Abstract
A reduction in dopaminergic innervation of the subventricular zone (SVZ) is responsible for the impaired proliferation of its resident precursor cells in this region in Parkinson's disease (PD). Here, we show that this effect involves EGF, but not FGF2. In particular, we demonstrate that dopamine increases the proliferation of SVZ-derived cells by releasing EGF in a PKC-dependent manner in vitro and that activation of the EGF receptor (EGFR) is required for this effect. We also show that dopamine selectively expands the GFAP+ multipotent stem cell population in vitro by promoting their self-renewal. Furthermore, in vivo dopamine depletion leads to a decrease in precursor cell proliferation in the SVZ concomitant with a reduction in local EGF production, which is reversed through the administration of the dopamine precursor levodopa (l-DOPA). Finally, we show that EGFR+ cells are depleted in the SVZ of human PD patients compared with age-matched controls. We have therefore demonstrated a unique role for EGF as a mediator of dopamine-induced precursor cell proliferation in the SVZ, which has potential implications for future therapies in PD.
Keywords: adult neural progenitor cell, Parkinson's disease, FGF2, EGF receptor, L-DOPA
The ability of neural stem and progenitor cells in the adult brain to continually proliferate and generate neuronal precursors is of great significance, because manipulation of this endogenous process may stimulate the replacement of cells lost as a consequence of disease. In the adult mammalian brain, the subventricular zone (SVZ) lining the lateral ventricles is 1 of the 2 primary sites of adult neurogenesis (1, 2), and it is in this niche that the first step in the process of neurogenesis (proliferation) occurs, involving neural stem cells (B cells), that proliferate slowly, giving rise to transit-amplifying progenitor cells (C cells) (3). Several locally-acting diffusible molecules, such as EGF, control proliferation in the SVZ (4–7). EGF influences SVZ expansion by binding to the EGF receptor (EGFR) that is present on “activated B cells” and rapidly dividing C cells (8).
The adult SVZ is innervated by dopaminergic fibers that originate in the substantia nigra (9, 10). These dopaminergic projections extending to the SVZ, predominantly contact the C cells and regulate their proliferative capacity (9). Thus in Parkinson's disease (PD), a dramatic reduction in SVZ precursor cell proliferation occurs as a consequence of dopamine depletion. However, the mechanism by which this occurs is unknown, but given that both dopamine and EGF receptors are coexpressed on the C cell, we sought to investigate the hypothesis that EGF was critical to this process. Using a range of in vitro and in vivo studies, we have now shown that dopamine stimulates the release of EGF from cells in the SVZ, which in turn acts on the EGFR to promote proliferation, and that EGFR expression in the SVZ is significantly depleted in PD patients.
Results
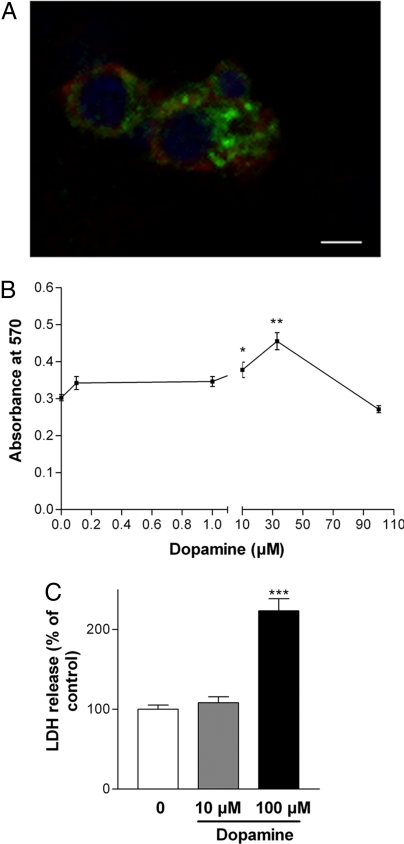
Adult SVZ-derived neural precursor cells (NPCs) displayed clear colocalization for the high-affinity dopamine receptor, the D2-like (D2L) receptor, and the EGFR, which suggests that dopamine and EGF could interact on the same cell via their respective receptors (Fig. 1A). The presence of the D2L receptor led us to examine the effect of dopamine on proliferation. To determine the optimal dose to administer, we set up a dose–response assay to dopamine treatment (0.1–100 μM) given over 4 days using adult SVZ cultures. Cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and lactate dehydrogenase (LDH) assays. Results show that at higher concentrations dopamine was toxic to the cells (Fig. 1 B and C); whereas at 10 μM it had a positive effect on cell viability (P < 0.05 and P < 0.01). However, MTT and LDH assays do not differentiate between survival and proliferation, so to assess the effect of dopamine on proliferation we used a BrdU labeling index. At first passage neurospheres were treated with dopamine (10 μM) daily for 4 days and pulsed with BrdU in the final 24 h. Dopamine addition resulted in a significant increase in BrdU-labeled cells (P < 0.01) (Fig. 2 A and B).
Fig. 1.
Adult SVZ-derived cells are responsive to dopamine receptor stimulation. Immunolabeling of expanded adult SVZ-derived cultures. (A) D2L receptor (green) and EGFR (red) demonstrates colocalization; Hoescht nuclei are blue. (Scale bar: 60 μm.) (B and C) Cell viability of cultures exposed to dopamine at differing concentrations assessed by MTT assay (B) and LDH assay (C). *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus control cultures. Values in the LDH assay are expressed as the mean ± SEM % of control levels.
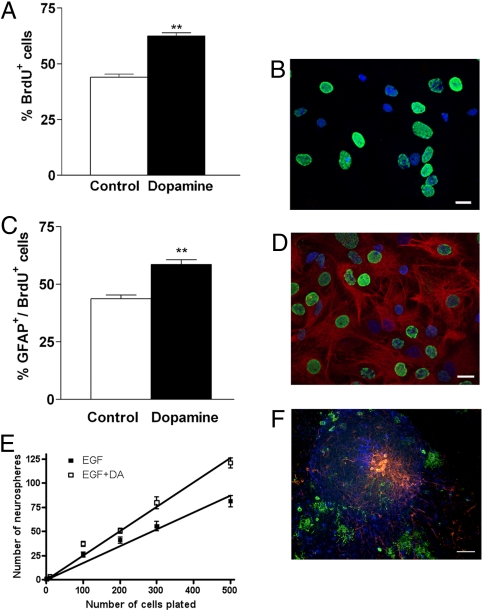
Fig. 2.
Dopamine increases proliferation of adult SVZ-NSCs in vitro. (A) The number of BrdU+ cells in dopamine-treated cultures was significantly increased compared with control cultures. (B) Representative photomicrograph of BrdU+ (green) and hoescht+ cells (blue) after dopamine stimulation. (Scale bar: 60 μm). (C) Significant increase in the number of newborn cells adopting a GFAP+ phenotype after dopamine exposure. (D) Representative photomicrograph of GFAP+ (red), BrdU+ (green), and hoescht+ cells (blue). *, P < 0.05; **, P < 0.01. (Scale bar: 60 μm.) (E) Clonal analysis of dopamine-responsive NSCs using limiting dilution analysis, with starting cell numbers ranging from 1 to 500 per well. The slope of the line reflects the proportion of cells plated that form neurospheres, i.e., display NSC characteristics. Linear regression values show that dopamine-treated cultures contain significantly more NSCs compared with untreated control cultures (***, P < 0.001). (F) Triple immunolabeling of a single neurosphere derived from dopamine-responsive NSCs demonstrate multipotentiality with the genesis of neurons (red), astrocytes (blue), and oligodendrocytes (green). (Scale bar: 160 μm.)
We next examined the effect of dopamine on the fate of newborn cells. Results demonstrated that the significant increase in proliferation in response to dopamine was paralleled by a significant increase in cells with a newborn astrocytic phenotype (Fig. 2C), as demonstrated by GFAP immunostaining (Fig. 2D). There was no significant change in β-III-tubulin-positive newborn neurons or O4-positive newborn oligodendrocytes in vitro (Fig. S1).
GFAP is both an astrocytic marker and a marker for multipotent NPCs/stem cells (3). To investigate the potential actions of dopamine on neural stem cell (NSC) behavior, we used the fact that the identification of NSCs is predominantly based on assays identifying NSCs as neurosphere-initiating cells (11). Thus, dopamine-treated and untreated adult SVZ-derived cultures were examined for their ability to generate neurospheres using a limiting dilution assay (12). Cells were seeded at a range of plating densities from 1 to 500 cells per well into 96-well plates, and the frequency of neurospheres generated 7 days later was counted. The slope of the best-fit line used to calculate the frequency of sphere-forming cells showed that neurosphere formation was significantly greater when cultures were stimulated with dopamine (Fig. 2E). In addition, the percentage of single cells that formed neurospheres demonstrated that clonal efficiency was increased in the presence of dopamine (Fig. S2). To further confirm that dopamine enriches for NSCs, the multipotentiality of dopamine-treated neurospheres was examined. Triple-labeling demonstrated the presence of neurons, astrocytes, and oligodendrocytes (Fig. 2F), further supporting a role for dopamine in increasing proliferation by promoting self-renewing divisions of stem/progenitor cells.
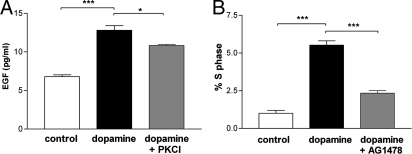
To address the mechanisms by which dopamine enhances neurosphere formation, we examined its possible interaction with EGF. Results show that dopamine, measured by ELISA, almost doubled the release of EGF from adult NPCs after 24 h of treatment (P < 0.001) (Fig. 3A). FGF2 is another major growth factor that is present in the SVZ and known to promote the proliferation of dividing cells in this region. We therefore next sought to examine the possible interaction of dopamine and FGF2 in neurospheres derived from the adult SVZ and found that stimulation of neurospheres with dopamine did not cause a release of FGF2 from these cultures (Fig. S3).
Fig. 3.
Dopamine stimulates the release of EGF and EGFR activation from adult SVZ NPCs. (A) EGF release after 24-h treatment with dopamine ± PKC inhibitor, compared with control using an ELISA. ***, P < 0.001; *, P < 0.05. (B) Cell cycle analysis of NPCs using propidium iodide shows that the percentage of cells in S phase treated with dopamine alone is increased and lost when used in combination with an EGFR inhibitor (AG1478). ***, P < 0.001.
EGF release via G protein-coupled receptor agonists has been shown to require signaling through the PKC pathway (13). We therefore sought to establish whether the observed EGF release, driven by dopamine, occurs via the PKC pathway. Inhibition of this pathway decreased the amount of dopamine-stimulated EGF release by one-third compared with control levels (Fig. 3A). Thus, taken together we show that secretion of EGF, but not FGF2, from NPCs is enhanced by dopamine stimulation in a PKC-dependent manner, although through which exact PKC isotype remains unresolved.
To investigate whether dopamine-induced EGF release acts to promote NPC proliferation, we assessed the effect of dopamine on cell cycle dynamics in the presence and absence of AG1478, a specific EGFR blocker (14). As expected, dopamine-treated cultures showed an increase in the percentage of cells in the S phase of the cell cycle after 12 h compared with the untreated control cultures (P < 0.001). Treatment of dopamine-stimulated cells with AG1478 markedly reduced the dopamine-induced increase in proliferation (P < 0.001) (Fig. 3B). Thus, dopamine causes EGF release from precursor cells, which activates its high-affinity EGFR and thereby drives proliferation.
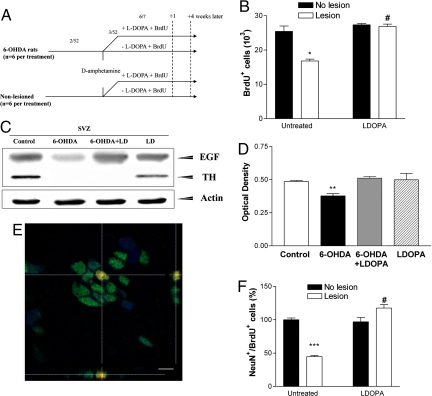
To examine the role of dopamine in vivo we produced an irreversible unilateral depletion of dopaminergic fibers innervating the SVZ by using 6-hydroxydopamine (6-OHDA). Three weeks postlesion, animals were examined for SVZ NPC proliferation by using BrdU (Fig. 4A) and in agreement with other studies dopaminergic denervation led to a significant reduction in the number of BrdU-labeled cells (P < 0.01) when compared with control rats (Fig. 4B) or the nonlesioned hemisphere (9, 10). We then treated lesioned animals with levodopa (l-DOPA) for the same period as BrdU and demonstrated that NPC proliferation in the SVZ had returned to normal (P < 0.001) (Fig. 4B). The control animals treated with l-DOPA showed no significant difference in proliferation. To ensure that l-DOPA was converted to dopamine in lesioned animals, we examined the concentration of dopamine in the striatum of lesioned animals treated with l-DOPA compared with untreated lesioned animals by HPLC. In agreement with other studies (15), we found a significant (4-fold) increase in striatal dopamine concentration after l-DOPA treatment of lesioned animals compared with vehicle-treated, lesioned animals (P < 0.05) (Table S1).
Fig. 4.
Elimination of dopaminergic fibers decreases proliferation in the SVZ caused by reduced local EGF production, and restoration of dopamine levels in the SVZ through l-DOPA treatment restores EGF levels to baseline. (A) Experimental paradigm. The dopaminergic nigrostriatal tract was lesioned by 6-OHDA. Two weeks later, animals were challenged with amphetamine, then1 week later animals received BrdU daily for 6 days, ± l-DOPA for the same 6 days and were killed 1 day or 21 days later. 2/52 and 3/52 represents 2 and 3 weeks later, respectively. 6/7 represents 6 days later. (B) Numbers of BrdU+ cells in the SVZ 1 day after the last BrdU injection. *, P < 0.05 versus control group; #, P < 0.001 versus untreated lesion group. A significant interaction effect was observed between treatment and lesion (***, P < 0.001). (C) Representative Western blots showing a significant decrease in EGF levels in the SVZ of lesioned animals, which could be restored to control levels with l-DOPA. The blotted membrane was reprobed with α-TH to show that the dopaminergic projections in the SVZ were depleted and the α-actin antibody as loading control. (D) Optical densitometry of band intensities for EGF normalized to actin (n = 3 per group) showing that EGF levels in lesioned animals were significantly decreased relative to the other 3 treatment groups. **, P < 0.05. (E) Representative confocal image shows colocalization of BrdU and NeuN in the OB of an l-DOPA-treated lesioned rodent. (Scale bar: 5 μm.) (F) 6-OHDA-lesioned animals show a significant reduction in the percentage of BrdU+ cells expressing NeuN in the OB 21 days after the last BrdU injection (***, P < 0.001). This was restored by l-DOPA (#, P < 0.001 versus untreated lesion group.)
We next wanted to determine whether dopamine had an effect on EGF levels in the SVZ in vivo, so we measured it by Western blot analysis 1 day after their last l-DOPA injection. The complete absence of tyrosine hydroxylase (TH) in the SVZ confirmed the extent of the 6-OHDA-induced dopamine denervation (Fig. 4C). The loss of this projection resulted in a significant decrease in EGF expression in the SVZ compared with control animals (P < 0.01) (Fig. 4 C and D). Dopaminergic replacement through the administration of l-DOPA, restored EGF expression to control levels in the SVZ of lesioned animals (Fig. 4 C and D), but l-DOPA did not alter the EGF levels in the SVZ of control (nonlesioned) animals.
NPCs born in the SVZ migrate out along the rostral migratory stream where they differentiate into neurons in the olfactory bulb (OB) (2, 16, 17). Non-neuronal precursors generate glia and migrate to the striatum, corpus callosum, or neocortex after injury (18). To determine whether the neuronal fate of the newly-generated precursors was affected as a result of alterations in dopamine and EGF levels, lesioned animals with or without l-DOPA and control animals were examined for levels of proliferation and newborn neuronal differentiation in the OB 21 days after the last BrdU injection. As expected, the total number of BrdU-labeled cells in the OB was decreased in the lesioned animals, compared with controls (P < 0.001) (Fig. S4). Using confocal microscopy we confirmed that this also was true for NeuN+/BrdU+ cells (Fig. 4E). Thus, lesioned animals with reduced EGF levels in the SVZ showed a 56% reduction in newborn neurons in the OB compared with control animals, a situation that was reversed back to normal by the administration of l-DOPA (Fig. 4F).
Our in vitro and in vivo experimental data have clearly indicated that NPC proliferation and neurogenesis are impaired in a rodent model of PD, as a consequence of a reduction in dopamine-mediated EGF release from NPCs in the SVZ. To determine whether similar processes occurred in PD, we examined the expression of the EGFR in the SVZ of PD patients and age-matched controls. We confirmed that EGFR+ cells were significantly decreased in the SVZ of these patients (P < 0.0001) in contrast to age-matched controls (Fig. 5) and that those remaining cells that did express the EGFR in the SVZ of PD patients demonstrated a very low expression profile. The subjects studied were 75–83 years of age, had a mini mental state examination (MMSE) score ranging from 12 to 21, had advanced PD (Hoehn and Yahr stage 3–5), and were taking a combination of l-dopa medications. At this stage of disease there is extensive dopaminergic denervation of the striatum and the ability to convert l-dopa to dopamine is compromised, accounting for the on–off phenomena that characterizes this stage of disease. As a result, the ability of the synthesized dopamine to release EGF in these patients would be significantly affected and this coupled with the absence of medication being taken in the agonal stages of the illness would account for the down-regulation of EGFR seen in these patients at postmortem. It would be very interesting to know whether PD patients in the early stages of the disease have similar abnormalities in the face of a more functionally-intact dopaminergic nigrostriatal pathway.
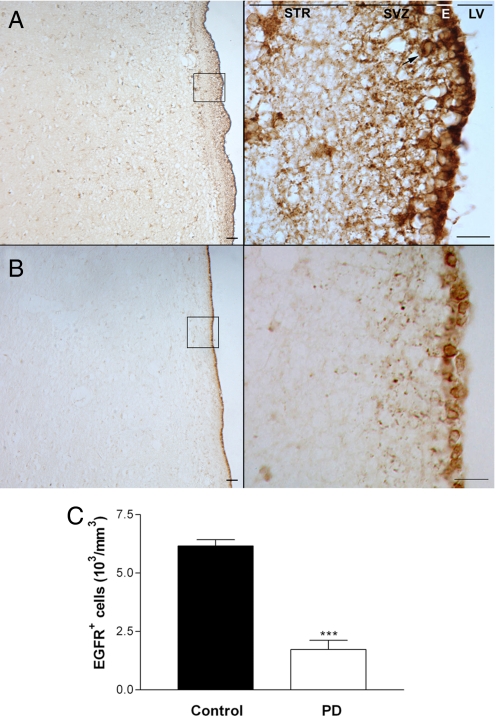
Fig. 5.
The number of EGFR+ cells in the SVZ of PD patients is significantly decreased compared with age-matched controls. (A) EGFR+ cells are present in the human SVZ, as demonstrated by a representative control human SVZ section at 10× (Left) and 63× (Right) magnification. (Scale bar: 60 μm.) (B) A representative SVZ section from a patient that died with PD at 10× (Left) and 63× (Right) magnification. The arrow points to an EGFR+ cell. STR, striatum; E, ependymal layer; LV, lateral ventricle. (C) The number of EGFR+ cells is significantly decreased in the SVZ of PD patients compared with age-and sex-matched controls (n = 6 per group). ***, P < 0.001.
Discussion
The present study has provided extensive evidence for a role for EGF as a key mediator of dopamine-induced neurogenesis in the adult SVZ, which in turn may have implications for PD. In vitro we have shown that EGF (but not FGF2), an endogenous regulator of SVZ proliferation, is released by NPCs in response to dopamine stimulation, a process that uses the PKC pathway. Furthermore, this dopamine-induced proliferation involves the EGFR, because its inhibition diminishes dopamine's proliferative effect and is selective in its effects on the GFAP+ multipotent stem/progenitor cell population. In vivo, l- DOPA increases the levels of EGF in the SVZ and stimulates the proliferation of NPCs in the SVZ of 6-OHDA-lesioned animals with a consequent effect on neurogenesis in the OB. Finally, we present data that demonstrates that EGFR+ cells are significantly depleted in the SVZ of individuals with PD.
Previous studies have identified EGF as a crucial regulator of SVZ expansion. Although the EGF-responsive cell in the SVZ corresponds mainly to rapidly cycling C cells, it also includes a subset of B cells (8). This EGFR+ cell in the SVZ has been identified as the anatomical and functional target of dopaminergic innervation (9). We have now shown that these NPCs express D2L receptors, which colocalize with the EGFR, suggesting that both dopamine and EGF can act on the same cell via their respective receptors.
In addition, we, and others have shown that dopaminergic receptor activation controls the proliferation of adult NPCs in vitro (9, 19, 20), suggesting that dopamine might interact with the EGFR in this neurogenic niche to promote proliferation. However, this is unlikely to be the only pathway, and indeed D2 receptor activation has recently been shown to promote neurogenesis through the regulation of ciliary neurotrophic factor expression in the SVZ (21). Our data clearly indicate that dopamine stimulates EGF (and not FGF2) release from NPCs, by doubling the amount of EGF released in 24 h. This result identifies dopamine as an endogenous regulator of EGF expression in the SVZ, which may be of crucial significance in diseases with impaired dopaminergic signaling.
Dopamine receptors are G protein-coupled receptors (GPCRs), and it is now well described that GPCR-induced transactivation of EGFR (22, 23), as would be the case for dopamine stimulation, regulates cellular functions such as proliferation. Transactivation of the EGFR via GPCRs requires specific downstream signaling pathways, of which the PKC pathway has been shown to be involved (13). We confirm that the PKC pathway is the mode of action of dopamine in NPC proliferation via EGF, because inhibition of this pathway decreased the amount of EGF released. However, the exact PKC isoform mediating this has yet to be identified.
Signaling through the EGFR has been shown to induce proliferation by selectively amplifying a subset of “activated” B cells (GFAP+/EGFR+) and C cells (GFAP−/EGFR+) in vivo without affecting the primary B cells (GFAP+/EGFR−) that are being maintained in a more quiescent state (8). C cells divide rapidly in vivo, and activation of the EGFR has been identified as the regulator of controlled C-cell amplification. The majority of dopaminergic fibers in the SVZ selectively contact the EGFR+ cell (9). Our data show that dopamine drives proliferation via the EGFR, because cell cycle analysis demonstrated that EGFR inhibition blocked the proliferative capacity of dopamine on NPCs.
Because a subset of B cells (“activated astrocytes”) and all C-cell populations have been shown to express the EGFR, the EGF released as a consequence of dopamine stimulation could act on either population of cells to activate the EGFR and selectively expand either, or both, of them. Our results showed that the dopamine-induced generation of newborn cells adopted primarily a GFAP+ phenotype after dopamine treatment, to an extent that paralleled dopamine's proliferative effect, suggesting that dopamine selectively induced a population of GFAP+ multipotent neural stem/progenitor cells. In other words, our results suggest that in vitro the EGF released as a consequence of dopaminergic stimulation acts to rapidly expand the proliferative pool, with cells adopting a multipotent GFAP+ phenotype. This result can easily be explained with reference to a previous study (8). First, C cells in the SVZ have been shown to retain stem cell properties when exposed to EGF, suggesting that dopamine stimulation releases EGF that ultimately causes C cells to become activated B cells that are GFAP+/EGFR+. Second, C cells correspond to the EGF-responsive cell in vivo but behave as multipotent B cells in vitro when exposed to EGF, possibly because of the absence of the glial microenvironmental signals (4, 8).
In summary, our in vitro data demonstrate that dopamine stimulates EGF release via the PKC pathway and the released EGF then produces its effects by binding to its cell surface receptor (EGFR), which in turn initiates multiple intracellular responses that culminate in the selective expansion of stem/progenitor cells.
In vivo studies then confirmed that dopamine denervation significantly reduced NPC proliferation in the adult SVZ as reported (9, 14). However, of greater interest is the finding that local EGF release is significantly reduced in the SVZ of dopamine-denervated animals. This results in a reduction in the capacity of EGF to expand the proliferating population of NPCs in the SVZ, an effect that is restored along with neurogenesis by l-DOPA. When l-DOPA was administered to control animals, however, the EGF levels in the SVZ did not change significantly. This may be because the intact system is already maximally activated with respect to this EGF pathway, an observation that may help explain why the SVZ NPCs are relatively insensitive to environmental manipulation in contrast to the NPCs of the dentate gyrus.
The significance of this alteration in SVZ NPC proliferation to the OB is an overall reduction in BrdU-labeled cells and newborn neuronal progeny in lesioned animals, which is reversed experimentally by dopamine replacement with l-DOPA. This increase in OB neuronal differentiation is different from that reported with exogenous EGF administration, in which there was a change in the astrocytic, not neuronal, fate of NPCs in vivo (5). These conflicting results may be explained by the fact that chronic high concentrations of exogenous EGF were used in these studies, doses that led to the induction of pronounced hyperplasias in the ventricular wall, whereas the EGF released as a consequence of dopamine stimulation is endogenous and at more physiological concentrations. Moreover, prolonged exposure to high doses of EGF has been shown to arrest neuroblast production and promote the genesis of highly-proliferative glia-like cells (8).
In conclusion, our in vivo data have shown that dopamine innervation of the SVZ modulates EGF release, which in turn has a proliferative effect on NPC in this site.
The contribution of neurogenesis to the pathology of neurodegenerative disorders is an area of intense interest. Neurogenesis has been shown to be altered in a number of neurodegenerative diseases including Alzheimer's disease (24–26), Huntington's disease (27, 28), ischaemic and traumatic brain injury (29, 30), and PD (10, 31). Odor memorization has been impaired in experimental animals with reduced neurogenesis (32), which is of considerable interest in PD as the identification and discrimination of odors is impaired in a large proportion of PD patients (33). Interestingly, fine olfactory discrimination is also impaired in waved-1 mutant mice (TGF-α) in which SVZ neurogenesis is significantly reduced (34). TGF-α is another ligand of the EGFR, and our experimental data suggest that in PD neurogenesis is reduced as a consequence of a decrease in local EGF in the SVZ. It is possible that this process may lead to olfactory dysfunction in PD.
All of this work has been done in either rodent cultures or rat models of PD, and its relevance to the human brain and PD is unknown. The EGFR has been identified in the human SVZ. Using postmortem tissue, our study has also shown that EGFR+ cells are largely absent in the SVZ of human PD patients in contrast to age-matched controls. This result is consistent with our experimental data and would support an interaction between dopamine and EGFR in human PD and could suggest that the pathway identified in rodents is conserved across all mammalian species, including humans.
In summary, the process of neurogenesis in the SVZ involves the orchestration of 4 distinct events to yield functional newborn neurons: proliferation, migration, differentiation, and survival. In the absence of dopamine, the first step in the process of neurogenesis (proliferation) is negatively affected such that the size of the proliferative pool in the SVZ is significantly reduced, and as a consequence the downstream events will also be affected in a similar way. Our data have now revealed a role for the dopamine-EGFR signaling loop in this process, by showing that the EGFR, through the dopamine-mediated release of EGF by NPC contributes to neurogenesis in this area of the adult brain. This, in turn, may have implications for the treatment and pathogenesis of dopaminergic disorders of the CNS such as PD.
Methods
6-OHDA-Treated Rats.
Experiments were carried out according to the U.K. Animals (Scientific Procedures) Act 1986, under appropriate Home Office license. Female Sprague–Dawley rats (250–300 g), received unilateral medial forebrain bundle injections of 6-OHDA as described (35). Two weeks later, rats were challenged with d-amphetamine (2.5 mg/kg; Sigma), and rats displaying ipsilateral rotations of >7 turns per min were included.
Rats were divided into 4 distinct treatment groups (n = 6/group): lesion, lesion + l-DOPA, l-DOPA, and nonlesioned, non-l-DOPA-treated control animals. 3 weeks postlesion, all animals received BrdU (50 mg/kg per day, i.p.) for 6 days. The l-DOPA-treated rats received l-DOPA (6 mg/kg per day, i.p.) stabilized by benserazide hydrochloride (12 mg/kg per day), for the same 6 days as BrdU. Rats were killed 1 or 21 days after the last BrdU injection and snap-frozen or perfused with 4% paraformaldehyde (PFA). Brains were postfixed and then cryoprotected in 30% sucrose/0.1 M PBS, after which coronal sections of 40-μm thickness were cut. For HPLC analysis, rats were killed 2 h after the last l-DOPA injection, and striata were immediately dissected. Dopamine concentration was analyzed by RP-HPLC and electrochemical detection as described (36).
Neurosphere Cultures.
Adult rodents were killed by CO2 asphyxiation, and the SVZ was dissected, diced with a scalpel blade, and digested with trypsin (0.1%; Worthington Biochemical) for 7 min at 37 °C. Digestion was stopped by 0.1% trypsin inhibitor/0.008% DNase. Cells were grown in neurosphere medium: DMEM/F-12 (7:3; Gibco), 2% B27 (Invitrogen), 1% penicillin/streptomycin/fungizone (Invitrogen), and 20 ng/mL EGF (Sigma) at a density of 3,500 cells per cm2. Primary neurospheres were passaged to produce secondary neurospheres on day 10.
For proliferation and differentiation experiments, primary neurospheres were dissociated and grown in neurosphere medium. Dopamine + 0.02% ascorbic acid (Sigma) was added to the cultures daily for 4 days (10 μM). On day 3, cultures were pulsed with 0.2 μM BrdU (Sigma) for 24 h. Cells were then plated at a density of 50,000 cells per coverslip in neurosphere medium minus EGF for 4 days, followed by fixation in 4% PFA.
Immunohistochemistry and Immunocytochemistry.
Primary antibodies were added to free-floating sections or neurosphere cultures after blocking with 3% normal goat serum (ST2). These were visualized by using biotinylated secondary antibodies (1:200 in Tx-TBS) and the diaminobenzidine system (Vectastain ABC kit; Vector Laboratories) or fluorescence-conjugated secondary antibodies (Alexa Fluor 488 and 568, 1:1,000; Molecular Probes). For antibodies used see Table S2.
MTT Assay.
Primary neurospheres were dissociated and grown in neurosphere medium. Dopamine + 0.02% ascorbic acid was administered (0.1–100 μM) daily for 4 days. Cells were incubated with MTT (5 mg/mL in PBS) for 2 h at 37 °C and reaction was stopped by addition of SDS solution (20% in 1:1 distilled water/N,N-dimethylformamide, pH 4.7). The absorbance of the formazan product was determined at a wavelength of 570 nm by using a plate reader.
LDH Assay.
Dopamine (10 or 100 μM + 0.02% ascorbic acid) was added to SVZ cultures for 24 h. LDH activity was quantified in 50-μL samples of supernatant, using an assay kit, according to the manufacturer's directions (Sigma–Aldrich).
Limiting Dilution Assay.
NSC numbers were analyzed as described (37, 38). Briefly, after 7 days in EGF or EGF + dopamine, neurospheres were dissociated with accutase and mechanical dissociation. Cells were serially diluted to achieve a range of cells from 500 to 1/100 μL of media. The next day, the plate containing 1 cell per well was examined and only wells with 1 cell per well were analyzed further. After 7 days, the number of neurospheres per well was counted and plotted against the number of cells plated per well. In addition, the number of wells in the 1 sphere per well plates that contained neurospheres was counted and expressed as a percentage of cells plated.
Cell Cycle Analysis.
Primary neurospheres were passaged and grown in DMEM/F-12 (7:3) supplemented with 1% N2 (Gibco) and 1% PSF, without growth factors for 24 h to enable synchrony. Dopamine (10 μM) ± AG 1478 (1 μM; Tocris) was added to cultures for 12 h. Cells were dissociated and fixed on ice. After resuspension in 820 μL of PBS and 100 μL of RNase (1 mg/mL), 80 μL of propidium iodide (0.5 mg/mL) was added and incubated in the dark for 30 min at 37 °C. Cell cycle analysis was carried out with a Moflo Flow cytometer (Dakocytomation). The DNA content of 10,000 events per sample was analyzed for n = 3 independent cultures. The percentage of cells in different stages of the cell cycle was determined with Summit 4.1 software (Cytomation).
EGF and FGF2 ELISA.
Cells were prepared as for cell cycle analysis. Twenty-four hours later, dopamine (10 μM) ± PKC inhibitor (Calbiochem) was added and supernatant was collected 24 h later. EGF and FGF2 levels were determined by using a quantikine assay for EGF and FGF2 (R&D Systems), according to the manufacturer's guidelines.
Western Blot Analysis.
Protein was extracted from the SVZ (n = 6) with lysis buffer [50 mM Tris, 150 mM NaCl, 1% Nonidet P-40 and protease inhibitors: pepstatin (Roche) and complete mini tablet (Roche)]. Primary antibodies rabbit αEGF (1:1,000; Upstate), α-actin (Sigma), and α-TH (Chemicon) were used in combination with a HRP-conjugated secondary antibody (Dako). Bands were detected by using an enhanced chemiluminescence substrate mixture (ECL Plus; Amersham). Each experiment was performed in triplicate, and the intensity of the EGF bands was quantified densitometrically and normalized to the corresponding actin control.
Image Analysis.
Counting was performed blind to treatment condition. The stereological estimation of the total number of BrdU+ cells in the SVZ was done by using the Olympus CAST-grid system equipped with an Olympus BX51 microscope. Absolute values for cell counts in the SVZ of each brain were calculated by multiplying the ratio of reference volume to sampling volume.
Quantification of the number of BrdU+/NeuN+ cells was carried out with a Leica LCS-SPE confocal microscope. LAS-SPE imaging software (Leica Microsystems) was used to quantify the number of colocalized cells per image. 3D reconstruction from z-series was used to verify colocalization in the x–y, y–z, and x–z planes.
For quantitative analysis of in vitro experiments, images were taken with a Leica DM6000 fluorescence microscope, FX350 camera, and FW4000 software.
Statistical Analysis.
All experiments were carried out a minimum of 3 times from independently-generated cultures for in vitro work and 6 times for in vivo experiments, unless otherwise stated. An average across all experiments was calculated, and data are presented as mean ± SEM. All statistics were calculated by using Student's t test or ANOVAs (1 or 2 way) followed by Neumann Kuels post hoc test where appropriate. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank David Story, Nigel Miller, and David Theobald for technical assistance and Elaine Perry and Mary Johnson (Newcastle University) for the human SVZ tissue. This work was supported by a Medical Research Council studentship (to G.C.O.), the Royal Society, and the Parkinson's Disease Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803955106/DCSupplemental.
References
- 1.Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration, and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 2.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morshead CM, et al. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 7.Craig CG, et al. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 9.Hoglinger GU, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 10.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 12.Garcion E, Halilagic A, Faissner A, ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131:3423–3432. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 13.Sahin U, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- 15.Karoum F, et al. d-dopa and l-dopa similarly elevate brain dopamine and produce turning behavior in rats. Brain Res. 1988;440:190–194. doi: 10.1016/0006-8993(88)91176-6. [DOI] [PubMed] [Google Scholar]
- 16.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 17.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nait-Oumesmar B, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coronas V, et al. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the postnatal subventricular zone. J Neurochem. 2004;91:1292–1301. doi: 10.1111/j.1471-4159.2004.02823.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang P, Arnold SA, Habas A, Hetman M, Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci. 2008;28:2231–2241. doi: 10.1523/JNEUROSCI.3574-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signaling by G protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 23.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: Epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20:1594–1600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- 24.Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C. Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J Neurosci. 2007;27:6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng R, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- 26.Haughey NJ, et al. Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 27.Curtis MA, et al. The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington's disease human brain. Neuroscience. 2005;132:777–788. doi: 10.1016/j.neuroscience.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Curtis MA, et al. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci USA. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 30.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 31.Winner B, et al. Mutant α-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol Aging. 2007;29:913–925. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berendse HW, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson's disease patients' relatives with a decreased sense of smell. Ann Neurol. 2001;50:34–41. doi: 10.1002/ana.1049. [DOI] [PubMed] [Google Scholar]
- 34.Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svendsen CN, et al. Long term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinsons disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 36.Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 37.Tropepe V, et al. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 38.Bellows CG, Aubin JE. Determination of numbers of osteoprogenitors present in isolated fetal rat calvaria cells in vitro. Dev Biol. 1989;133:8–13. doi: 10.1016/0012-1606(89)90291-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.