV-infected aphids was DplDNV free.
Testing of Koch's Postulates.
Production of genetically identical rosy apple aphid clones infected with different virus combinations were required to complete Koch's postulates. We established both the virus-free clone (clone 2D) and the DplDNV-infected clone (clone 10A) from clone WS (DplDNV and RAAV infected) by propagation and selection on an artificial diet. In addition, we established clone R3, which is infected only with RAAV, from clone WS by selection on plants (Fig. 1A). Aphid clones that are virus free or infected with only one of the viruses are susceptible to the other virus/viruses, namely RAAV and/or DplDNV. The virus purification included homogenization of aphids in 0.1 M sodium phosphate buffer (pH 7.5) followed by filtration through a 0.8/0.2-μm filter (Pall Gelman Laboratory), to exclude bacterial and fungal pathogens. An additional CsCl gradient centrifugation step was included in the preparation of virus inocula for microinjection and diet transmission experiments. The fraction with a buoyant density of 1.35–1.45 g/cm3 in CsCl contained only DplDNV and/or RAAV. It was diluted 5-fold with 0.1 M sodium phosphate buffer (pH 7.5), and the virus particles were pelleted by centrifugation (30,000 g, 3 h, 4 °C). The virus concentration in the preparations was determined by real-time PCR. Aphids could be infected (i) by feeding on artificial diet containing a DplDNV virus preparation (Table 1), (ii) by injection of a DplDNV or RAAV virus preparation into the aphid hemolymph (Table 1), (iii) via plant tissue, either by rearing the recipient aphids on leaves previously exposed to virus-infected aphids, or (iv) by direct application of a virus preparation onto the leaf surface. Plant-mediated infection with DplDNV or RAAV occurred in all of the triplicated experiments where groups of 5 virus-free aphids were placed for 7 days, either on the leaves previously exposed to the aphids infected with DplDNV or RAAV or on leaves onto which virus preparations had been applied. No virus infections resulted when leaves were exposed to the virus-free aphid cultures or coated with a preparation from the virus-free aphids.
Fig. 1.
RAAV and DplDNV in the rosy apple aphid. (A) Genetically identical aphids infected with different virus combinations. (B) Accumulation of RAAV and DplDNV in individual aphids. Clone WS was originated from a single doubly infected aphid; clones 2D, R3, and 10A were produced by selecting progeny of clone WS. Clone Inj-9 was produced from a virus-free aphid of clone 2D following injection with a DplDNV virus preparation from clone 10A.
Table 1.
Infection of rosy apple aphids with RAAV and DplDNV virus preparationsa
| Inoculumb | Colonies with the winged morphc |
Colonies without the winged morphd |
||||
|---|---|---|---|---|---|---|
| Virus free | DplDNV | RAAV | Virus free | DplDNV | RAAV | |
| Artificial diet inoculatione | ||||||
| Control | 0 | 0 | 0 | 12 | 0 | 0 |
| DplDNV | 0 | 5 | 0 | 7 | 0 | 0 |
| Inoculation by injectionf | ||||||
| Control | 0 | 0 | 0 | 11 | 0 | 0 |
| DplDNV | 0 | 3 | 0 | 6 | 0 | 0 |
| RAAV | 0 | 0 | 0 | 4 | 0 | 6 |
aFifteen fifth instars from virus-free clone 2D were used for infection for each group. Inoculation and progeny rearing on plantain plants was carried out at 20 °C ± 1 °C on a 16/8-h light/dark cycle. Virus accumulation was tested in the established colonies 4 weeks postinoculation (w.p.i.) by RT-PCR in pooled samples extracted from 20 randomly selected fourth and fifth instars.
bVirus preparations from DplDNV-infected aphids, clone 10A (DplDNV), RAAV-infected aphids, clone R3 (RAAV), or DplDNV-free aphids, clone 2D (control).
cNumber of established colonies with the winged morph, first appeared 2 w.p.i..
dNumber of established colonies without the winged morph, monitored up to 4 w.p.i..
eIndividual aphids were fed on artificial diet containing DplDNV virus preparation (20 pg/μL of DplDNV DNA) or a control virus-free preparation for 72 h. The surviving parent female aphid and progeny nymphs from each chamber were transferred to individual plantain plants to establish a colony.
fFifth instars aphid were injected with 2.3 ± 0.2 nL of virus or control suspensions in Schneider's cell culture medium. The DplDNV virus preparation contained 1 ng/mL of DplDNV DNA; the RAAV virus preparation contained 5 ng/μL of RAAV RNA; and the control virus-free suspension was isolated from virus-free aphids using the same method as that for virus isolation.
Characteristics of the Aphid Clones.
Clone WS contained 2 phenotypes: dark with developing wing buds, and light (Fig. 1A), both of which were smaller than the virus-free aphids of clone 2D. The levels of DplDNV in the dark aphids were 1,000–10,000× higher than in the light aphids (Figs. 1B and 2G, Fig. S1F, and Table S3). The low levels of DplDNV observed in the light aphids, which were higher than the detection threshold, could have been the result of either early-stage infection or surface contamination. It is also possible that some individuals can resist DplDNV replication because of differences in immune response. Indeed, the DplDNV-free clone R3 originated from a nymph of a light aphid adult from clone WS. When 10 light aphids from clone WS with low DplDNV levels were used to establish a colony, the resulting colony contained both dark and light aphids with high and low levels of DplDNV, respectively, after 2 weeks of rearing (Table 2). Significantly, dark aphids with the high levels of DplDNV produced nymphs, which developed into both dark (high DplDNV levels) and light (low DplDNV levels) phenotypes (Table 2). The proportion of dark and light aphids in individual colonies from clone WS was variable. Usually, the proportion of the dark aphids (with high levels of DplDNV) was lower in younger colonies compared with more established colonies.
Fig. 2.
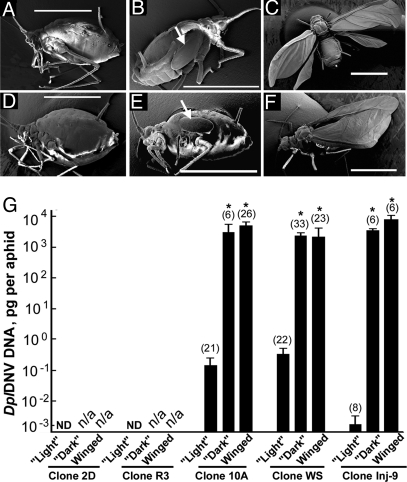
DplDNV-induced wing development in asexual clones of the rosy apple aphid. Scanning electron microscopy. (A) Adult aphid from clone 2D (virus free). (D) Adult aphid from clone R3 (RAAV infected). Aphids from clone 10A (DplDNV infected): (B) fourth instar with wing buds and (C) winged adult. Aphids from the clone WS (DplDNV and RAAV infections): (E) fourth instar with wing buds and (F) winged adult. Wing buds are indicated with arrows. (Scale bar: 1 mm.) (G) Accumulation of DplDNV DNA in individual light, dark (with wing buds), and winged aphids. ND, nondetectable levels of DplDNV DNA; n/a, not applicable. Clone Inj-9 was produced from a single aphid from clone 2D injected with a DplDNV virus preparation; the progeny was sampled 4 weeks postinjection. Bars depict the accumulation of DplDNV DNA (mean ± standard error) in individual aphids. Numbers of the sampled aphids are shown in parentheses. Values without significant difference (ANOVA) are indicated with asterisks (*).
Table 2.
Reproduction rate, wing development, and DplDNV infection in rosy apple aphid clones
| Clonea | Aphidsb | Wingedc | DplDNVd | High DplDNV levele |
|
|---|---|---|---|---|---|
| Wingless | Winged | ||||
| 2D | 1,550 | 0 | − | 0/20 | n.a. |
| R3 | 1,050 | 0 | − | 0/20 | n.a. |
| 10A | 335 | 10 | + | 6/20 | 9/10 |
| WS light | 1,382 | 39 | + | 5/20 | 4/10 |
| WS dark | 917 | 10 | + | 3/20 | 5/10 |
aTen adult aphids were placed on 20-cm-high plantain plants at 20 °C ± 1 °C on a 16/8-h light/dark cycle. Figures refer to day 14 after start of experiment.
bTotal number of parent and progeny aphids.
cNumber of aphids with fully developed wings.
dDplDNV DNV was tested by qPCR in the pooled samples extracted from 100 randomly selected wingless aphids.
eNumber of aphids with more than 10 pg of DplDNV DNA per aphid per number of aphids tested.
Aphids from clone 10A (infected with DplDNV but RAAV free) were smaller and darker than the virus-free aphids of the clone 2D (Fig. 1A). All of the darker aphids from clone 10A had developing wing buds, as well as high levels of DplDNV, equivalent to those for the dark aphids in the clone WS. The remainder of the aphids from clone 10A had much lower levels of DplDNV, similar to those in the light aphids of the clone WS (ANOVA, LSD test, P < 0.05; Fig. 1B). In aphids from clone Inj-9, originating from a single aphid from clone 2D after injection with a DplDNV virus preparation, the levels of DplDNV accumulation in dark aphids from the resulting progeny were similar to those in clone 10A (Fig. 1B). RAAV infection did not have a significant impact on the accumulation of DplDNV (Fig. 1B; ANOVA, LSD test, P < 0.05).
The levels of RAAV in both light and dark aphids from clone WS were similar (Fig. 1B). Interestingly, the levels of RAAV in the aphids from the DplDNV-free clone R3 were slightly lower than in the clone WS (ANOVA, LSD test, P < 0.05; Fig. 1B). Therefore, we cannot exclude the possibility that DplDNV may have a positive effect on RAAV replication.
DplDNV infection resulted in increased movement and local dispersal of wingless aphids. Though the DplDNV-free aphids (clones 2D and R3) congregated at the plant base (Fig. 3A), the DplDNV-infected aphids (clones WS and 10A) dispersed over the whole plant (Fig. 3 B and C). In triplicate experiments, 10 aphids from each clone were placed at the base of 15-cm-long plantain leaves and allowed to establish colonies. The numbers of aphids were recorded for the upper and the lower (up to 5 cm from the leaf base) parts of the leaves from each plant. A significant difference was observed between aphid numbers for DplDNV-infected and DplDNV-free clones found in the upper areas of the leaves (ANOVA, LSD test, P < 0.05). In the case of DplDNV-free clones 2D and R3, 7.0 ± 2.08 and 6.0 ± 1.53 aphids (which was 2.67% ± 0.79% and 2.40% ± 0.65% of the total aphid number on a plant) were located in the upper areas, which was lower than in the case of DplDNV-infected clones 10A and WS, 23 ± 2.65 and 20.3 ± 1.2 aphids (45.18% ± 6.34% and 27.48% ± 4.71% of the total aphids on a plant; Fig. S3).
Fig. 3.
Dispersal of DplDNV-infected aphids. Plantain 2 weeks postinfestation with 10 aphids from clones (A) R3 (localized) and (B and C) WS (dispersed). (Scale bars: 1 cm.)
Effect of Virus Infection on Rosy Apple Aphid Fecundity.
The DplDNV had a negative effect on fecundity in the case of both single DplDNV infection and mixed infection (DplDNV with RAAV). Plant aphid propagation experiments showed that there was a significant reduction in the offspring from clones infected with DplDNV (10A and WS) compared with aphids from uninfected clone 2D and RAAV-infected clone R3 (Tables 2 and 3; ANOVA, LSD test, P < 0.05). Such a reduction in the production of nymphs in DplDNV-infected clones could be the result of either a pathological effect of DplDNV infection on reproduction or a consequence of the high proportion of insects undergoing wing development. Indeed, it has been reported that the number of nymphs produced by alates is lower than that of apterae (5).
Table 3.
Effect of DplDNV and RAAV on the reproduction and dispersal of rosy apple aphids in a controlled environmenta
| Clone (viruses) | Dispersalb | Donor plantc | Recipient plantd | Wingede | % dark aphidsf |
|---|---|---|---|---|---|
| 2D (none) | 0/5 | 215.8 ± 45.8 | 0 | 0 | 0 |
| R3 (RAAV) | 0/5 | 163.6 ± 49.5 | 0 | 0 | 0 |
| 10A (DplDNV) | 5/5 | 71.2 ± 14.3 | 11.4 ± 3.5 | 12.6 ± 3.6 | 35.4 ± 7.1 |
| WS (DplDNV, RAAV) | 3/5 | 55.4 ± 25.0 | 9.6 ± 5.7 | 3.4 ± 0.7 | 11.1 ± 2.6 |
aFive cages were used for each aphid clone. Ten aphids were placed on donor plants in each cage and reared at 20 °C ± 1 °C on a 16/8-h light/dark cycle. All figures in table refer to day 11 after start of experiment.
bNumber of cages where colonization of the recipient plant took place/total number of cages.
cNumber of aphids on donor plants (mean ± standard error).
dNumber of aphids on recipient plants (mean ± standard error), only for infested plants.
eNumber of winged aphids (including winged aphids on both plants, and in the cage but not on the plants).
fPercentage of dark-colored aphids in cage, except aphids with developed wings (mean ± standard error).
DplDNV Induces Development of the Winged Morph in Asexual Clones of the Rosy Apple Aphid.
Winged morphs are essential for aphid dispersal, and it is reported that they are produced in response to high population densities and poor plant quality (1). No winged morphs were observed in the case of the virus-free clone 2D and the RAAV-infected clone R3, even at high population densities and poor plant quality when reared under long-day conditions. However, our rosy apple aphid clones, including 2D and R3, all produced sexual winged morphs under short-day “autumn” conditions (8 h light/16 h dark, +15 °C). Under long-day conditions, winged rosy apple aphids (Fig. 2 C and F) and aphids with wing buds (Fig. 2 B and E) were found only in clones, which were infected with DplDNV, clones WS and 10A (Fig. 1B; Table S3). Also, the infection of virus-free clone 2D with DplDNV by injection, artificial feeding, or via plant tissue resulted in the induction of the winged morph production, which was observed first approximately 2 weeks postinoculation (Table 1 and Fig. 1B). Regardless of how DplDNV was introduced, aphids with high levels of DplDNV from clones WS, 10A, and Inj-9 reared under long-day conditions were darker and showed the presence of developing wing buds (Fig. 2 B, E, and G) or had wings (Fig. 2 C, F, and G). There was no significant difference in DplDNV DNA accumulation between these groups (ANOVA; Fig. 2G). All aphids without wing buds or wings in the same clones were lighter and had low levels of DplDNV (Figs. 1B and 2G). This was also observed in the DplDNV-free clones 2D and R3 (Fig. 2 A, D, and G). The proportion of the aphids with high levels of DplDNV (dark fourth instars with wing buds and the winged aphids) was significantly higher in clone 10A (50.72% ± 4.14%) than in clone WS (24.98% ± 3.99%) (ANOVA, LSD test, P < 0.05; Table 3 and Table S5). We suggest that the difference in DplDNV infection rates in clones 10A and WS (affecting the proportion of dark and winged aphids in the population) may be attributed to the presence of RAAV in clone WS. It may be possible that RAAV reduces the infectivity of DplDNV in mixed infections by reducing the rate of transmission and ultimately the proportion of dark and winged aphids in the population.
Wing development in DplDNV-infected aphids is not induced by crowding but occurs in DplDNV-infected aphids from the clones 10A and WS even when reared singly from the first instar on detached leaves. No wing development was observed in aphids from DplDNV-free clones 2D and R3 under the same conditions (Table S6).
DplDNV-Induced Dispersal of Rosy Apple Aphid.
The presence of the winged morph exclusively in DplDNV-infected clones of rosy apple aphid prompted us to test whether these aphids had an increased ability to disperse and colonize neighboring plants. Tests were carried out under controlled environmental conditions, as well as in the field. In the controlled environment experiments, we tested the ability of aphids to fly from one plant to another inside an insect-proof chamber (Table 3 and Fig. S4A). One plant was infested with 10 aphids (adults or fourth instars) and acted as the aphid donor plant; the other plant, located 85 cm apart, was the recipient plant. Five replicate chambers were set up for each of the rosy apple aphid clones. After 11 days under long-day conditions, colonization of the recipient plants occurred in all 5 chambers where the donor plant was infested with clone 10A (DplDNV-infected). In the case of clone WS (DplDNV and RAAV infected), colonization of the recipient plants was observed in 3 of 5 chambers. No dispersal was observed in the 5 chambers where the donor plants were infested with aphids from virus-free clone 2D or the RAAV-infected clone R3 (Table 3 and Table S5).
We also assessed the effect of plant quality on wing production under controlled environmental conditions. The experiment was set up using the same design, except that the donor plants were deprived of water after placing the aphids on the donor plant. By day 11 the majority of donor plants had died. We observed colonization of 3 of 5 recipient plants in the case of clones WS and 10A. No colonization took place in the case of clones 2D and R3.
The field dispersal test involved 4 outdoor chambers. Each chamber contained a donor plant infested with one of the model clones (2D, 10A, R3, or WS). The donor and recipient plants were placed 2 m apart in each chamber (Fig. S4 B and C). The recipient plants were colonized by aphids after 18 days in the case of the DplDNV-infected clones 10A and WS, but not in the case of clones R3 and 2D, which were free from DplDNV. DplDNV infection was detected in the aphids from the new colonies established on the colonized plants in the case of clones 10A and WS.
Discussion
There is limited information on the physiological and ecological impacts of viruses on insects, in particular those that cause sublethal infections, due to the paucity of studies on insect viral diversity. We used molecular screening to determine virus diversity in the rosy apple aphid, which resulted in the identification of 2 previously undescribed viruses, an RNA virus, RAAV, and a DNA virus, DplDNV.
Asexual propagation in aphids is of the ameiotic type and does not involve endomeiosis or internal chromosomal recombination (21–23). Therefore, it is possible to produce and maintain rosy apple aphid clones with identical genotypes that are infected with different combinations of viruses. A surprising result was the difference in the development of the winged morph in virus-infected and virus-free cultures. Crowding and poor quality diet was reported as cues responsible for inducing wing development in asexual aphids as early as the 1920s (24). However, these cues are not universal inducers for wing development in all aphid species or even in different lines of the same species (4, 5). We found that winged morphs are not produced in clones that are DplDNV free (including RAAV-infected clone R3), even in response to crowding and poor plant quality under long-day conditions. Conversely, winged morphs are produced by aphids with the same genetic background, even at low population density in the presence of DplDNV, either as a single infection (clone 10A) or together with RAAV (clone WS). High levels of DplDNV are detected in clones WS and 10A, exclusively in dark colored aphids (fourth instars), which also have clearly developed wing buds, and in winged adults. We found that though RAAV infection has no effect on the accumulation of DplDNV in individual aphids, there is a possibility that RAAV may lessen the negative impact of DplDNV for the whole colony by inhibiting the development of DplDNV infection in some individuals. Indeed, a reduction in the proportion of dark aphids (all of which had wing buds and high levels of DplDNV) was observed in the case of the clone WS compared with the clone 10A (Table 2). This suggests that the proportion of aphids with wing buds is reduced in the presence of RAAV, which may account for the increased fecundity of clone WS. Alternatively, DplDNV may have a direct effect on fecundity.
Winged morphs are essential for the dispersal of an aphid clone and, ultimately, for its survival. We hypothesize that despite the negative impact of DplDNV on the fecundity of the rosy apple aphid, DplDNV infection has a net positive effect in regard to dispersal. The glasshouse and the field dispersal experiments clearly showed that only the DplDNV-infected aphids from clones WS and 10A were able to produce the winged morph, fly, and colonize new plants (Table 3). In no cases were DplDNV-free aphids found on recipient plants. The increased spread of aphids in DplDNV-infected clones on the host plant (Fig. 3 B and C) may be more advantageous to the virus rather than the aphid. Behavioral changes aiding virus dissemination have been reported for virus infections in other orders of insects (25). Even vertebrate viruses cause changes in behavior (e.g., rabies virus), which are advantageous for the dissemination of the virus (26).
We propose the following model for the role of a virus in aphid dispersal. The progeny from a single winged aphid infected with both RAAV and DplDNV includes insects with both high and low DplDNV levels. The higher fecundity of the aphids with the lower levels of DplDNV leads to an increase in the proportion of these aphids on a plant. However, because the rate of horizontal transmission for DplDNV increases with the population density, the proportion of the aphids with high levels of DplDNV also increases. These virus-infected aphids are likely to develop wings and colonize neighboring plants. Density-dependent induction of the winged morph development has been reported for asexual clones of other aphid species (1, 2, 4) and may also be the result of the increased incidence of virus due to increased horizontal transmission under conditions of crowding. Densovirus ESTs were derived from aphid laboratory cultures with winged asexual females (Table S1 and Table S2, and Fig. S2), suggesting that densovirus-induced wing formation may occur in other aphid species.
The induction of winged morph development as a result of virus infection offers some advantages. All of the offspring from an asexually reproducing aphid clone are genetically identical and will respond in a similar way to changes in external factors. However, if a virus infection is present in a proportion of individuals in a colony, any resulting epigenetic changes may modify their response to environmental cues. Indeed, the nonstructural proteins of vertebrate parvoviruses can activate transcription factors (27) as well as induce epigenetic modification through histone acetylation (28).
Our data suggests that a mutualistic relationship exists between the rosy apple aphid, RAAV, and DplDNV. The subliminal nature of the DplDNV and RAAV infections ensures the survival of both viruses and the host. The DplDNV induces the winged morph and increases mobility, which facilitates the dispersal of the host as well as the viruses. The presence of RAAV decreases the DplDNV-associated loss of the host fecundity. Such interdependence and existence of mutualistic relationships may indicate a long coevolution of the 3 components of this system, which has resulted in the minimization of the virus-induced harm to the host and the development of dependence on a virus for some physiological processes (wing development). Indeed, recent reports have indicated that viruses may be beneficial to their hosts. For example, a latent herpesvirus infection confers resistance to harmful bacteria in mice and possibly humans by activating the immune system (29). Pathogens, including viruses, are an integral part of natural systems; therefore they are likely to play a role in “normal” physiology.
Materials and Methods
Aphid Rearing.
The clones of rosy apple aphid, Dysaphis plantaginea (Passerini) (Hemiptera: Aphididae), were reared on Plantago longifolia (plantain) in isolated growth chambers at 20 °C ± 1 °C under long-day conditions, 16/8-h light/dark cycle. In artificial diet experiments, aphids were placed in the chambers containing artificial aphid diet (Bio-Serv Inc.) under stretched Parafilm.
Virus Discovery and Sequencing.
Virus screening and sequencing were carried as described previously (6, 7). The amplifications of the full-length RAAV cDNA and the complete coding sequence of DplDNV were carried out with Phusion DNA polymerase (Finnzyme) using cDNA to RNA and DNA, which were extracted from virus preparations from clone WS, and the primers 5′-GCTATAATACGACTCACTATAGGCGAAAATAAGTATATATTGCTTTTATTTCG-3′ and 5′-CGGTGTTTAAAC(T)27ATTTTGCCCAAAATATGCTTTGCATAAACTATATAC-3′ for RAAV or the primer 5′-GAACAAGTAACCAGTCGTAAGGTGC-3′ for DplDNV. Mapping of the DplDNV transcripts included amplification of the fragments of the DplDNV mRNA with a series of primer pairs covering the DplDNV genome, using the cDNA to DNAseI-treated total RNA extracted from the DplDNV-infected aphids. The 3′ termini of the DplDNV transcripts were mapped using a 3′-RACE RLM kit (Ambion).
Virus Detection and Quantification.
The total RNA and DNA samples were isolated from the individual aphids, either fourth instars or adults, using the AllPrep DNA/RNA/Protein Mini Kit (Qiagen). The cDNA to the total RNA was synthesized using the random hexanucleotides and SuperScript II Reverse Transcriptase Kit (Invitrogen). Detection of RAAV was carried out using the cDNA to total RNA with the primers 5′-ATGAGCGGCGCGCCAATGAATAGATCGGCTCCTAATAAC-3′ and 5′-CACCATTGCTGAGGAaaagtttaaagaataacctttctttg-3′. Detection of DplDNV was carried out using total DNA extracts with the primers 5′-GAAAGCGGAGGTTCAAATGCAAGAC-3′ and 5′-GAACCAGTTTGTCGACAATTG-3′, which flank the intron region in the nonstructural protein gene of DplDNV. PCR was performed using Taq polymerase (Roche). Amplification included 5 min at 94 °C and 35 cycles (94 °C for 30 sec, 53 °C for 1 min, 72 °C for 1 min). Real-time quantitative PCR was performed in duplicate using the SYBR Green kit (Eurogentech). RAAV RNA was quantified using the cDNA with the primers 5′-AGAGAACGGAGTTGTTTATTACTACGAA-3′ and 5′-TATGGAAATACCATCTTGGGAGTTG-3′. The DplDNV DNA was quantified from total DNA with the primers 5′-CGCCCGCGTAAATGGATATTATGGCG-3′ and 5′-GATGGTCGTGACGCTGTTGTTT-3′. Amplification was performed in 20-μL reactions using ABI Prism 7900HT system (Applied Biosystems) and included 2 min at 50 °C, 10 min at 95 °C, and 40 cycles (95 °C for 15 sec, 60 °C for 1 min). Viral DNA and RNA levels were determined using the comparative Ct analytical method with a cloned part of DplDNV genomic DNA and an in vitro RNA transcript produced from the cloned cDNA to genomic RNA of RAAV as reference standards for qPCR detection of DplDNV and qRT-PCR detection of RAAV, respectively.
Microinjection.
The virus and control preparations were resuspended in Schneider's cell culture medium and injected into the hemolymph of fifth instars of rosy apple aphid using the Femtojet (Eppendorf). Approximately 2.3 ± 0.2 nL of the virus suspension (DplDNV concentration 1 ng/μL or RAAV concentration 5 ng/μL) was administered using a capillary inserted in the lower side of insect abdomen using a compensatory pressure of 20 hPa, five 1-sec pulses, and an injection pressure of 60 hPa.
Aphid Dispersal.
Dispersal tests in a controlled environment were carried out at 20 °C ± 1 °C under 16/8-h light/dark cycles. Each pair of the donor and the recipient plantain plants, ≈10 cm high, were located 85 cm apart within a separate insect-proof chamber, 100 cm long, 30 cm high, and 20 cm wide, made from transparent plastic with nylon mesh-covered openings on 2 sides to ensure access and ventilation (Fig. S4A). Potted plants were placed in water traps to prevent movement of aphids from one plant to another by crawling. Watering of the plants and filling of the water traps was carried out through built-in piping.
The field dispersal tests were carried out in Warwickshire, U.K., from June 13 to July 1, 2008. The donor plantain plant with an aphid culture (which was developed for 2 weeks from 10 aphids) was placed at a distance of 2 m from the recipient plantain plant. The donor and recipient plants were placed inside an insect-proof tent-shaped chamber made of a nylon mesh, 2.5 m long, 1 m wide, and 0.85 m high (Fig. S4 B and C). The plants were placed inside water traps to prevent aphids from crawling between plants (Fig. S4C).
Microscopy.
For transmission electron microscopy, the virus suspension deposited on a carbon-coated grid was negatively stained with 2% potassium phosphotungstate (pH 7.0). For scanning electron microscopy, the anesthetized aphids were attached to the stub with carbon paste, cryofixed and gold coated on a cryostage, and viewed in the frozen hydrated state.
Supplementary Material
Acknowledgments.
We thank Dr. Julie Jones for statistical analysis and Miss Charline Alenda for her assistance in aphid dispersal experiments. This work was supported by grants to Warwick HRI from the Biotechnology and Biological Sciences Research Council, U.K., and the Department for Environment, Food, and Rural Affairs, U.K.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EU851411, FJ040397, and DQ286292).
This article contains supporting information online at www.pnas.org/cgi/content/full/0901389106/DCSupplemental.
References
- 1.Dixon AFG. Aphid Ecology. London: Chapman and Hall; 1998. [Google Scholar]
- 2.Zera AJ, Denno RF. Physiology and ecology of dispersal polymorphism in insects. Annu Rev Entomol. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa A, Hongo S, Miura T. Morphological and histological examination of polyphonic wing formation in the pea aphid Acyrthosiphon pisum (Hemiptera, Hexapoda) Zoomorphology. 2008;127:121–133. [Google Scholar]
- 4.Müller CB, Williams IS, Hardie J. The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol Entomol. 2001;26:330–340. [Google Scholar]
- 5.Braendle CG, Davis K, Brisson JA, Stern DL. Wing dimorphism in aphids. Heredity. 2006;97:192–199. doi: 10.1038/sj.hdy.6800863. [DOI] [PubMed] [Google Scholar]
- 6.Sloggett JJ, Weisser WW. Parasitoids induce production of the dispersal morph of the pea aphid, Acyrthosiphon pisum. Oikos. 2001;98:323–333. [Google Scholar]
- 7.Gildow FE. Increased production of alate by aphids (Hemiptera, Aphididae) reared on oats infected with Barley yellow dwarf virus. Ann Entomol Soc Am. 1980;73:343–347. [Google Scholar]
- 8.Hodge S, Powell G. Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance? Environ Entomol. 2008;37:1573–1581. doi: 10.1603/0046-225x-37.6.1573. [DOI] [PubMed] [Google Scholar]
- 9.Fereres A, Moreno A. Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 2009;141:158–168. doi: 10.1016/j.virusres.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 10.van Munster M, et al. A new virus infecting Myzus persicae has a genome organization similar to the species of the genus Densovirus. J Gen Virol. 2003;84:165–172. doi: 10.1099/vir.0.18650-0. [DOI] [PubMed] [Google Scholar]
- 11.van Munster M, et al. Sequence analysis and genomic organization of Aphid lethal paralysis virus: A new member of the family Dicistroviridae. J Gen Virol. 2002;83:3131–3138. doi: 10.1099/0022-1317-83-12-3131. [DOI] [PubMed] [Google Scholar]
- 12.Moon JS, Domier LL, McCoppin NK, D'Arcy CJ, Jin H. Nucleotide sequence analysis shows that Rhopalosiphum padi virus is a member of a novel group of insect-infecting RNA viruses. Virology. 1998;243:54–65. doi: 10.1006/viro.1998.9043. [DOI] [PubMed] [Google Scholar]
- 13.Ryabov EV. A novel virus isolated from the aphid Brevicoryne brassicae with similarity to picorna-like viruses infecting Hymenoptera. J Gen Virol. 2007;88:2590–2595. doi: 10.1099/vir.0.83050-0. [DOI] [PubMed] [Google Scholar]
- 14.Van der Wilk F, Dullemans AM, Verbeeck M, Van den Huevel JFJM. Nucleotide sequence and genome organisation of Acyrthosiphon pisum virus. Virology. 1997;238:353–362. doi: 10.1006/viro.1997.8835. [DOI] [PubMed] [Google Scholar]
- 15.Ban L, Ahmed E, Ninkovic V, Delp G, Glinwood R. Infection with an insect virus affects olfactory behaviour and interactions with host plant and natural enemies in an aphid. Entomologia Experimentalis et Applicata. 2008;127:108–117. [Google Scholar]
- 16.Allander T, et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tattersall P, et al. Family Parvoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. London: Elsevier/Academic; 2005. pp. 353–369. [Google Scholar]
- 18.Ban L, Didon A, Jonsson LMV, Glinwood R, Delp G. An improved detection method for the Rhopalosiphum padi virus (RhPV) allows monitoring of its presence in aphids and movements within plants. J Virol Meth. 2007;142:136–142. doi: 10.1016/j.jviromet.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Gildow FE, D'Arcy CJ. Barley and oats as reservoirs for an aphid virus and the influence on barley yellow dwarf virus transmission. Phytopathology. 1988;78:811–816. [Google Scholar]
- 20.Ofori FA, Francki RIB. Transmission of leafhopper A virus, vertically through eggs and horizontally through maize in which it does not multiply. Virology. 1985;144:152–157. doi: 10.1016/0042-6822(85)90313-7. [DOI] [PubMed] [Google Scholar]
- 21.Blackman RL. Stability and variation in aphid clonal lineages. Biol J Linn Soc Lond. 1979;11:259–277. [Google Scholar]
- 22.Blackman RL. Reproduction, cytogenetics and development. In: Minks AK, Harrewijn P, editors. Aphids: Their Biology, Natural Enemies and Control. Amsterdam: Elsevier; 1987. pp. 163–195. [Google Scholar]
- 23.Hales DF, et al. Lack of detectable genetic recombination on the X chromosome during the parthenogenetic production of female and male aphids. Genet Res. 2002;79:203–209. doi: 10.1017/s0016672302005657. [DOI] [PubMed] [Google Scholar]
- 24.Wadley FM. Factors affecting the proportion of alate and apterous forms of aphids. Ann Entomol Soc Am. 1923;16:229–232. [Google Scholar]
- 25.Kamita SG, et al. A baculovirus-encoded protein tyrosine phosphatase gene induces enhanced locomotory activity in a lepidopteran host. Proc Natl Acad Sci USA. 2005;102:2584–2589. doi: 10.1073/pnas.0409457102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu ZF, Jackson AC. Neuronal dysfunction and death in rabies virus infection. J Neurovirol. 2005;11:101–106. doi: 10.1080/13550280590900445. [DOI] [PubMed] [Google Scholar]
- 27.Duechting A, et al. Human parvovirus B19 NS1 protein modulates inflammatory signalling by activation of STAT3/PIAS3 in human endothelial cells. J Virol. 2008;82:7942–7952. doi: 10.1128/JVI.00891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iseki H, et al. Parvovirus nonstructural proteins induce an epigenetic modification through histone acetylation in host genes and revert tumor. J Virol. 2005;79:8886–8893. doi: 10.1128/JVI.79.14.8886-8893.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton ES, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.