Abstract
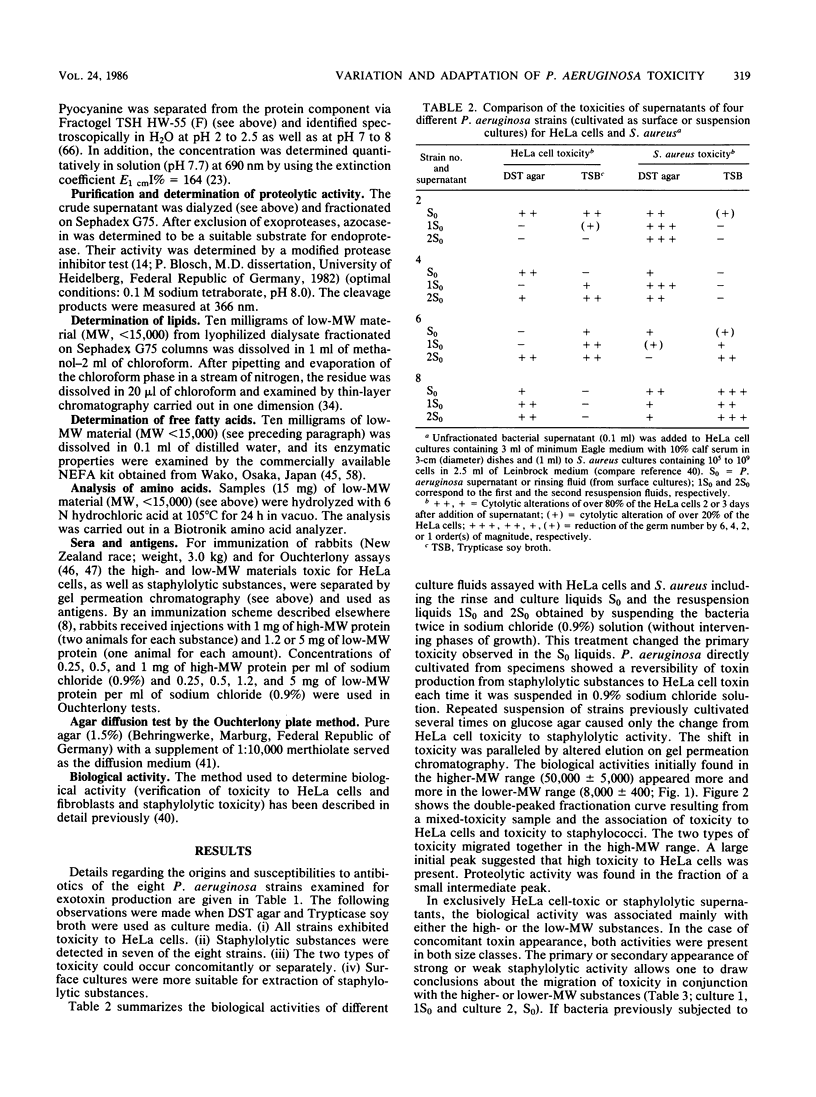
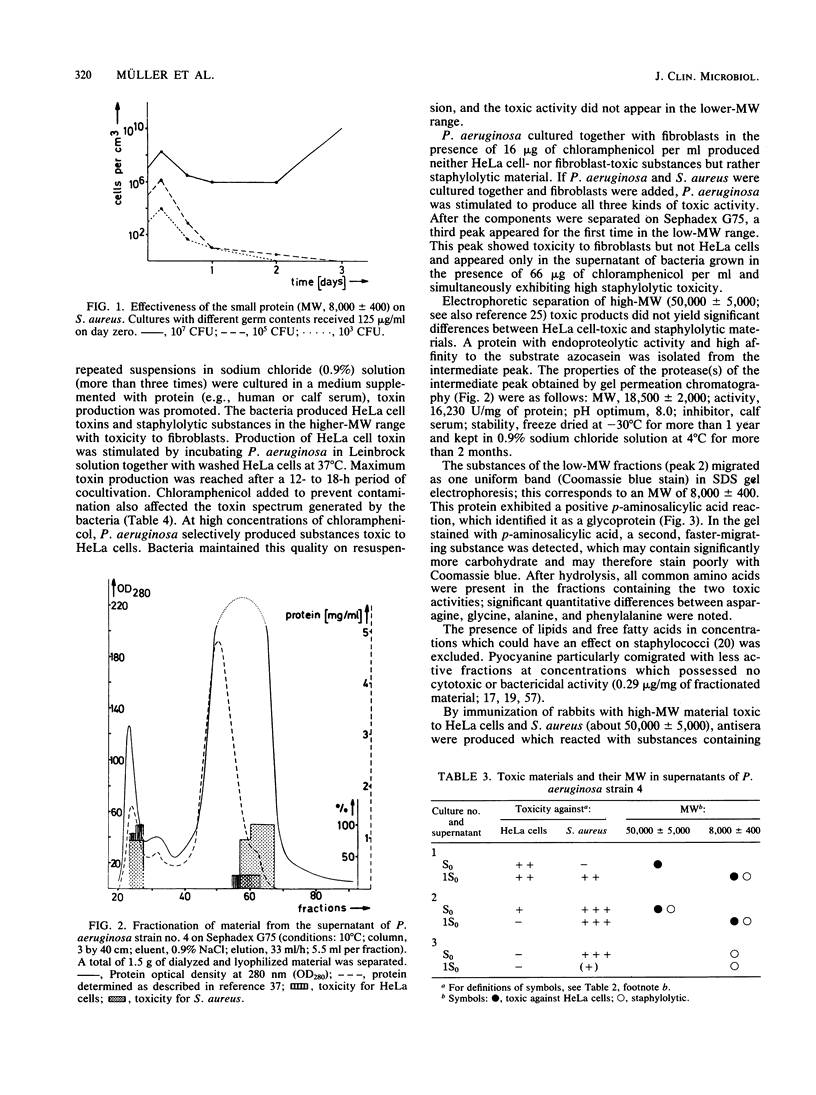
The toxic components of supernatants from Pseudomonas aeruginosa cultures directed against HeLa cells and Staphylococcus aureus were evaluated with the aim of discovering interactions. Supernatants of eight different strains of P. aeruginosa were assayed for cytotoxic activity. All were active against HeLa cells; seven were toxic for S. aureus. On repeated suspension of P. aeruginosa in 0.9% sodium chloride solution, a shift from HeLa cell toxicity to staphylococcal lytic activity occurred along with a change of toxic activity from a high (50,000 +/- 5,000) to a low (8,000 +/- 400) molecular weight (MW) range on gel filtration. Addition of protein to the minimal medium of cultures producing material toxic only for S. aureus reactivated the generation of HeLa cell-toxic material. Cultivation of P. aeruginosa in the presence of HeLa cells and a chloramphenicol supplement produced suppression of the generation of material toxic for S. aureus but facilitated that of HeLa-toxic material of high MW. Adaptation of toxicity against fibroblasts developed only on cocultivation of P. aeruginosa together with S. aureus and in the presence of fibroblasts. Under these conditions a strong lytic activity for S. aureus appeared, even in the presence of chloramphenicol. Chloramphenicol caused the material toxic for fibroblasts to elute at a low MW well separated from that toxic for HeLa cells. In contrast to the high-MW toxic substances, the low-MW material did not induce antibodies after injection into rabbits. This may explain failures of vaccination against P. aeruginosa infection and of serum therapy of homologous sepsis in humans.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S. Prospects for a mucoid exopolysaccharide vaccine for the prevention of infection due to mucoid strains of Pseudomonas aeruginosa. Antibiot Chemother (1971) 1985;36:147–156. doi: 10.1159/000410479. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Berger U., Piotrowski H. D. Zur Diagnostik der nicht-fermentierenden gramnegativen Stäbchenbakterien. Erfahrungen an 676 apyocyaninogenen Stämmen. Zentralbl Bakteriol A. 1981 Feb;248(4):509–525. [PubMed] [Google Scholar]
- Borderon E., Vargues R. Action des lysines de Pseudomonas aeruginosa sur les membranes bacteriennes. C R Seances Soc Biol Fil. 1972;166(11):1531–1534. [PubMed] [Google Scholar]
- Botzenhart K., Ebel E. A. Zytotoxische Aktivität von Pseudomonas aeruginosa in Beziehung zu Lecithinase, Lipase, Proteinase, Hämolysin und Oberflächenaktivität. Zentralbl Bakteriol Orig A. 1973 Dec;225(2):387–397. [PubMed] [Google Scholar]
- Burke M. E., Pattee P. A. Purification and characterization of a staphylolytic enzyme from Pseudomonas aeruginosa. J Bacteriol. 1967 Mar;93(3):860–865. doi: 10.1128/jb.93.3.860-865.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd Pseudomonas aeruginosa exotoxin: purification by preparative polyacrylamide gel electrophoresis and the development of a highly specific antitoxin serum. Infect Immun. 1976 Jul;14(1):55–61. doi: 10.1128/iai.14.1.55-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd Purification and characterization of Pseudomonas aeruginosa exotoxin. Infect Immun. 1974 Jan;9(1):113–118. doi: 10.1128/iai.9.1.113-118.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K. Extracellular toxins of Pseudomonas aeruginosa. III. Radioimmunoassay for detection of alkaline protease. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):239–247. [PubMed] [Google Scholar]
- Farkas-Himsley H., Cheung R. Bacterial proteinaceous products (bacteriocins) as cytotoxic agents of neoplasia. Cancer Res. 1976 Oct;36(10):3561–3567. [PubMed] [Google Scholar]
- Gilbert D. N. An evaluation of antipseudomonal antimicrobic agents. Antibiot Chemother (1971) 1985;36:111–133. doi: 10.1159/000410477. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Parker M. G., Matthews J. M., Berg R. D. Use of a purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984 Apr;44(1):49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Mechanism of the antibiotic action pyocyanine. J Bacteriol. 1980 Jan;141(1):156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R. C., Pavlovskis O. R., Galloway D. R. Antibody response of infected mice to outer membrane proteins of Pseudomonas aeruginosa. Infect Immun. 1984 Jan;43(1):49–53. doi: 10.1128/iai.43.1.49-53.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. M., Campbell J. J. A new resuspension medium for pyocyanine production. Can J Microbiol. 1969 Jun;15(6):595–598. doi: 10.1139/m69-101. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Phillippe L., Teng Tseng J., Stemke G. W., Campbell J. N. Purification and characterization of exocellular proteases produced by a clinical isolate and a laboratory strain of Pseudomonas aeruginosa. Can J Microbiol. 1980 Jan;26(1):77–86. doi: 10.1139/m80-012. [DOI] [PubMed] [Google Scholar]
- Keilich G., Brossmer R., Ehrhardt T., Pech H., Müller H., Sonntag H. G., Spring H., Kinzel V. Exotoxine von Pseudomonas aeruginosa. I. Mitteilung: Substanzgewinnung, Reinigung und biochemische Charakterisierung. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 May;252(1):57–73. [PubMed] [Google Scholar]
- Keilich G., Brossmer R., Müller H., Pech H., Franke W. W., Spring H. Das Schüttelverfahren als Methode für Zellwanduntersuchungen bei Pseudomonas aeruginosa. II. Mitteilung: Die mechanisch abtennnbaren und chemisch nachweisbaren Substanzen. Zentralbl Bakteriol Orig A. 1976 Apr;234(3):317–326. [PubMed] [Google Scholar]
- LIU P. V., ABE Y., BATES J. L. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. J Infect Dis. 1961 Mar-Apr;108:218–228. doi: 10.1093/infdis/108.2.218. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lache M., Hearn W. R., Zyskind J. W., Tipper D. J., Strominger J. L. Specificity of a bacteriolytic enzyme from Pseudomonas aeruginosa. J Bacteriol. 1969 Oct;100(1):254–259. doi: 10.1128/jb.100.1.254-259.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Yoshii S., Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1973 Oct;128(4):514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- Lory S., Collier R. J. Expression of enzymic activity by exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1980 May;28(2):494–501. doi: 10.1128/iai.28.2.494-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S., Tai P. C., Davis B. D. Mechanism of protein excretion by gram-negative bacteria: Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1983 Nov;156(2):695–702. doi: 10.1128/jb.156.2.695-702.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER H., KLEINMAIER H. Zur Serostruktur der O-Antigene von Pseudomonas aeruginosa. Z Hyg Infektionskr. 1958;145(1):85–91. [PubMed] [Google Scholar]
- Marks M. I. The pathogenesis and treatment of pulmonary infections in patients with cystic fibrosis. J Pediatr. 1981 Feb;98(2):173–179. doi: 10.1016/s0022-3476(81)80631-2. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Production of protease and elastase by Pseudomonas aeruginosa strains isolated from patients. Infect Immun. 1977 Mar;15(3):679–685. doi: 10.1128/iai.15.3.679-685.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H., Kinzel V., Richards J., Pech H., Keilich G., Brossmer R., Ehrhardt T., Sonntag H. G. Exotoxine von Pseudomonas aeruginosa II. Mitteilung: Cytostatische und bakteriostatische Aktivität. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 May;252(1):74–82. [PubMed] [Google Scholar]
- Müller H., Pech H., Keilich G., Brossmer R., Franke W. W., Spring H. Das Schüttelverfahren als Methode für Zellwanduntersuchungen bei Pseudomonas aeruginosa. I. Mitteilung: Serologisch nachweisbare Oberflächenänderungen. Zentralbl Bakteriol Orig A. 1976 Apr;234(3):305–316. [PubMed] [Google Scholar]
- Nicas T. I., Iglewski B. H. Genetic approaches to study Pseudomonas aeruginosa protein antigens. Adv Exp Med Biol. 1985;185:223–232. doi: 10.1007/978-1-4684-7974-4_15. [DOI] [PubMed] [Google Scholar]
- Obernesser H. J., Döring G. Extracellular toxins of Pseudomonas aeruginosa. IV. Radioimmunoassay for detection of elastase. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):248–256. [PubMed] [Google Scholar]
- Okabe H., Uji Y., Nagashima K., Noma A. Enzymic determination of free fatty acids in serum. Clin Chem. 1980 Oct;26(11):1540–1543. [PubMed] [Google Scholar]
- Parmely M. J., Iglewski B. H., Horvat R. T. Identification of the principal T lymphocyte-stimulating antigens of Pseudomonas aeruginosa. J Exp Med. 1984 Nov 1;160(5):1338–1349. doi: 10.1084/jem.160.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Edman D. C., Leppla S. H., Wretlind B., Lewis L. R., Martin K. E. Protection against experimental Pseudomonas aeruginosa infection in mice by active immunization with exotoxin A toxoids. Infect Immun. 1981 May;32(2):681–689. doi: 10.1128/iai.32.2.681-689.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Gordon F. B. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J Infect Dis. 1972 Jun;125(6):631–636. doi: 10.1093/infdis/125.6.631. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Pollack M., Callahan L. T., 3rd, Iglewski B. H. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect Immun. 1977 Dec;18(3):596–602. doi: 10.1128/iai.18.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflüger R., Scharmann W., Blobel H. Toxizitätsuntersuchungen an Kulturüberständen von Pseudomonas aeruginosa. Zentralbl Bakteriol Orig A. 1975 Oct;233(2):236–244. [PubMed] [Google Scholar]
- Pier G. B. Pseudomonas aeruginosa surface polysaccharide vaccines. New therapeutic approaches from basic research. Antibiot Chemother (1971) 1985;36:157–167. [PubMed] [Google Scholar]
- SCHNEIERSON S. S., AMSTERDAM D., PERLMAN E. Inhibition of Pseudomonas aeruginosa pigment formation by chloramphenicol and erythromycin. Antibiot Chemother (Northfield) 1960 Jan;10:30–33. [PubMed] [Google Scholar]
- Sadoff J. C., Wright D. C., Futrovsky S., Sidberry H., Collins H., Kaufmann B. Characterization of mouse monoclonal antibodies directed against Pseudomonas aeruginosa lipopolysaccharides. Antibiot Chemother (1971) 1985;36:134–146. doi: 10.1159/000410478. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Tani Y., Yamada H., Tabata M., Murachi T. Enzymatic determination of serum-free fatty acids: a colorimetric method. Anal Biochem. 1980 Sep 1;107(1):193–198. doi: 10.1016/0003-2697(80)90511-4. [DOI] [PubMed] [Google Scholar]
- Stanislavsky E. S., Joo I., Mashilova G. M., Boolk V. F., Boltutsy L. G., Zakgeim D. A., Severtśova M. K., Yedvabnaya L. S., Gladus M. A., Fatkhinurova T. I. Vaccines against Pseudomonas aeruginosa infection: 1. Experimental studies. Vaccine. 1985 Jun;3(2):128–136. doi: 10.1016/0264-410x(85)90062-3. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Iglewski B. H. Comparative toxicities of diphtherial toxin and Pseudomonas aeruginosa exotoxin A: evidence for different cell receptors. J Gen Microbiol. 1978 Oct;108(2):333–337. doi: 10.1099/00221287-108-2-333. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Wadström T. Purification and properties of a protease with elastase activity from Pseudomonas aeruginosa. J Gen Microbiol. 1977 Dec;103(2):319–327. doi: 10.1099/00221287-103-2-319. [DOI] [PubMed] [Google Scholar]
- ZAUGG W. S. SPECTROSCOPIC CHARACTERISTICS AND SOME CHEMICAL PROPERTIES OF N-METHYLPHENAZINIUM METHYL SULFATE (PHENAZINE METHOSULFATE) AND PYOCYANINE AT THE SEMIQUIDNOID OXIDATION LEVEL. J Biol Chem. 1964 Nov;239:3964–3970. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- de Repentigny J., Mathieu L. G. Metabolism and pathogenicity in Staphylococcus single and mixed cultures and infections. Ann N Y Acad Sci. 1974 Jul 31;236(0):144–159. doi: 10.1111/j.1749-6632.1974.tb41488.x. [DOI] [PubMed] [Google Scholar]