Abstract
Although mind wandering occupies a large proportion of our waking life, its neural basis and relation to ongoing behavior remain controversial. We report an fMRI study that used experience sampling to provide an online measure of mind wandering during a concurrent task. Analyses focused on the interval of time immediately preceding experience sampling probes demonstrate activation of default network regions during mind wandering, a finding consistent with theoretical accounts of default network functions. Activation in medial prefrontal default network regions was observed both in association with subjective self-reports of mind wandering and an independent behavioral measure (performance errors on the concurrent task). In addition to default network activation, mind wandering was associated with executive network recruitment, a finding predicted by behavioral theories of off-task thought and its relation to executive resources. Finally, neural recruitment in both default and executive network regions was strongest when subjects were unaware of their own mind wandering, suggesting that mind wandering is most pronounced when it lacks meta-awareness. The observed parallel recruitment of executive and default network regions—two brain systems that so far have been assumed to work in opposition—suggests that mind wandering may evoke a unique mental state that may allow otherwise opposing networks to work in cooperation. The ability of this study to reveal a number of crucial aspects of the neural recruitment associated with mind wandering underscores the value of combining subjective self-reports with online measures of brain function for advancing our understanding of the neurophenomenology of subjective experience.
When unoccupied by external demands, the human mind often works with particular rigor. Indeed, one of the most intriguing neuroscientific findings of the past decade has been the observation that certain regions of the brain become increasingly recruited with decreasing external task demands. This group of brain regions has collectively been termed the “default network” (1–3), and includes, most prominently, the medial prefrontal cortex (PFC), posterior cingulate cortex/precuneus region, and the temporoparietal junction. The mental processes that keep the brain so busy when it is not occupied by external demands have been a source of significant theoretical conjecture. A particularly prominent view is that default network recruitment reflects internally focused thought (2, 3) that can occur in the form of mind wandering (4–6) if it takes place simultaneously with, and yet is unrelated to an ongoing task. Indirect support for this view comes from neuroimaging studies (6–8), demonstrating correlations between reported frequency of task-unrelated thoughts and default network activation during conditions of low cognitive demand, as well as stronger default network activation during highly practiced compared with novel tasks in people with higher propensity for mind wandering (6).
However, neuroimaging studies so far have inferred mind wandering only indirectly, by varying task demands to influence the probability of task-unrelated thoughts and collecting mind wandering reports during a separate session outside the scanner. Because there were no online measures of mind wandering taken during scanning, it is possible that the observed default network recruitment could be due to factors other than mind wandering. In this vein, Gilbert et al. (9) recently argued that instead of mind wandering, activations in the medial PFC part of the default network may reflect stimulus-related thought such as enhanced watchfulness toward the external environment that is also likely to occur during highly practiced tasks.
A key objective of the present study was to provide a direct empirical test for the hypothesis that default network recruitment, including medial PFC regions, occurs during the precise moments when the mind wanders away from the task at hand. To this end, we introduced the method of experience sampling (10, 11) to fMRI research on mind wandering. Experience sampling involves intermittently probing individuals to provide self-reports about their current mental state; thus, enabling online assessment of momentary changes in the contents of consciousness. By collecting self-reports in an online fashion during scanning while keeping cognitive demands constant, and by examining differences in neural recruitment immediately before self-reports of being off versus on task, the present study was well positioned to overcome some of the limitations that have prevented previous research from drawing conclusive inferences about the role of the default network in mind wandering. To provide additional corroborative evidence for these self-reports, they were collected while participants performed a sustained attention to response task (SART) (12), during which performance errors have been linked to mind wandering (12–17). This procedure allowed us to assess the convergence in brain activations between behavioral and subjective indices of mind wandering.
A second goal of the present study was to examine the role of the executive system of the brain during mind wandering. Executive brain regions, most notably the dorsal anterior cingulate cortex (ACC) and the dorsolateral PFC (DLPFC), become consistently activated when individuals engage in demanding mental activity (18–20). Currently, behavioral and neuroimaging studies offer disparate views on the likely contribution of the executive system to mind wandering. Behavioral studies employing experience sampling (5, 21, 22) indicate that mind wandering is a complex mental activity that often interferes with cognitively demanding tasks, suggesting a processing overlap with the executive system of the brain. In contrast, neuroimaging findings (6–8) have predominantly implicated the default network. So far, however, neuroimaging studies have inferred mind wandering by contrasting highly practiced tasks and conditions of “rest” (known to be associated with relatively high incidence of mind wandering) to novel, cognitively demanding tasks (known to be associated with relatively low incidence of mind wandering) (5, 6). The associated decrease in cognitive demand from activation to baseline in those contrasts conceivably could have obscured the involvement of executive regions during mind wandering. Therefore, the second aim of the present study was to hold task demands constant and use experience sampling to test the theoretical prediction from behavioral research (5, 21, 22) that the executive network of the brain would be recruited during mind wandering.
Finally, there is accumulating evidence that individuals fluctuate in their explicit awareness of the contents of their own thought, a phenomenon termed meta-awareness or metaconsciousness (23–25). In response to an experience-sampling probe, participants sometimes report having been aware that their mind had wandered, whereas at other times they report having mind wandered without being aware of it (26–29). Recent research indicates that task performance is more disrupted by unaware than by aware mind wandering episodes (27–29), suggesting that the mental processes associated with mind wandering may be most pronounced when it goes unnoticed. Therefore, the third aim of this study was to use experience sampling to examine the relationship between meta-awareness and the neural recruitment associated with mind wandering.
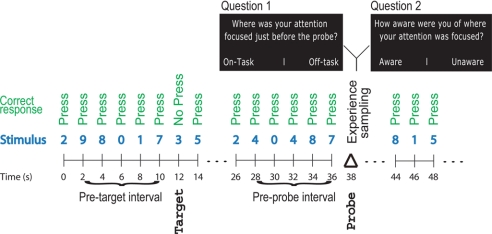
To collect experience sampling reports of mind wandering during fMRI scanning, subjects were presented with thought probes (30) while performing a simple go/no-go task (Fig. 1) known as the SART (12). Thought probes provided subjective reports of mind wandering, whereas task performance errors provided a behavioral index of mind wandering (12–17). Each thought probe asked subjects 2 questions (Fig. 1) about their mental state immediately preceding the probe. First, whether their attention was focused on the task or on something unrelated to the task, and second, whether or not they were aware of where their attention was focused. “Awareness” was defined to subjects as the experience of not recognizing that mind wandering had occurred until the moment that the probe was presented (26–28). Subjects answered using a 7-point Likert scale, ranging from “completely on task” to “completely off task” for the first question, and from “completely aware” to “completely unaware” for the second question (with the scale direction counterbalanced across subjects). Probes occurred pseudorandomly, at a rate of ≈1 per minute and at moments unpredictable to the subjects. A total of 96 probes per subject were presented throughout the experiment. For details on behavioral procedure, see SI Materials and Methods.
Fig. 1.
Experimental paradigm. An experience sampling approach was used to collect self-reports about the subjects' focus of attention while their performed a concurrent task (the SART). Also, task accuracy at targets was used as a behavioral index of mind wandering. Analyses focused on the interval of time immediately preceding experience sampling probes to dissociate the effects of mind wandering from the effects of answering a probe.
The 10-s interval of time directly preceding a probe was subsequently categorized according to the subject's response as “on-task” (responses 1–3 on the 7-point scale) or “off-task” (responses 5–7). Probes that were responded to with a 4, corresponding to the middle of the scale, were excluded from analysis. Also, off-task intervals were divided into “off-task aware” (mind wandering with meta-awareness; responses 1–3 on the 7-point scale) and “off-task unaware” (mind wandering in the absence of meta-awareness; responses 5–7). Probes that were responded to with a 4 to the second question were also excluded. Finally, the 10-s interval directly preceding each target was categorized according to the subject's response as either “correct” (correct withhold) or “incorrect” (commission error). To dissociate the effects of mind wandering from the effects of answering a probe or making an error, the analyses focused on the intervals of time immediately preceding probes or targets (Fig. 1).
Results
Behavioral Data.
During the intervals before off-task probes, subjects made significantly more errors on the SART than during the intervals before on-task probes (t = 2.4, df = 14, P < 0.05). No significant difference was observed in reaction time during on-task and off-task periods. Also, there were no significant differences in reaction time or accuracy during the intervals before off-task aware and off-task unaware probes. Subjects reported being off-task on 43% of probes (SE = 3.7%) and on-task on 54% of the probes (SE = 3.4%). Of the off-task intervals, subjects reported being unaware of where their attention was focused on 45% of probes (SE = 3.7%) and aware of where it was focused on 44% of the probes (SE = 3.9%). The remaining probes were answered with the midpoint (4) of the 7-point scale.
Functional MRI Data.
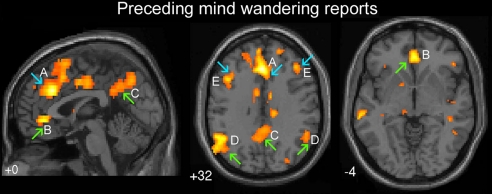
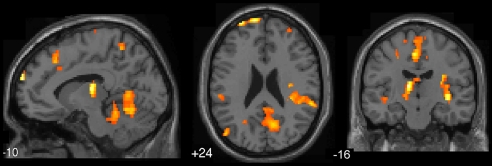
When episodes of mind wandering were compared with episodes of being on task (intervals before off-task probes versus intervals before on-task probes), a robust recruitment of both default and executive network regions was observed (Fig. 2 and Table 1). Activations were observed in the 3 most prominent default network regions, including the ventral ACC [Brodmann area (BA) 24/32], the precuneus (BA 7), and the temporoparietal junction (BA 39) (upward green arrows in Fig. 2 B–D). Also, the 2 main executive regions were activated, namely, the dorsal ACC (BA 32) and DLPFC (BA 9) (downward blue arrows in Fig. 2 A and E). Activations in regions outside those typically associated with the executive and default network were also observed, including the temporopolar cortex (BA 38), inferior and middle temporal gyri (BA 20/21), anterior insula, and caudate nucleus.
Fig. 2.
Activations preceding reports of mind wandering (intervals prior to off-task versus on-task probes). Upward green arrows, default network regions; downward blue arrows, executive network regions. Regions of activation included: (A) dorsal ACC (BA 32), (B) ventral ACC (BA 24/32), (C) precuneus (BA 7), (D) bilateral temporoparietal junction (BA 39), and (E) bilateral DLPFC (BA 9). Height threshold P < 0.005, extent threshold k > 5 voxels.
Table 1.
Activation peaks prior to reports of mind wandering
| Region | BA | Number of voxels | Z-value | Talairach coordinates,x, y, z |
|---|---|---|---|---|
| Dorsal ACC | 32 | 141 | 4.1 | 0, 30, 32 |
| Ventral ACC | 24/32 | 47 | 3.69 | 2, 40, -4 |
| Mid cingulate | 24/31 | 52 | 3.76 | -8, -6, 40 |
| Left MFG | 9 | 23 | 3.44 | -42, 16, 36 |
| Right MFG | 9 | 6 | 3.30 | 36, 24, 44 |
| Left IFG | 45 | 12 | 3.35 | -38, 22, 4 |
| Premotor cortex | 6 | 67 | 3.6 | 6, 12, 56 |
| PCC/Precuneus | 31/7 | 24 | 3.44 | -6, -52, 40 |
| Left Posterior parietal | 39 | 210 | 3.82 | -60, -64, 24 |
| Left Temporopolar cortex | 38 | 18 | 3.48 | -40, 12, -28 |
| Right Temporopolar cortex | 38 | 47 | 4.24 | 44, 14, -20 |
| Left ITG | 20 | 15 | 3.42 | -56, -24, -20 |
| Left MTG | 21 | 10 | 3.36 | -64, -32, -4 |
| Right ITG | 20 | 41 | 3.73 | 62, -34, -16 |
| Right MTG | 21 | 12 | 3.46 | 48, -48, 4 |
| Left Anterior insula | - | 12 | 3.46 | -32, 32, 0 |
| Caudate nucleus | - | 17 | 3.52 | -6, 10, 4 |
Intervals prior to off-task reports vs. intervals prior to on-task reports. All activations were significant at the P < 0.001 level (k > 5). ACC, anterior cingulate cortex; BA, Brodmann area; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; PCC, posterior cingulate cortex; ITG, inferior temporal gyrus; MTG, middle temporal gyrus.
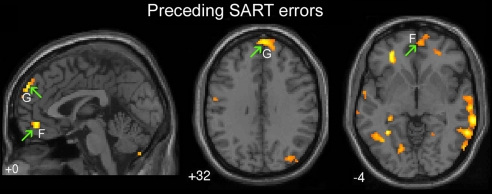
Next, we examined brain recruitment preceding SART performance errors (intervals before SART commission errors compared with intervals before correct target withholds) (Fig. 3 and Table 2). Activations were observed in 2 default network regions: the dorsomedial PFC (BA 9) and ventromedial PFC (BA 10/11). Also, activations in the temporopolar cortex (BA 38), fusiform gyrus, and extrastriate visual cortex were observed (Table 2). Importantly, both mind wandering reports and performance errors were preceded by activation in default network regions (upward green arrows in Figs. 2 and 3, respectively), suggesting a convergence between subjective and behavioral measures of mind wandering. Activation preceding mind wandering reports extended throughout the default network, including the medial frontal, posterior cingulate, and temporoparietal cortices (Fig. 2 B–D), whereas error-preceding activations (Fig. 3 F and G) were concentrated within the medial frontal subcomponents of the default network. Fianlly, both mind wandering reports and performance errors were preceded by recruitment of the temporopolar cortex.
Fig. 3.
Activations preceding SART errors (intervals prior to incorrect versus correct targets). Upward green arrows, default network regions. Regions of activation included: (F) Ventromedial PFC (BA 10/11) and (G) Dorsomedial PFC (BA 9). Height threshold P < 0.005, extent threshold k > 5 voxels.
Table 2.
Activations prior to SART task errors
| Region | BA | Number of voxels | Z-value | Talairach coordinates, x, y, z |
|---|---|---|---|---|
| Dorsomedial PFC | 9 | 83 | 3.58 | -4, 62, 36 |
| Ventromedial PFC | 10 | 80 | 3.43 | -4, 48, -8 |
| 11 | - | - | -8, 40, -12 | |
| Right MTG | 37/39 | 93 | 3.13 | 56, -66, 8 |
| Left Fusiform gyrus | 37 | 101 | 3.1 | -60, -64, -12 |
| Left Temporopolar cortex | 38 | 24 | 3.04 | -34, 8, -40 |
| Left PHG | 28/36 | 30 | 3.17 | -24, -12, -16 |
| Left Extrastriate visual | 18 | 24 | 3.02 | -18, -72, 0 |
| Right Extrastriate visual | 19 | 36 | 3.29 | 40, -78, 28 |
| Right Tail of Caudate | - | 14 | 3.11 | 28, -38, 8 |
Intervals prior to incorrect vs. correct responses to SART targets. All activations were significant at the P < 0.001 level (k > 5). PHG, parahippocampal gyrus.
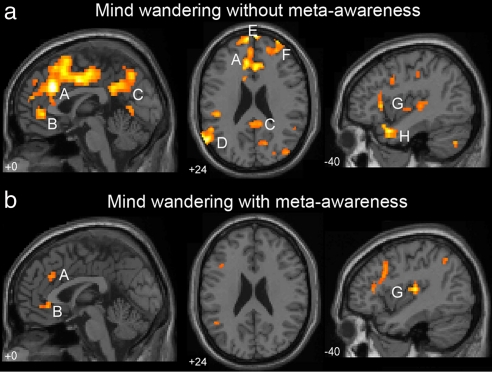
To examine the effect of meta-awareness on mind wandering, we assessed unaware and aware mind wandering intervals separately, comparing each to on-task intervals. Reports of mind wandering in the absence of meta-awareness (Fig. 4a; Table S1) were preceded by robust activation of both the executive network (dorsal ACC and lateral PFC) and the default network (ventral ACC, posterior cingulate/precuneus, and temporoparietal cortex). Reports of mind wandering with meta-awareness (Fig. 4b; Table S2) were associated with similar, but weaker activation in both networks. When mind wandering in the absence of meta-awareness was directly compared to mind wandering with meta-awareness (Fig. 5 and Table 3), significant activations in a number of regions in the executive and default network were observed, including the medial and lateral aspects of the anterior PFC (BA 10), dorsal ACC (BA 32), right DLPFC (BA 9/10), and posterior cingulate/precuneus (BA 31/7). Additional regions of activation included the superior temporal cortex, the fusiform and parahippocampal gyri, the posterior insula, thalamus, and cerebellum. No brain regions were significantly more activated during mind wandering with meta-awareness than during mind wandering in the absence of meta-awareness.
Fig. 4.
Mind wandering in the absence (a) and presence (b) of meta-awareness. (a) Regions of activation associated with mind wandering in the absence of awareness (intervals prior to off-task unaware vs. on-task probes) included: (A) Dorsal ACC (BA 32), (B) Ventral ACC (BA 32), (C) Precuneus (BA 7), (D) Temporoparietal Junction (BA 39), (E) Dorsal Rostromedial PFC (BA 10), (F) Right Rostrolateral PFC (BA 10), (G) Posterior and Anterior Insula, and (H) Bilateral Temporopolar Cortex. (b) Similar regions were activated during mind wandering with awareness (intervals prior to off-task aware vs. on-task probes), but to a lesser degree, including: (A) Dorsal ACC (BA 32), (B) Ventral ACC (BA 24/32), and (G) Posterior and Anterior insula. Height threshold P < 0.005, extent threshold k > 5 voxels.
Fig. 5.
Regions showing greater activation during mind wandering in the absence of meta-awareness compared to mind wandering with meta-awareness (intervals prior to off-task unaware vs. off-task aware probes). Height threshold P < 0.005, extent threshold k > 5.
Table 3.
Activation peaks for mind wandering without meta-awareness vs. mind wandering with meta-awareness
| Region | BA | Number of voxels | Z-value | Talairach coordinates, x, y, z |
|---|---|---|---|---|
| Left RMPFC | 10 | 12 | 3.86 | -12, 66, 24 |
| Left RLPFC | 10 | 7 | 3.8 | -28, 62, 24 |
| Left Dorsal ACC | 32 | 4 | 3.23 | -14, 22, 36 |
| Right MFG | 9 | 6 | 3.47 | 30, 58, 32 |
| PCC/precuneus | 31/7 | 42 | 3.59 | 16, -38, 48 |
| PCC | 30 | 13 | 3.5 | 12, -66, 28 |
| Left STG | 12/21 | 20 | 3.52 | -54, 2, -8 |
| 22 | - | 3.23 | -48, -2, -4 | |
| Right STG | 22 | 5 | 3.35 | 66, -24, 4 |
| Left Fusiform gyrus | 20 | 11 | 3.41 | -30, -36, -16 |
| Left PHG | 36 | - | 3.34 | -36, -32, -20 |
| Right Lingual gyrus | 18 | 9 | 3.3 | 16, -62, 4 |
| Left Lingual gyrus | 18 | 10 | 3.25 | -16, -56, 4 |
| Right Posterior Insula | - | 38 | 4.53 | 38, -20, -4 |
| Thalamus | - | 17 | 3.81 | -10, -16, 12 |
| Left Medial cerebellum | - | 30 | 3.63 | -14, -62, -16 |
| Left Lateral cerebellum | - | 16 | 3.43 | -48, -66, -20 |
Intervals prior to off-task unaware vs. off-task aware reports. All activations were significant at the P < 0.001 level. RMPFC, rostromedial prefrontal cortex; RLPFC, rostrolateral prefrontal cortex; STG, superior temporal gyrus.
Discussion
This study set out to explore the neural recruitment that occurs when the mind wanders away from the task at hand. A key finding was that mind wandering was associated with recruitment of medial PFC, posterior cingulate/precuneus, and posterior temporoparietal cortex, regions that form the core of the default network (1–3). This finding is consistent with prior theorizing about the functions of the default network (2, 3), and with neuroimaging studies (6–8) that varied task demands to explore the link between default network functions and the mind wandering process. Also, the results demonstrate that mind wandering entails recruitment of the executive system of the brain, a finding predicted by behavioral theories of off-task thought. Finally, individuals' self-reports of whether or not they were aware of their mind wandering indicated that brain recruitment associated with off-task thinking is most pronounced in the absence of meta-awareness.
The medial prefrontal part of the default network, whose involvement in mind wandering has been the subject of a recent controversy (6, 9, 31), was activated both when subjects reported having their attention turned away from the task and before making an error, a condition associated with less effective task-directed attention (5). To our knowledge, these findings of medial PFC recruitment for 2 independent measures of mind wandering provide the strongest empirical evidence to date for a link between default network functions and mind wandering. They also suggest that the observed activations do not necessarily reflect stimulus-related attentional focus that contributes to effective task-related processing, as previously argued (9). Nonetheless, the present results do not entirely rule out a role for the medial PFC in stimulus-oriented thought, because some aspects of mind wandering could involve a preoccupation with task-irrelevant stimulus features (e.g., the scanner noise or the rhythm of stimulus presentation). Although our findings yield strong support to the notion that the medial PFC is involved in mind wandering (6), they do not specify whether it is involved in stimulus-independent or stimulus-oriented mind wandering, an important question that remains subject for further research.
In addition to default network recruitment, mind wandering was also associated with recruitment of the dorsal ACC and the DLPFC, the 2 main regions of the executive network (18, 20, 32). Although previous neuroimaging studies (6–8) have primarily linked mind wandering to default network recruitment, this executive system recruitment is consistent with behavioral findings indicating a processing overlap between mind wandering and central executive resources (5, 21, 22, 26), and may help explain why mind wandering can undermine performance on demanding tasks (5, 14, 15, 26, 29, 33). Prior neuroimaging studies have generally assessed mind wandering indirectly, by comparing brain activation between tasks shown to vary in the extent to which they facilitate mind wandering. In contrast, by holding the task constant and examining on-line fluctuations in individuals' self-reported mind wandering, the present investigation may have been able to detect executive system involvement where others have not (6, 7).
Intriguingly, brain recruitment in both default and executive network regions was strongest when mind wandering occurred in the absence of meta-awareness. These findings complement behavioral results, demonstrating that mind wandering is most disruptive to concurrent task performance when it goes unnoticed (5, 27, 29). The observed pattern of interaction between mind wandering and meta-awareness offers a number of constraints for interpreting the recruitment of executive resources during mind wandering. From a theoretical perspective, executive recruitment during mind wandering could reflect several possible processes, including (i) multitasking, or the conscious coordination of task performance and mind wandering; (ii) increased conflict detection and cognitive control aimed toward bringing attention back to the experimental task; or (iii) detecting conflict within the content of mind wandering itself, i.e., thoughts and memories from the stream of consciousness that pertain to discrepancies between one's more general personal goals and the current state of affairs. Because the first 2 possibilities would entail meta-awareness of the mind wandering process, they would predict stronger executive recruitment when subjects are meta-aware. However, the finding that both the anterior PFC, a region previously linked to multitasking (34–37), and the dorsal ACC, a region closely linked to conflict detection (32, 38–40), were more active when subjects were unaware of their own mind wandering, suggests that the third possibility may be most likely, namely, that executive recruitment reflects, at least in part, the presence of conflict inherent to the content of mind wandering. This possibility would also be consistent with observations that the content of mind wandering is closely related to current personal concerns and unresolved matters (41–43).
Also, the anterior PFC (BA 10), which has previously been implicated in meta-awareness of one's own internal mental contents (2, 37, 44–50), was significantly more active when subjects were unaware of their own mind wandering than when meta-awareness was present. This finding suggests that subjects may have been aware of the contents of their consciousness, without being aware of the fact that they were mind wandering, or the process of mind wandering itself. Although this suggested distinction between meta-awareness of process and content is not part of current theoretical models of anterior PFC function (34, 36, 44–46, 49, 51) and metaconsciousness (23, 52), it may prove instrumental for their future development. Also, the finding that mind wandering with meta-awareness did not result in significant activations above and beyond those observed during mind wandering without meta-awareness suggests that the same brain regions that implement awareness of the mind wandering process may also underlie meta-awareness of the contents of mind wandering when subjects are unaware of the process itself.
However, it must be acknowledged that the above interpretations of mental processes underlying the neural recruitment associated with mind wandering involve “reverse inference” (53–56), or inferring the engagement of a particular cognitive process from the activation of a particular brain region. Although reverse inference can be informative, and is widely used in the neuroimaging literature, it has limited validity (53–56), especially for higher order brain regions such as the anterior PFC that can be activated by multiple, diverse mental processes (46, 55). Future research might profitably overcome the limitations of such reverse inference by using in-depth experience sampling to gain insight into the specific content of participants' thoughts during mind wandering such as, for example, whether they were engaging in meta-cognitive reflection.
Although this study was not specifically designed to reveal the potential functions of mind wandering, the observation of parallel recruitment of the executive and default network regions presents an intriguing possibility. In general, the executive and default networks are thought to act in opposition to each other so that when the executive network becomes activated, the default network becomes deactivated or actively suppressed (57–59). In contrast, here we observed a parallel recruitment of the 2 networks. Although this activation pattern differs from the pattern of results observed during many tasks and baseline conditions (1–3), it is reminiscent of the neural recruitment observed during creative thinking (60–62), where executive regions such as the dorsal ACC and default network regions such as the PCC are activated before solving problems with insight. Also, a similar parallel recruitment of executive and default regions has also been observed during naturalistic film viewing (63), which is related to immersive simulative mental experience (64). Thus, mind wandering may be part of a larger class of mental phenomena that enable executive processes to occur without diminishing the potential contribution of the default network for creative thought (60–62, 65) and mental simulation (66–68). Although it may undermine our immediate goals, mind wandering may enable the parallel operation of diverse brain areas in the service of distal goals that extend beyond the current task.
The ability of this study to provide a direct empirical examination of the neural recruitment associated with mind wandering underscores the value of combining experience sampling with the tools of cognitive neuroscience for advancing our understanding of the neurophenomenology of subjective experience (69). Also, the finding of default network recruitment in association with 2 independent measures, one relying on subjective experience sampling and the other on behavioral performance, helps to validate the use of first-person introspective reports in the study of mind wandering (14, 24, 70). In general, experimental investigations in cognitive neuroscience try to minimize their reliance on subjective reports, and instead, aim to establish a task-based control over subjects' mental processes. However, this approach seems too limited for investigating spontaneously occurring, highly subjective mental experiences such as mind wandering (69, 71, 72). Instead, by using introspective reports alongside behavioral and brain imaging measures (71), and by creating experimental situations in which they reciprocally constrain each other (24, 69, 73), we may be able to further enrich our understanding of the elusive nature of subjective experience. Thus, by capitalizing on the ability of our species for self-reflection and combining it with the detailed measures of brain function that modern technology allows, cognitive neuroscience may be better able to reveal what is unique about the human mind.
Materials and Methods
Subjects.
Fifteen right-handed students from the University of British Columbia (UBC) (mean age 22; age range 19–29 years; 10 female) gave their written consent to participate and received $20 per hour as compensation. Procedures were approved by the UBC Clinical Research Ethics Board and by the UBC High Field Magnetic Imaging Centre.
SART.
During the SART, 1 digit (0–9) was presented every 2 s. Targets, consisting of the number 3, appeared in 5% of trials. This relatively low target frequency was chosen as it helps establish an automaticity of response and promotes relatively high incidence of mind wandering (70).
Functional MRI Data Acquisition.
Data were collected using a 3.0 Tesla Philips Intera MRI scanner. Head movement was restricted using foam padding around the head. Five functional runs, each consisting of 800 dynamics, were acquired for each participant using a time of repetition (TR) of 1,000 ms. The functional volumes contained BOLD contrast intensity values and were acquired using a T2*-weighted single shot echo-planar imaging (EPI) gradient echo sequence sensitive to BOLD contrast [echo time (TE) = 30 ms; flip angle (FA) = 90°; field of view (FOV) = 24 × 24 × 13.2 cm; matrix size 80 × 80, reconstructed to 128 × 128; SENSE factor = 1.0; inplane resolution = 3 mm]. The volumes covered the whole brain and consisted of 19 slices (each, 6-mm thick; separated by a 1-mm interslice gap) acquired parallel to the AC/PC line. Before functional imaging, an inversion recovery prepared T1-weighted fast spin-echo anatomic volume was obtained for each participant (TR = 2000 ms; TE = 10 ms; spin echo turbo factor = 5; FA = 90; FOV = 24 × 24 cm2; 512 × 512 voxels; inversion delay IR = 800 ms). It contained 19 slices (6-mm thick, separated by 1-mm skip) acquired in the same slice locations as that used for the functional images.
Functional MRI Analysis.
Data were preprocessed and analyzed using SPM5. Voxel time series were interpolated using sinc interpolation and resampled using the middle (tenth) slice as a reference point. All functional volumes were realigned to the first one in the time series. The structural T1-weighted volume was segmented to extract a gray matter image for each subject, which was spatially normalized to a gray matter image of the MNI template. The derived spatial transformations for each subject were applied to the realigned functional volumes, to bring them into standardized MNI space. After normalization, all volumes were resampled in 2 × 2 × 4 mm voxels using sinc interpolation in space. Fianlly, all T2*-weighted volumes were smoothed with an 8-mm FWHM isotropic Gaussian kernel.
Statistical analysis was performed at each voxel to assess the magnitude of differences between conditions of interest. An anatomically defined gray-matter mask was created and explicitly specified to ensure that statistical analysis was performed in all brain regions, including those where signal may have been low due to susceptibility artifacts. To remove low-frequency drift in the BOLD signal, data were high-pass filtered using an upper cut-off period of 164 s. No global scaling was performed.
Condition effects at each voxel were estimated according to the general linear model (for details, see SI Materials and Methods). Regressors of interests were compared in pairwise comparisons, and the resulting t maps were subsequently transformed to the unit normal Z-distribution to create a statistical parametric map for each contrast. The foci of maximum activation were localized on an anatomical image created by averaging the normalized individual T1-weighted images. Threshold for significance in the brain was set at voxel level P < 0.001.
Supplementary Material
Acknowledgments.
We thank Brian Makinen for his help with data collection and analysis; Matt Botvinick, Todd Handy, Alan Kingstone, John Kounios, Raymond Mar, Masataka Watanabe, and 2 anonymous reviewers for their thoughtful comments and feedback on earlier versions of the manuscript. K.C. was supported by grant awards from the Natural Sciences and Engineering Research Council, the Canadian Institutes of Health Research, and the Michael Smith Foundation for Health Research. J.W.S. and J.S. were supported by the Bower Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900234106/DCSupplemental.
References
- 1.Shulman GL, et al. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 4.Wegner DM. In: Scientific approaches to consciousness. Cohen JD, Schooler JW, editors. Mahwah, NJ: Erlbaum; 1997. pp. 295–315. [Google Scholar]
- 5.Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- 6.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. Neuroreport. 1996;7:2095–2099. [PubMed] [Google Scholar]
- 8.McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: The default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. [DOI] [PubMed] [Google Scholar]
- 10.Hurlburt RT. Sampling Normal and Schizophrenic Inner Experience. New York: Plenum Press; 1990. [Google Scholar]
- 11.Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA. A survey method for characterizing daily life experience: The day reconstruction method. Science. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- 12.Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. 'Oops!': Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 13.Manly T, Robertson IH, Galloway M, Hawkins K. The absent mind: Further investigations of sustained attention to response. Neuropsychologia. 1999;37:661–670. doi: 10.1016/s0028-3932(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 14.Smallwood J, et al. Subjective experience and the attentional lapse: Task engagement and disengagement during sustained attention. Conscious Cogn. 2004;13:657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Smallwood J, Beach E, Schooler JW, Handy TC. Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. J Cogn Neurosci. 2008;20:458–469. doi: 10.1162/jocn.2008.20037. [DOI] [PubMed] [Google Scholar]
- 16.Cheyne JA, Carriere JSA, Smilek D. Absent-mindedness: Lapses of conscious awareness and everyday cognitive failures. Conscious Cogn. 2006;15:578–592. doi: 10.1016/j.concog.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: A comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 20.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Cogn Sci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 21.Kane MJ, et al. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychol Sci. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 22.Teasdale JD, et al. Stimulus-independent thought depends on central executive resources. Mem Cognit. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- 23.Schooler JW. Re-representing consciousness: Dissociations between experience and meta-consciousness. Trends Cogn Sci. 2002;6:339–344. doi: 10.1016/s1364-6613(02)01949-6. [DOI] [PubMed] [Google Scholar]
- 24.Schooler JW, Schreiber CA. Experience, meta-consciousness, and the paradox of introspection. J Conscious Stud. 2004;11:17–39. [Google Scholar]
- 25.Winkielman PW, Schooler JW. In: Social Cognition: The basis of Human Interaction. Strack F, Förster J, editors. Philadelphia: Psychology Press; 2009. in press. [Google Scholar]
- 26.Schooler JW, Reichle ED, Halpern DV. In: Thinking and Seeing: Visual meta-cognition in Adults and Children. Levine DT, editor. Cambridge, MA: MIT Press; pp. 203–226. [Google Scholar]
- 27.Smallwood J, McSpadden M, Schooler JW. The lights are on but no one's home: Meta-awareness and the decoupling of attention when the mind wanders. Psychon Bull Rev. 2007;14:527–533. doi: 10.3758/bf03194102. [DOI] [PubMed] [Google Scholar]
- 28.Smallwood J, McSpadden M, Luus B, Schooler J. Segmenting the stream of consciousness: The psychological correlates of temporal structures in the time series data of a continuous performance task. Brain Cogn. 2008;66:50–56. doi: 10.1016/j.bandc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Smallwood J, Schooler J. When attention matters: The curious incident of the wandering mind. Mem Cognit. 2008;36:1144–1150. doi: 10.3758/MC.36.6.1144. [DOI] [PubMed] [Google Scholar]
- 30.Antrobus JS. Information theory and stimulus-independent thought. Br J Psychol. 1968;59:423–430. [Google Scholar]
- 31.Mason MF, et al. Response to comment on “Wandering minds: The default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. [DOI] [PubMed] [Google Scholar]
- 32.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 33.Smallwood J, O'Connor RC, Sudberry MV, Haskell C, Ballantyne C. The consequences of encoding information on the maintenance of internally generated images and thoughts: The role of meaning complexes. Conscious Cogn. 2004;13:789–820. doi: 10.1016/j.concog.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 35.Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- 36.Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert SJ, et al. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 38.Carter CS, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 39.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 40.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 41.Antrobus JS, Singer JL, Goldstein S, Fortgang M. Mindwandering and cognitive structure. Trans N Y Acad Sci. 1970;32:242–252. doi: 10.1111/j.2164-0947.1970.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 42.Klinger E, Cox WM. Dimensions of thought flow in everyday life. Imagination Cognition Personality. 1987;7:105–128. [Google Scholar]
- 43.Smallwood J, Fitzgerald A, Miles LM, L. P Shifting moods, wandering minds: Negative moods lead the mind to wander. Emotion. 2009;9:271–276. doi: 10.1037/a0014855. [DOI] [PubMed] [Google Scholar]
- 44.Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- 45.Ochsner KN, et al. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 46.Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- 47.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christoff K, Ream JM, Geddes LPT, Gabrieli JDE. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- 49.Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. In: Measuring the Mind: Speed, Control, and Age. Duncan J, Phillips L, McLeod P, editors. Oxford: Oxford Univ Press; 2005. pp. 217–248. [Google Scholar]
- 50.Smith R, Keramatian K, Christoff K. Localizing the rostrolateral prefrontal cortex at the individual level. Neuroimage. 2007;36:1387–1396. doi: 10.1016/j.neuroimage.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 51.Christoff K, Ream JM, Gabrieli JD. Neural basis of spontaneous thought processes. Cortex. 2004;40:623–630. doi: 10.1016/s0010-9452(08)70158-8. [DOI] [PubMed] [Google Scholar]
- 52.Lambie JA, Marcel AJ. Consciousness and the varieties of emotion experience: A theoretical framework. Psychol Rev. 2002;109:219–259. doi: 10.1037/0033-295x.109.2.219. [DOI] [PubMed] [Google Scholar]
- 53.D'Esposito M, Ballard D, Aguirre GK, Zarahn E. Human prefrontal cortex is not specific for working memory: A functional MRI study. Neuroimage. 1998;8:274–282. doi: 10.1006/nimg.1998.0364. [DOI] [PubMed] [Google Scholar]
- 54.Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Christoff K, Owen AM. Improving reverse neuroimaging inference: Cognitive domain versus cognitive complexity. Trends Cogn Sci. 2006;10:352–353. doi: 10.1016/j.tics.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Van Horn JD, Poldrack RA. Functional MRI at the crossroads. Int J Psychophysiol. 2008 Nov 18; doi: 10.1016/j.ijpsycho.2008.11.003. doi: 10.1016/j.ijpsycho.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 60.Kounios J, et al. The prepared mind: neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychol Sci. 2006;17:882–890. doi: 10.1111/j.1467-9280.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- 61.Kounios J, et al. The origins of insight in resting-state brain activity. Neuropsychologia. 2008;46:281–291. doi: 10.1016/j.neuropsychologia.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subramaniam K, Kounios J, Parrish TB, Jung-Beeman M. A brain mechanism for facilitation of insight by positive affect. J Cogn Neurosci. 2009;21:415–432. doi: 10.1162/jocn.2009.21057. [DOI] [PubMed] [Google Scholar]
- 63.Golland Y, et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- 64.Mar RA, Oatley K. The function of fiction is the abstraction and simulation of social experience. Perspect Psychol Sci. 2008;3:173–192. doi: 10.1111/j.1745-6924.2008.00073.x. [DOI] [PubMed] [Google Scholar]
- 65.Christoff K, Gordon AM, Smith R. In: Neuroscience of Decision Making. Vartanian O, Mandel DR, editors. New York: Psychology Press; 2009. in press. [Google Scholar]
- 66.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 67.Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 68.Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 69.Lutz A, Thompson E. Neurophenomenology: Integrating subjective experience and brain dynamics in the neuroscience of consciousness. J Conscious Stud. 2003;10:31–52. [Google Scholar]
- 70.Smallwood JM, Baracaia SF, Lowe M, Obonsawin M. Task unrelated thought whilst encoding information. Conscious Cogn. 2003;12:452–484. doi: 10.1016/s1053-8100(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 71.Jack AI, Roepstorff A. Introspection and cognitive brain mapping: From stimulus-response to script-report. Trends Cogn Sci. 2002;6:333–339. doi: 10.1016/s1364-6613(02)01941-1. [DOI] [PubMed] [Google Scholar]
- 72.Cosmelli D, Lachaux J-P, Thompson E. In: The Cambridge Handbook of Consciousness. Zelazo PD, Moscovitch M, Thompson E, editors. New York: Cambridge Univ Press; 2007. pp. 731–772. [Google Scholar]
- 73.Schooler JW. Establishing a legitimate relationship with introspection: Response to Jack and Roepstorf. Trends Cogn Sci. 2002;6:372–373. doi: 10.1016/s1364-6613(02)01970-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.