Abstract
Immunodominance refers to the highly selective peptide reactivity of T cells during an immune response. In this study, we tested the hypothesis that persistence of peptide:class II complexes is one key parameter that selects the final specificity of CD4 T cells. We found that low-stability peptide:class II complexes support the initial priming and expansion of CD4 T cells, but the expansion becomes strikingly aborted in the presence of competitive T cell responses to unrelated peptides. Our experiments revealed that for inhibition to occur, the competitive responses must be initiated by the same antigen presenting cell, and it is not because of competition for MHC binding. These studies not only provide an insight into the events that regulate competitive CD4 T cell priming in vivo, but also provide a previously undescribed conceptual framework to understand the parameters that select the final specificity of the T cell repertoire during pathogen or vaccine-induced immune responses.
Keywords: immunodominance, competition, T cell activation, MHC, peptide
Antigen presenting cells (APC) ingest antigens in peripheral tissues and, in response to inflammatory signals, migrate to lymph nodes (LNs), where they present peptide antigens bound to MHC class II molecules to recirculating CD4 T cells. The majority of T cells that are primed during an immune response are selectively skewed to one or a few of the many possible peptides from an antigen, resulting in a phenomenon known as immunodominance. Our laboratory has shown that an intrinsic biochemical property of the ligand recognized by the responding T cells, the kinetic stability of the peptide:MHC class II complexes, has the major role in both predicting and determining immunodominance (1). High-stability peptides elicit dominant responses, whereas low-stability peptides do not recruit detectable responses and are thus, “cryptic” (1). Selectivity in the repertoire of the presented peptide:class II complexes was shown to be modulated by DM editing in APC (2–4), and to be independent of the protein context in which those peptides resided (5).
Recent findings describing the dynamic interactions of antigen-bearing dendritic cells (DC) and T cells suggest that peptide off-rates from class II molecules may impact the immune response (6–8). The quality of signals received through the T cell antigen receptor (TcR) by peptides of different affinity for MHC class II has recently been suggested to regulate the fate and function of CD4 T cells (9, 10). Despite new methods that allow visualization of different phases of DC:T cell interactions to antigenic stimulus in vivo (11–18), the factors that contribute to efficient T cell activation and how the kinetic features of those interactions governs the fate of the responding T cells is still unknown. The decision for a T cell to “stop” and form long-lived contacts with a DC when scanning for cognate antigen may depend on multiple parameters, but antigen density (12) and peptide affinity for class II (19) have been shown to augment the frequency of prolonged DC:T cell contacts, increasing the efficiency of the T cell stop signal. Recent studies have also suggested the requirement of stable DC:T cell interactions for the induction of immunity, rather than tolerance (15, 20–22). Strikingly, recent publications suggest that peptide:class II complexes that persist for long periods of time may be an essential feature of a germinal center reaction (23, 24). All these studies are consistent with the possibility that peptides that have long-lived interactions with class II may have a selective advantage during competitive CD4 T cell priming in vivo.
In the present study we have asked how the final magnitude and specificity of primary CD4 T cell responses are influenced by peptide persistence on the class II molecule. We have devised an immunization strategy to prime T cells of disparate antigen specificities by using the natural endogenous TcR repertoire with its correspondingly low precursor frequencies as the responding population. Collectively, our studies revealed that during competitive CD4 T cell responses, peptides with low-stability interactions with class II molecules lose the ability to sustain T cell responses. Also, we demonstrate that CD4 T cells that are specific for rapidly decaying peptides from class II molecules are initially recruited into an immune response, but fail to progress further in the activation program by an apparent stall in proliferation when in the presence of other ongoing T cell responses to dominant peptides.
Results
Peptides with Fast Off-Rates from Class II Elicit Diminished T Cell Responses, but Only When Immunized with Dominant Peptides.
Cryptic peptides fail to recruit CD4 T cells, at least in part because DM editing within endosomes removes them from class II molecules. However, it is unknown whether these low-stability peptide:class II complexes are otherwise competent to recruit CD4 T cells if intracellular antigen processing is bypassed. The need for extended interactions of antigen-bearing APC by CD4 T cells (15, 20–22) suggests that persistence of cell surface complexes may be a generalized feature of the ability of a peptide to recruit T cells. To explore this issue, we evaluated whether CD4 T cell responses to low-stability peptide:class II complexes can be sustained over time. We also asked whether the presence of ongoing CD4 responses to unrelated peptides influenced the full expansion of CD4 T cells during a primary response. We have chosen to study this issue in the context of the endogenous CD4 T cell repertoire, with its naturally low precursor frequency and heterogeneity in TcR structure and affinity. The strategy in all of the studies described here, unless otherwise stated, is to immunize BALB/c mice in the hind footpad with wild-type or variant peptides (Table 1) alone or with a mixture of defined dominant peptides that are known to persist on the class II molecule I-Ad (1). CD4 T cell expansion is then evaluated by assessing the number of responding T cells at the peak of the immune response via IL-2 ELISpot assays. All peptides were emulsified in IFA containing LPS as an adjuvant.
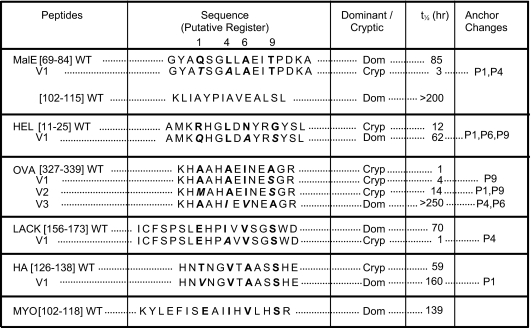
Table 1.
Kinetic stability of wild-type and variant peptide epitopes in association with I-Ad
Half-lifes of peptide:I-Ad complexes were calculated from the time required to dissociate 50% of the FITC-labeled peptide initially bound to soluble I-Ad at pH 7.4. The immunodominance of different peptide epitopes was determined from protein immunizations and denoted as Dom or Cryp, respectively. Putative pocket residues for peptides are indicated in bold, and the mutations at pocket residues that modulate kinetic stability are indicated in bold italics. Lys in OVA[327-339] was substituted for Val to eliminate binding of an alternate register.
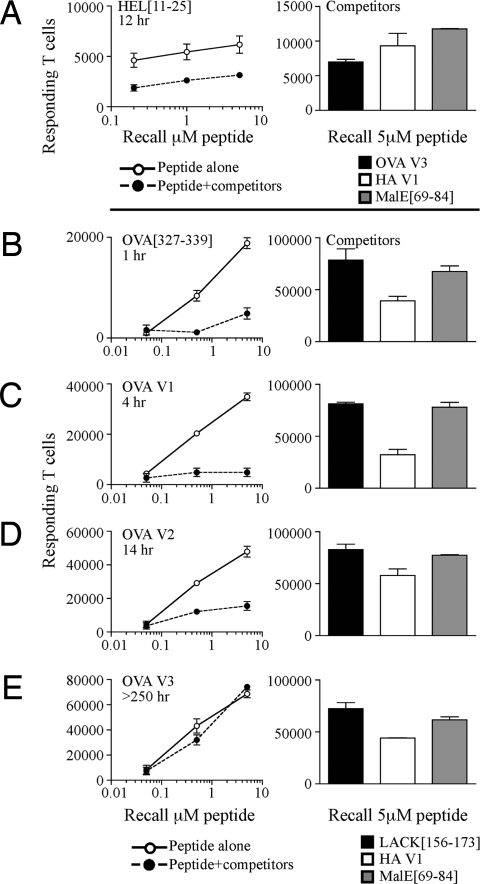
Initial studies used the well-defined, cryptic peptide HEL[11-25], which has very unstable interactions with I-Ad (t1/2 12 h). When introduced alone, the HEL peptide elicited a robust CD4 T cell response (Fig. 1A). Strikingly, however, when the expansion of HEL[11-25]-specific T cells was monitored after coadministration with several high-stability dominant peptides, there was a dramatic loss in the number of T cells detected. To verify this important finding, a peptide from OVA, for which several peptide variants of altered kinetic stabilities with class II have been defined (1) and known priming doses so they are loaded at similar initial epitope densities (Fig. S1), was tested in this competitive T cell assay. When this peptide alone was used for immunization, a robust CD4 T cell response was elicited, despite the fact that this peptide has a very fast off-rate from I-Ad (t1/2 < 2 h) (Fig. 1B). However, when the OVA peptide was coadministered with 3 dominant peptides, the OVA-specific response became almost undetectable (>75% reduction). Other low-stability variant OVA peptides (Table 1) exhibited the same truncated expansion when they were administered with other unrelated high-stability peptides (Fig. 1 C and D). Interestingly, however, CD4 T cell responses to the OVA peptide were sustained in the face of competition when a variant that had been engineered to have enhanced anchor interactions with the class II molecule (t1/2 > 250 h) (1) was used (Fig. 1E). This striking result indicates that extended persistence of peptide:class II complexes is needed to maintain recruitment of CD4 T cells during concurrent responses. Independent of the test OVA peptide used, the 3 heterologous peptides, LACK[156-173], HA V1, and MalE[69-84], recruited similar numbers of IL-2 secreting T cells (Fig. 1 B–E). Similar patterns of responses were observed when IFNγ-producing, rather than IL-2-producing, CD4 T cells were quantified as an indication of T cell priming. Also, the phenomenon can be recapitulated when complete Freund's adjuvant (CFA) is used as an adjuvant.
Fig. 1.
Expansion of T cells to low-stability peptide:class II complexes is lost in the presence of ongoing responses to unrelated peptide antigens. (A) Mice were immunized in the hind footpad with 25 nmol of HEL[11-25] in an emulsion containing IFA/PBS and 0.6 μg/mL LPS either alone (○) or in a mixture containing 5 nmol of each of the dominant peptides LACK[156-173], HA V1, and MalE[69-84] (●). (B–E) Mice were immunized with 25 nmol of OVA[327-339], or 5 nmol of higher-stability variants, OVA V1, OVA V2, and OVA V3 alone (○) or in a mixture containing dominant peptides (●). Draining LNs from individual mice were pooled, n = 2 mice per group. Shown is the total number of IL-2 secreting antigen-specific cells at day 10 after restimulation with increasing doses of the test peptides or 5 μM of competing dominant peptides (Right in A–E). The results are representative of 2–7 independent experiments.
Kinetic Stability of the Peptide:Class II Complexes Predicts Resistance to Competitive T Cell Activation.
To determine whether the susceptibility to abortive T cell priming during competitive T cell responses was a general phenomenon of low-stability peptides, an independent set of unrelated peptides were coadministered with competing dominant peptides (Table 1). These peptides each recruit distinct subsets of CD4 T cells, at different precursor frequencies and TcR repertoire. In each experiment, the ability of the test peptide to sustain expansion of CD4 T cells was measured with or without “competitor” peptides in the immunizing emulsion. For each peptide, we present the data as the percentage response gained or lost when coadministered with competing peptides. Fig. 2A shows there was a dramatic and consistent segregation of the peptide epitopes based on their persistence with the class II molecule. Highly stable peptides (black) elicited similar or enhanced T cell responses while in competition, whereas peptides with rapid off-rates from class II (white) consistently showed diminished T cell responses, even though all of the peptides were able to recruit CD4 T cells when they were introduced alone. The selective loss of T cell responses to rapidly dissociating peptides while in competition was highly reproducible and significant (Spearman r2 = 0.7818, P < 0.0064) (Fig. 2B). Interestingly, we found that as few as 1 immunodominant peptide is sufficient to induce the observed loss in T cell responses (Fig. S2). These losses were still observed even if the low-stability peptide was given a 25-fold advantage in priming dose.
Fig. 2.
The persistence of peptide:class II ligand controls the ability of CD4 T cells to expand during responses to unrelated peptide antigens. (A) Mice were immunized with the low-stability peptides OVA[327-339], OVA V1, OVA V2, LACK V1, HEL[11-25], MalE V1 (white bars) or high-stability peptides OVA V3, LACK[156-173], HEL V1, MalE[69-84], and MalE[102-115] (black bars) to assess the ability of these peptides to elicit T cell responses when immunized with a mixture of dominant peptides. The results are presented as the percentage of the response gained or lost with competitor peptides in the immunization, relative to the peptide alone responses and are an average, from triplicate wells of IL-2 ELISpots of the indicated number of experiments ± SEM. Shown beneath each bar is the number of experiments performed. (B) Percentage loss or gain of T cell activation of the indicated peptides was quantified across experiments by comparing the total number of antigen-specific IL-2 responders from test peptide immunized alone to test peptide immunized with competitors at the peak of the immune response. The percentage of the response with competitors was then plotted against the t1/2 in association with I-Ad of the peptide:class II complex under study. The data points were subjected to Spearman's nonparametric test, resulting in an r2 value = 0.7818, P < 0.0064 (significant correlation).
Competitive T Cell Activation Is a Local Event That Is Limited to the Draining LN.
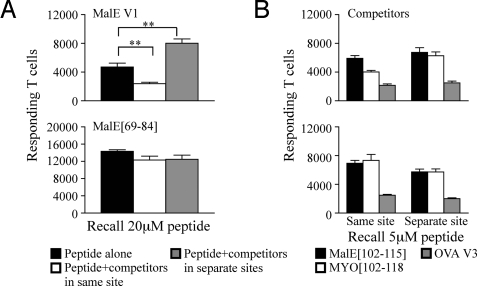
To evaluate the factors that contribute to the diminished recruitment of CD4 T cells to low-stability peptides in the face of ongoing responses to other peptides, it was important to determine whether the negative effect occurred systemically or locally. To address this issue, mice were immunized with test and competitor peptides in 2 peripheral sites that drained to separate LNs. These studies revealed that inhibition of expansion required the responses to the competitors to be in the same LN as the low-stability complexes (Fig. S3). To determine whether the competitor and low-stability peptide needed to be introduced on the same APC in order for the loss in expansion to occur, the peptides were introduced at separate sites that drained to the same LN. We reasoned that if an abrogation in the loss in T cell responsiveness to unstable peptides were observed only when competitor peptides were administered at the same site, it would imply a key role for the antigen-bearing cell in competition. Mice were immunized in the footpad using 3 different conditions: (i) a test peptide alone on one side of the footpad, with IFA/LPS on the opposite side; (ii) a test peptide in a mixture of dominant peptides on one side with IFA/LPS on the other; or (iii) a low or high-stability peptide alone on one side and the mixture of dominant peptides in the same footpad, but on the opposite side. Incomplete Freund's adjuvant (IFA)/LPS sham injections were used to control for the cellular influx from the site of immunization and a possible increase of mature APC entering the LN. Remarkably, when the test peptides and competing peptides were administered in separate emulsions at 2 different sites in the same footpad, both low- and high-stability peptides primed similar numbers of T cells as the peptide alone group (gray bars in Fig. 3A Upper and Lower, respectively). Collectively, these data show that for the competitive loss in T cell priming to occur, it must be within the same draining LN microenvironment, and the same antigen-bearing cell must present the competing peptides.
Fig. 3.
Competitive loss of responses to low-stability complexes requires that the competitor and test peptides be introduced at the same immunization site. (A) Total number of IL-2 producing cells at the peak of the immune response after restimulation with 20 μM of the test peptide when mice were immunized with 10 μL of 25 nmol of the low-stability variant MalE V1 (Upper) or 5 nmol of MalE[69-84] (Lower) in an emulsion containing IFA/PBS and 0.6 μg/mL LPS alone (black), as a mixture with 5 nmol of the dominant peptides MalE[102-115], OVA V3 and MYO[102-118] (white), or the test peptide alone in one side of the footpad and the dominant peptides in the other side of the same hind footpad (gray). (B) Total number of IL-2-producing cells after restimulation with 5 μM of competing dominant peptides. Draining LNs from individual mice were pooled, n = 2 mice per group. The results are an average of 2 independent experiments ± SEM. Three other independent experiments using alternate test peptides also show similar results. Results were analyzed with the 1-way Student's t test; **, P < 0.005.
Peptide Competition for Binding of Available Class II Does Not Account for Diminished T Cell Responses.
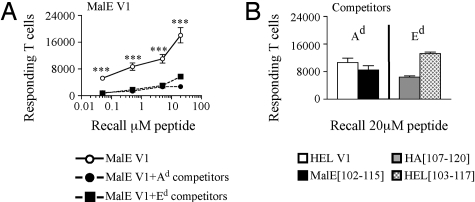
One of the simplest explanations for the failure of low-stability complexes to recruit CD4 T cells under competitive conditions is that the presence of the competitor peptides diminishes presentation of the low-affinity peptides on the priming APC. To address whether there is competition for peptide binding to class II molecules on the surface of the APC, several approaches were taken. First, purified CD11c+ dendritic cells were incubated with HA[126-138] alone or in a mixture of 3 dominant peptides in vitro. IL-2 production by T cell hybridomas was used to compare the efficiency of peptide:class II presentation. Fig. S4A shows that there was no difference in the dose–response curves of HA alone, HA with the addition of 3 dominant peptides, or when a kinetic advantage was given to the competitor peptides by prepulsing the APC with the 3 dominant peptides. Because it could be argued that the local APC have a more limited MHC class II density than the APC used in vitro, we tested the impact of competition on the initial epitope density on the priming APC by using CFSE-labeled TcR transgenic T cells. There was no difference in the initial expansion of the transferred T cells when in the presence or absence of competitor peptides (Fig. S4 B and C). To verify this important issue using an alternative method, the effect of competitor peptides that are restricted by a different class II molecule with the endogenous pool of T cells was used. Mice were immunized with a low-stability I-Ad restricted peptide alone or with either I-Ad or I-Ed restricted dominant peptides. Published data on both HA[107-120] (25) and HEL[103-117] (26) indicate neither peptide detectably binds to I-Ad. If there is competition for available class II, then the I-Ed restricted peptides should not inhibit the T cells responding to the I-Ad peptide. The results of this experiment show that both the MHC matched (I-Ad) and unmatched (I-Ed) competitor peptides elicited robust responses (Fig. 4B), and were equally potent in attenuating the expansion of I-Ad restricted T cells (Fig. 4A closed circles and closed squares). Collectively, these results argue that peptide competition for class II does not account for the dramatic loss of T cells responding to low-stability peptides.
Fig. 4.
Attenuation of the T cell responses is not because of competition for peptide binding to MHC class II molecules. (A) To assess competition for peptide binding in vivo, BALB/c mice were immunized with 25 nmol of the low-stability variant MalE V1 in an emulsion containing IFA/PBS and 0.6 μg/mL LPS alone (○) or as a mixture with 5 nmol of the dominant peptides that bind I-Ad HEL V1 and MalE[102-115] (A, ●) or as a mixture with 5 nmol of the dominant peptides that bind I-Ed HA[107-120] and HEL[103-117] (■) in the hind footpad with increasing doses of the test peptides. (B) CD4 T cell responses after restimulation with 20 μM of competing dominant peptides. Draining LNs from individual mice were pooled, n = 2 mice per group. The results are presented as the total number of responding T cells and are an average of 2 independent experiments ± SEM. Results were analyzed with the 1-way Student's t test; ***, P < 0.0005.
Abortive T Cell Activation by Low-Stability Peptide:Class II Complexes Occurs During the Expansion Phase of the Primary Immune Response.
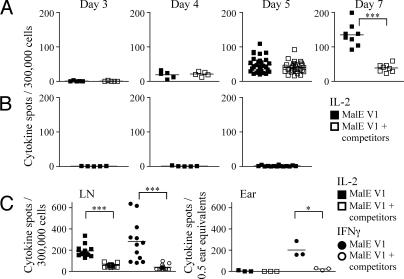
Recently, the kinetics of competition during the priming of CD8 T cell responses was shown to occur within the first few hours after antigen exposure (27). To determine when during an evolving immune response the CD4 T cell recruitment to unstable peptides became attenuated, we performed kinetic analyses. Mice were immunized in the ear pinnae, and at different days, the draining LNs were harvested, and antigen-specific CD4 responses were assessed for the production of IL-2 and IFNγ by ELISpot assays. The CD4 T cells accumulating at the site of immunization after priming in the LN were also examined for number and phenotype at days 3, 4, 5, 7, and 10. The results shown in Fig. 5 indicate that late in the response, after day 7, the disparity in T cell expansion from mice immunized with peptide alone vs. peptide in competition was striking (P < 0.0005). However, responses to unstable peptides measured at early time points, days 3, 4, and 5, were indistinguishable with or without additional peptides in the emulsion. Initial priming and expansion was apparent when either IL-2 (Fig. 5A) or IFNγ was used to quantify T cells above IFA/LPS alone (Fig. 5B). The later loss in responses to low-stability complexes in the context of competitive responses to other peptides was not only detected by CD4 T cells in the draining LN (Fig. 5 A and C Left), but also by CD4 T cells that migrate back to the peripheral site of immunization (Fig. 5C Right). This striking result indicates that when an unstable peptide is introduced concurrently with dominant peptides, the complexes initially recruit and prime antigen-specific CD4 T cells, but this response fails to progress, where other responses to dominant peptides continue to expand.
Fig. 5.
Abortive CD4 T cell expansion to low-stability peptide:class II complexes. Mice were immunized in the ear with 10 μL of 25 nmol of MalE V1 in an emulsion containing IFA/PBS and 0.6 μg/mL LPS alone or in a mixture with 5 nmol of the dominant peptides MalE[102-115], OVA V3, and MYO[102-118]. At days 3, 4, 5, 7, and 10, CD4 T cells purified by negative selection from deep cervical LNs (A and C) or unpurified ear pinnae tissue extract (C) were restimulated with 20 μM of the test peptide in the presence of T cell depleted splenocytes in IL-2 (A–C, □) and IFNγ (C, ○) ELISpot assays. There was no antigen-specific T cell response detected at the peripheral site of immunization before day 7. (B) Response of CD4 T cells purified from LN of mice immunized with IFA/LPS at the early time points for production of IL-2 when restimulated with 20 μM of the test peptide. CD4 T cells enriched from the LN were plated at 300,000 cells/well, and unpurified cell suspensions from the ear were plated at 0.5 ear equivalents/well. Draining LNs from individual mice were pooled and there were 5 mice per group. The results are presented as the number of cytokine spots/300,000 T cells per pooled LN of individual mice from triplicate wells or as the number of cytokine spots/0.5 ear equivalents from triplicate wells at each time point, with the mean number of spots indicated. Results were analyzed with the 1-way Student's t test; *, P < 0.05; ***, P < 0.0005.
Discussion
In the current study, we evaluated the role of peptide persistence on MHC class II molecules for CD4 T cell priming and expansion in vivo. Our results show that peptides with rapid decay from class II molecules are able to initially expand CD4 T cells, but fail to sustain T cell activation throughout the full course of the response. Importantly, this failure is only manifested when the unstable peptides are coadministered with other dominant peptides. Peptides that naturally possess stable interactions with class II molecules or those that are engineered to have this property were able to maintain, or show an enhanced ability to recruit their respective T cells under these conditions. Collectively, these results argue that during an immune response, even when antigen processing and DM editing is bypassed, there are other potent regulatory events that drive the selectivity in the T cell repertoire to peptides that possess highly stable interactions with the presenting MHC class II molecules.
In evaluating the potential mechanism(s) that could account for the loss in T cell expansion in vivo, we must first consider the complexities of the cell–cell associations needed for optimal T cell activation and continued cell division. First, on administration of peptides and adjuvant, peripheral APC will mature and peptides within the emulsion will be loaded onto surface MHC class II molecules. Once peptide is acquired, it takes ≈18 h for the antigen-bearing DC to migrate and enter the LNs (28). Here, CD4 T cells sample the microenvironment, and if cognate antigen is present, form many serial engagements with antigen-bearing dendritic cells. Within 1–3 h, the CD4 T cells apparently reach a threshold of activation, stop, and make stable (10–20 h) interactions with the antigen-bearing DC (11–17). After the initial signaling events that drive antigen-specific CD4 T cells into cell division have occurred, it is thought that T cells may require only transient interactions with peptide:class II complexes to continue expanding for several generations until they are capable of exiting the LN (29).
Heterologous peptides cointroduced in vivo with single peptide immunogens and the concomitant immune responses that these competitor peptides induce might down-modulate or arrest responses at any of the preceeding stages. At the peptide loading stage, if MHC class II molecules were limiting, there could be diminished recruitment of antigen-reactive CD4 T cells simply by competitive binding to the class II molecule. Evidence presented in this manuscript, both in vitro and in vivo, argues that the competitors are not acting at this level. Also arguing against this early effect of the competitor peptides is that the initial activation and priming of T cells to low-stability peptides is unaffected by the presence of other dominant peptides in the emulsion. If the competitor peptides are not acting here, then the subsequent events in recruitment and expansion must be considered. Because the CD4 T cell response matures through expansion and affinity maturation of antigen-specific T cells (30–35), there is thought to be a coincident loss in cognate antigen on the APC through trogocytosis that can transfer MHC-peptide, costimulatory molecules, or other plasma membrane proteins from the APC to the antigen-specific T cells (36, 37). This loss in peptide:class II molecules because of trogocytosis will be compounded for unstable peptides through the additional factor of peptide:class II dissociation. This decay in available peptide:class II will be kinetically linked to the increase in the number of T cells, driven by antigen-dependent expansion throughout the response (38, 39). Thus, it is possible that competition among CD4 T cells for available peptide:MHC may explain the failure of CD4 T cells reactive to low-stability complexes to continue to expand. This simple kinetic model is consistent with our data showing little effect of competitor peptides at the early time points, and an apparent failure of T cells to progress at later time points.
Also, there is the possibility that CD4 T cells responding to stable and unstable peptides may differ with respect to the number of serial engagements with the APC that they need to reach a similar threshold of activation. There is some evidence that very stable peptide:class II complexes may drive T cells into more rapid cell cycles, leading to asymmetric progression of these T cells through their proliferative program (19). Therefore, T cells recruited by very stable peptide:class II complexes may have already reached a level of activation where only transient interactions with antigen, class II molecules, or other activation molecules of the APC are adequate to sustain expansion of these T cells. Last, one must also consider the possibility that the T cells elicited by low-stability peptides may be inherently more sensitive to the normal regulatory mechanisms involved in the contraction of the immune response (40, 41). Suppression may be most selective for T cells elicited by low-stability determinants because of the diminished peptide:class II complexes density available to these T cells later in the immune response. It is also possible that the diminished responses in the presence of competitors might be accounted for, at least in part, by a deviation from the classical T helper subsets. We have begun to address this issue and have found no IL-4, IL-5, or IL-10 producing T cell responses, and are now pursuing the quantification of all possible antigen-specific T cells elicited under these conditions by MHC class II-tetramer analyses.
The immune system has evolved multiple, often interconnected mechanisms to regulate the expansion and contraction of T cells to foreign antigens. We have shown in earlier work that peptides with differing off-rates from class II are distinguished during intracellular antigen processing in dendritic cells (4), where the DM protein selectively removes unstable peptides from the class II molecule. The data presented here identifies an additional level of control, where peptide persistence on class II molecules controls the ability of CD4 T cells to continue to expand during priming, a level of control that occurs selectively in the face of ongoing responses to other peptides. These findings raise the important question of why the immune system might have evolved to select only stable peptide:class II complexes for the focus of CD4 T cells. One possibility is that persistence of a peptide on the class II molecule provides an advantage for stable CD4 memory generation and maintenance. Although it is known that antigen persistence is not required to generate memory cells (42–45), it is possible that continued opportunities to engage the CD4 T cells agonist ligand provides some advantage in the quantity or quality of memory T cells (29, 46–49). Also, engagement of CD4 T cells, particularly follicular helper T cells, with antigen-specific B cells in the germinal center may be most efficient and long-lived for stable peptide:class II complexes (23). These prolonged interactions between CD4 T cells with antigen-bearing B cells may be essential for the formation of a productive germinal center reaction (24). Last, delivery of effector function during pathogen-specific responses may be more efficient for CD4 T cells if their TcR ligand on the target cells persists at high epitope density after pathogen protein synthesis has diminished, allowing for complete removal of pathogen-bearing cells. Any or all of these activities of CD4 T cells may be most efficiently delivered if the ligand for the TcR persists for extended periods of time on the antigen-bearing APC. If so, then it may be counterproductive to initially prime and enrich for CD4 T cells that are specific for low-stability peptide:class II ligands, because these CD4 T cells may ultimately be less “fit” in effector function activity or for providing long-term memory responses to pathogens.
Materials and Methods
For details, see SI Methods.
Immunizations.
BALB/c mice (National Cancer Institute) were immunized in the hind footpad with 50 μL (unless otherwise indicated) or ear pinna with 10 μL [modified from Itano et al. (28)] of an IFA/PBS emulsion containing peptides (25 nm or 5 nm/mouse) and 0.6 μg/mL LPS (Sigma-Aldrich). Unless otherwise indicated, 10 days post immunization, mice were killed, and draining LNs of individual mice (at least 2 mice per group) were harvested and used as the source of CD4 T cells for ELISpot analyses. All animal handling was performed according to the regulations set by the University Committee on Animal Care at the University of Rochester.
Cell Purification.
A single cell suspension from the LN of an individual mouse was prepared and depleted of non-CD4 T cells as described previously (50). During kinetic experiments, LN from an individual mouse was enriched for CD4 T cells by negative selection (Miltenyi Biotech). CD4 T cell purity was typically 90–95%. Splenocytes from naive mice were depleted of red blood cells by treatment with ACK lysis buffer (0.15M NH4Cl/1 mM KHCO3/0.1 mM Na2-EDTA in H2O, pH 7.2; Sigma-Aldrich) and depleted of T cells by using the monoclonal antibody J1j.10. Viable cells were recovered and used as stimulators in ELISpot assays.
Isolation of T Cells from Ear Dermis.
The ear pinnae of 5 mice were removed, cut into pieces, and then digested with 1 mg/mL collagenase/dispase for 1 h at 37 °C. The tissue was mashed through a metal strainer, releasing a single cell suspension, which was washed 2 times with media. Staining for CD4 (RM4-4) typically yielded 0.5–1.8% CD4 T cells in the cell suspension. Cells were resuspended in media at 0.5 ear equivalents/well (≈5 × 106 cells/well) and were stimulated with freshly isolated, T cell depleted, syngeneic splenic APC in the presence or absence of peptide.
ELISpot Assay.
Cytokine production by the restimulated cell populations was measured by a 6 h (ear tissue extracts) or 18 h (purified LN) ELISpot assay as described previously (1). The mean number of spots for each condition was determined from triplicate wells. The quantification of spots was performed with an Immunospot Reader Series 2A, by using Immunospot Software V.2.0. The total number of responders/pooled LN in a mouse was calculated by using spots/300,000 cells and back calculating to the total number of LN cells and the starting percentage CD4 in the LN.
Antibodies and Peptides.
Purified rat anti-mouse IL-2 (JES6-1A12), biotinylated rat anti-mouse IL-2 (JES6-5H4), purified rat anti-mouse IFNγ (AN-18), biotinylated rat anti-mouse IFNγ (XMG1.2), anti-CD4-FITC (RM4-4) antibodies were obtained from BD PharMingen. The monoclonal antibody producing hybridomas MKD6, M5/114, 3.155, RA3-3A1/6.1, and J1j.10 were acquired from American Type Culture Collection. All synthetic peptides were acquired from BioPeptides or Invitrogen.
Supplementary Material
Acknowledgments.
This work was supported by the National Institutes of Health Grants R01-AI051542 (to A.J.S.) and T32-AI0785 (to J.M.W.) and by Health and Human Services Grant HHSN266200700008C (to A.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811584106/DCSupplemental.
References
- 1.Lazarski CL, et al. The kinetic stability of MHC class II:peptide complexes is a key parameter that dictates immunodominance. Immunity. 2005;23:29–40. doi: 10.1016/j.immuni.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Nanda N, Sant AJ. DM determines the cryptic and immunodominant fate of T cell epitopes. J Exp Med. 2000;192:781–788. doi: 10.1084/jem.192.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanda NK, Bikoff EK. DM peptide-editing function leads to immunodominance in CD4 T cell responses in vivo. J Immunol. 2005;175:6473–6480. doi: 10.4049/jimmunol.175.10.6473. [DOI] [PubMed] [Google Scholar]
- 4.Lazarski CA, Chaves FA, Sant AJ. The impact of DM on MHC class II-restricted antigen presentation can be altered by manipulation of MHC-peptide kinetic stability. J Exp Med. 2006;203:1319–1328. doi: 10.1084/jem.20060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver JM, et al. Immunodominance of CD4 T cells to foreign antigens is peptide intrinsic and independent of molecular context: Implications for vaccine design. J Immunol. 2008;181:3039–3048. doi: 10.4049/jimmunol.181.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Local development of effector and memory T helper cells. Curr Opin Immunol. 2007;19:259–267. doi: 10.1016/j.coi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Germain RN, Miller MJ, Dustin M, Nussenzweig MC. Dynamic imaging of the immune system: Progress, pitfalls and promise. Nat Rev Immunol. 2006;6:497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- 8.Henrickson S, von Andrian U. Single-cell dynamics of T-cell priming. Curr Opin Immunol. 2007;19:249–258. doi: 10.1016/j.coi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Margot CD, Ford ML, Evavold BD. Amelioration of established experimental autoimmune encephalomyelitis by an MHC anchor-substituted variant proteolipid protein 139-151. J Immunol. 2005;174:3352–3358. doi: 10.4049/jimmunol.174.6.3352. [DOI] [PubMed] [Google Scholar]
- 10.Skokos D, et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 11.Miller M, Safrina O, Parker I, Cahalan M. Imaging the single cell dynamics of CD4 T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 13.Gunzer M, et al. Antigen presentation in extracellular matrix: Interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 14.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell–dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4 T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Hugues S, et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 16.Shakhar G, et al. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mempel T, Henrickson S, von Andrian U. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 18.Huang AY, Qi H, Germain RN. Illuminating the landscape of in vivo immunity: Insights from dynamic in situ imaging of secondary lymphoid tissues. Immunity. 2004;21:331–339. doi: 10.1016/j.immuni.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Henrickson S, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tadokoro C, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:489–492. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinselmeyer BH, et al. In situ characterization of CD4 T cell behavior in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005;201:1815–1823. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazilleau N, et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8:753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 24.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buus S, Sette A, Colon SM, Miles C, Grey HM. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987;235:1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- 26.Adorini L, et al. Interaction of an immunodominant epitope with Ia molecules in T-cell activation. Proc Natl Acad Sci USA. 1988;85:5181–5185. doi: 10.1073/pnas.85.14.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis RA, Kappler J, Marrack P. CD8 T cell competition for dendritic cells in vivo is an early event in activation. Proc Natl Acad Sci USA. 2006;103:12063–12068. doi: 10.1073/pnas.0605130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 29.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panus JF, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific T helper cell function: Differential cytokine expression in primary and memory responses. J Exp Med. 2000;192:1301–1316. doi: 10.1084/jem.192.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 32.Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikszta JA, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-driven selection of TCR in vivo: Related TCR α-chains pair with diverse TCR β-chains. J Immunol. 1999;163:5978–5988. [PubMed] [Google Scholar]
- 35.McHeyzer-Williams LJ, Panus JF, Mikszta JA, McHeyzer-Williams MG. Evolution of antigen-specific T cell receptors in vivo: Preimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J Exp Med. 1999;189:1823–1838. doi: 10.1084/jem.189.11.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudrisier D, Bongrand P. Intercellular transfer of antigen-presenting cell determinants onto T cells: Molecular mechanisms and biological significance. FASEB J. 2002;16:477–486. doi: 10.1096/fj.01-0933rev. [DOI] [PubMed] [Google Scholar]
- 37.LeMaoult J, Caumartin J, Carosella ED. Exchanges of membrane patches (trogocytosis) split theoretical and actual functions of immune cells. Hum Immunol. 2007;68:240–243. doi: 10.1016/j.humimm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Obst R, van Santen H, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarke CA, et al. Proliferating CD4 T cells undergo immediate growth arrest upon cessation of TCR signaling in vivo. J Immunol. 2008;180:156–162. doi: 10.4049/jimmunol.180.1.156. [DOI] [PubMed] [Google Scholar]
- 40.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 41.Shevach E, et al. The lifestyle of naturally occurring CD4+CD25+Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 42.Hu H, et al. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat Immunol. 2001;2:705–710. doi: 10.1038/90643. [DOI] [PubMed] [Google Scholar]
- 43.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 44.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 45.Purton JF, et al. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 47.Kassiotis G, Gray D, Kiafard Z, Zwirner J, Stockinger B. Functional specialization of memory Th cells revealed by expression of integrin CD49b. J Immunol. 2006;177:968–975. doi: 10.4049/jimmunol.177.2.968. [DOI] [PubMed] [Google Scholar]
- 48.Godebu E, Summers-Torres D, Lin MM, Baaten BJ, Bradley LM. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J Immunol. 2008;181:1798–1805. doi: 10.4049/jimmunol.181.3.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 50.Richards K, et al. Direct ex vivo analyses of HLA-DR1 transgenic mice reveal an exceptionally broad pattern of immunodominance in the primary HLA-DR1-restricted CD4 T-cell response to influenza virus hemagglutinin. J Virol. 2007;81:7608–7619. doi: 10.1128/JVI.02834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.