Abstract
In contrast to adults, the murine neonatal CD4+ compartment contains a high frequency of recent thymic emigrants (RTEs). However, the functional capabilities of these cells in neonates are relatively unknown. Moreover, it has not been determined whether RTEs from neonates and adults are comparable. Here we have directly compared neonatal and adult CD4+ RTEs for the first time, using a transgenic mouse strain that allows for the identification and purification of RTEs. Our data demonstrate that RTEs from murine neonates and adults are phenotypically and functionally distinct. In particular, although the magnitude of RTEs cytokine responses from both age groups is dependent on the conditions of activation, neonatal RTEs always exhibited higher levels of effector Th1/Th2 cytokine production than adult RTEs. In addition, neonatal, but not adult, RTEs showed early proliferation in response to stimulation with interleukin-7 alone. This was associated with faster kinetics of interleukin-7Rα down-regulation and higher levels of pSTAT5 in neonatal RTEs. These quantitative and qualitative differences in the neonatal and adult RTEs populations may at least partially explain the diverse responses that are elicited in vivo in neonates in response to different conditions of antigen exposure.
Introduction
Newborn (≤ 1-day-old) mice are considered lymphopenic, as their peripheral lymphoid organs contain only small numbers of cells.1 During early life, the number of cells in the periphery is gradually increased by the constant output of newly exported T cells from the thymus, referred to as recent thymic emigrants (RTEs). Although thymic output is similar in neonates and adults,2 at day 7 of life, there remains a paucity of T cells in the neonatal lymph nodes (LNs) and spleen. Because of this reduced number of total cells, a greater proportion of T cells in the neonatal periphery are RTEs, compared with adults.2–4 Little is currently known about the characteristics of neonatal RTEs, as the majority of studies examine only adult RTEs.3,5–9 Because RTEs are more abundant among neonatal T-cell populations, distinct properties of neonatal RTEs may contribute to the diverse patterns of neonatal T helper (Th) responses in neonates.1 Moreover, because there has been no direct comparison of purified RTEs from neonates and adults, it is unknown whether RTEs from these 2 age groups are similar or different.
In adult mice, Bendelac et al5 demonstrated that RTEs are functionally mature, producing higher levels of both interleukin-4 (IL-4) and interferon-γ (IFN-γ) than resident cells. Clise-Dwyer et al also showed that adult RTEs are functionally mature,7 producing equivalent levels of IL-2, and with the same proliferative capacity as resident cells. However, a dichotomy exists in the literature, as there is also evidence that adult RTEs are functionally deficient compared with resident cells. In these studies, adult RTEs produced less IL-2 and had a lower proliferative capacity than resident cells.3,6
In contrast to adult RTEs, there are little available data on the function of neonatal RTEs. Several studies have demonstrated that neonatal CD4+ cord blood (CB) cells, which are enriched in RTEs by T-cell receptor (TCR) excision circle analysis,10 proliferate in response to the homeostatic cytokine IL-7 in the absence of stimulation through the TCR.10,11 In contrast, total adult naive CD4+ peripheral blood cells did not proliferate.10 Importantly, although these studies did not directly compare purified neonatal and adult RTEs, the data suggest that they may be functionally distinct. In this report, we have undertaken the first direct comparison of both the phenotype and function of neonatal and adult RTEs.
Although RTEs have traditionally been studied using intrathymic injection of fluorescein isothiocyanate,5,12–15 this method produces the potentially undesirable complication of surgical stress. Therefore, for these studies, we have used a transgenic mouse model developed by Nussenzweig16 that allows for the identification of RTEs in the peripheral lymphoid organs. These mice express green fluorescent protein (GFP) under the control of the Rag2 promoter (RAG2p-GFP). This leads to the expression of GFP mRNA only where Rag is expressed (thymus and bone marrow [BM]). However, Boursalian et al demonstrated in adult RAG2p-GFP mice that high levels of GFP are maintained on peripheral T cells for at least 7 days after emigration from the thymus.3 Therefore, this transgenic mouse model provides a useful tool to directly compare purified CD4+GFPhi RTEs from neonates and adults, without surgical stress.
Using RAG2p-GFP mice, we demonstrate that neonatal and adult RTEs are phenotypically and functionally distinct. Importantly, we observed that the relative Th cytokine responses of CD4+ RTEs and resident cells in both age groups are highly dependent on the conditions of activation. Under some conditions, both neonatal and adult RTEs produced less effector cytokine (IL-4 and IFN-γ) than their resident cell counterparts, but under other conditions, RTEs produced mature levels. Nevertheless, neonatal RTEs always exhibited higher levels of effector cytokine production than adult RTEs, regardless of the activation conditions. We also found that neonatal RTEs proliferated to IL-7 in the absence of TCR stimulation, whereas adult RTEs did not. This was associated with a faster down-regulation of IL-7Rα on neonatal RTEs and higher levels of pSTAT5 activation on exposure to IL-7. Finally, using an adoptive transfer system, we found that the functional properties of neonatal RTEs are not solely the result of the developmental age of the hematopoietic stem cells.
Methods
Mice
RAG2p-GFP (FVB-H-2q; generously provided by M. Nussenzweig, Rockefeller University, New York, NY) mice were bred and housed under barrier conditions at the Division of Veterinary Resources, University of Miami Miller School of Medicine. All animal procedures were reviewed and approved by the University of Miami Institutional Animal Care and Use Committee. The colonies were free of commonly occurring infectious agents. RAG2p-GFP+/− heterozygous neonatal and adult experimental animals were generated by mating wild-type RAG2p-GFP−/− mice with homozygous RAG2p-GFP+/+ mice. RAG2p-GFP females from timed matings were monitored from days 19 to 21 of gestation; the day of birth was called day 0 of life.
Flow cytometry
Phycoerythrin (PE)–conjugated anti–(α)-CD24 (M1/69), αCD8α (53-6.7), α-αβTCR; biotinylated (BN)–αQa2 (1-1-2), αIL-7Rα (B12-1); Alexa Fluor 647–conjugated αpSTAT5 (47); cychrome (Cy)–conjugated αCD4 (RM4-5); and strepavidin-Cy were from BD Pharmingen (San Diego, CA). αCD3-PE (145-2C11) and α-αβTCR-BN (21577) were from Invitrogen (Carlsbad, CA). αCD28-PE (37.51) was from eBioscience (San Diego, CA). AV-PE was from Jackson ImmunoResearch Laboratories (West Grove, PA). Samples were analyzed on a Becton Dickinson LSR I using CellQuest software (BD Biosciences Pharmingen).
Preparation of cells from neonatal and adult RAG2p-GFP+/− mice
CD4+ lymph node cells from neonatal (7-day-old) and adult (6- to 8-week old) RAG2p-GFP+/− mice were positively selected using the Miltenyi Biotec MACS system (Auburn, CA). GFPhi RTEs, GFPlo intermediate, and GFP− resident cells were identified by gating on RAG2p-GFP+/− thymocytes (> 90% GFPhi) and wild-type RAG2p-GFP−/− thymocytes (> 99% GFP−), and sorted using a BD FACSAria and FACSDiva software (BD Biosciences, San Jose, CA). For some experiments, total neonatal LN cells were stained first with αCD4-Cy, then sorted for CD4+GFPhi RTEs, CD4+GFPlo intermediate, and CD4+GFP− resident cells. Purity of cell populations was routinely more than 98%. After isolation, all cell populations were washed and resuspended in culture media.
Culture conditions for cytokine enzyme-linked immunosorbent spot and enzyme-linked immunosorbent assays
A total of 2 × 105 sorted CD4+ RTEs, intermediate, and resident cells were plated in 200 μL of culture media and activated with 0.5 μg/well plate-bound (PB) αCD3 (145-2C11) and 0.5 μg/mL soluble αCD28 (37.51; BD Pharmingen). Culture medium consisted of RPMI 1640 containing 1 mM sodium pyruvate, 2 mM L-glutamine, 1% penicillin-streptomycin, 5 × 10−2 mM 2-mercaptoethanol (Invitrogen), and 10% heat-inactivated (56°C, 30 minutes) fetal bovine serum (HyClone Laboratories, Logan, UT). For the antigen-presenting cell (APC) costimulation experiments, sorted cells were activated with 0.5 μg/well PBαCD3 in the presence of 4 × 105 splenic APCs. APCs were prepared from naive RAG2p-GFP+/− adults, using a protocol previously described by Adkins and Hamilton.17 Briefly, splenocytes were treated with anti–Thy-1 mAb plus complement to deplete T cells; the remaining cells were used as APCs. For the IL-7 costimulation experiments, sorted cells were activated with PBαCD3 and αCD28 in the presence or absence of 10 ng/mL recombinant murine IL-7 (PeproTech, Rocky Hill, NJ). Supernatants from all experimental conditions were harvested after 48 or 68 hours, and IFN-γ and IL-4 content was assessed using mouse-specific cytokine enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer's directions. To measure IL-2 production, purified αIL-2 (JES6-1A12) and αIL-2-BN (JES6-5H4; BD Pharmingen) were substituted into the ELISA protocol. To determine the frequency of cytokine secretors, cells were harvested 44 hours after activation, washed, and replated with 10 nM phorbol-12-myristate-13-acetate and 1 μM ionomycin (Calbiochem, San Diego, CA) in enzyme-linked immunosorbent spot (ELISPOT) wells. The cells were then incubated at 37°C, 5% CO2 for an additional 4 hours before developing as described previously.18
Measurement of proliferation and cell cycle
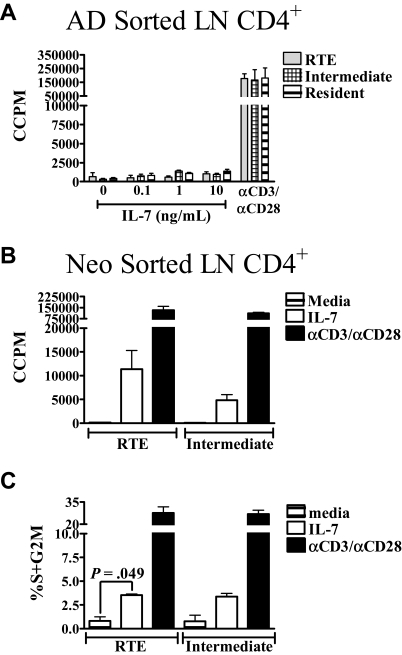
To measure IL-7–dependent proliferative responses, 2 × 105 sorted RTEs and intermediate cells from neonates were stimulated for 48 hours with 10 ng/mL IL-7. For adult responses, 2 × 105 sorted RTEs, intermediate, and resident cells from adults were stimulated with 0 to 100 ng/mL IL-7. During the last 20 to 24 hours of culture, 3H-thymidine (1 μC/well) was added to all cultures to measure proliferation. To examine cell-cycle entry, sorted neonatal RTEs and intermediate cells were stimulated with 10 ng/mL IL-7 for 72 hours. At each time point, cells were harvested, stained with propidium iodide (Sigma-Aldrich, St Louis, MO), and the percentages of cells in S plus G2M were determined as described previously in detail.19
Staining of RAG2p-GFP+/− LN cells for IL-7Rα and pSTAT5 expression
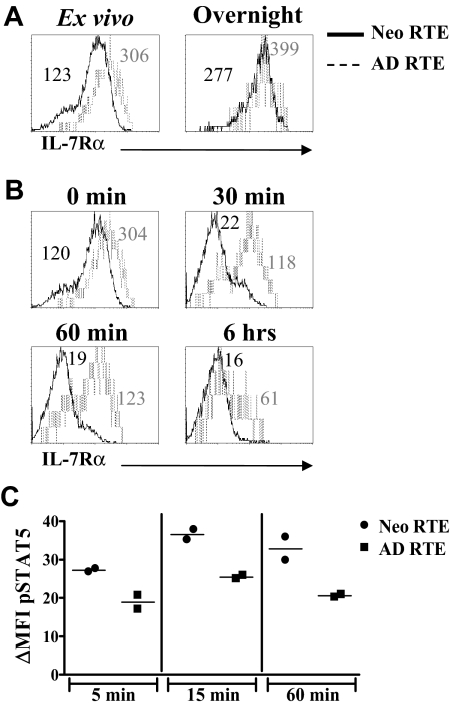
Purified CD4+ LN cells were stained with αIL-7Rα either directly ex vivo or after overnight culture in culture medium. Expression of the receptor on RTEs was then determined by gating on CD4+GFPhi cells. The kinetics of IL-7Rα down-regulation was assayed directly ex vivo on purified CD4+ cells in a similar fashion, after incubation with 10 ng/mL IL-7 for 30 minutes, 60 minutes, or 6 hours at 37°C, 5% CO2. Phospho-STAT5 expression in response to IL-7 was measured on sorted RTEs from RAG2p-GFP+/− neonates and adults. Sorted cells were cultured overnight in culture medium and then stimulated with 10 ng/mL IL-7 for 5, 15, or 60 minutes at 37°C, 5% CO2. At the end of each time point, the cells were fixed with 2% paraformaldehyde at 37°C for 10 minutes. The cells were then harvested, pelleted, and permeabilized by addition of 90% methanol with vigorous vortexing. After a 30-minute incubation on ice, the cells were washed twice with staining buffer (1× phosphate-buffered saline, 0.5% bovine serum albumin), then stained with αpSTAT5 (0.06 μg per 106 cells) for 30 minutes at room temperature. After staining, the cells were washed twice and then fixed in 1% paraformaldehyde. All samples were analyzed on a BD LSRI, using CellQuest software.
Adoptive transfer of FL and adult BM cells
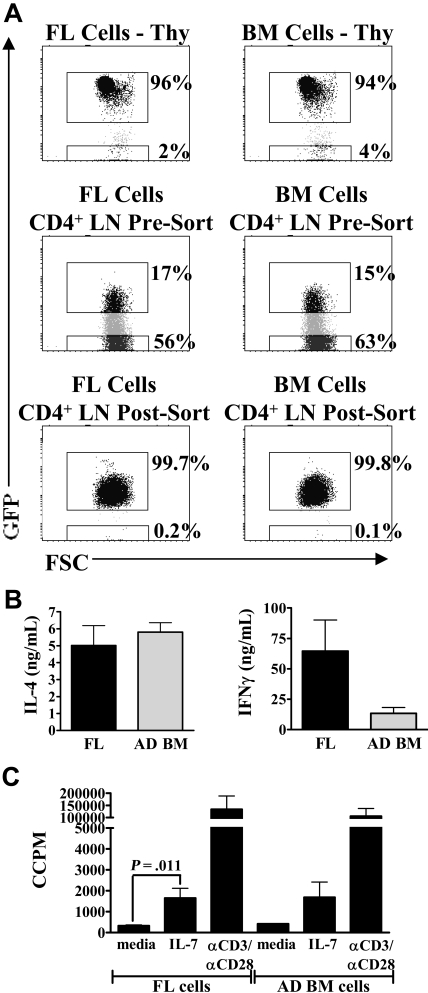
Liver tissue from RAG2p-GFP+/− fetuses (day 14 of gestation) was homogenized by passage through fine steel mesh (45 μm). BM was harvested from the tibia and femur of 6- to 8-week-old RAG2p-GFP+/− adults. The cells were washed twice in Hank balanced salt solution (HBSS) (Invitrogen), counted, and resuspended in HBSS for injection. A total of 2 × 107 fetal liver (FL) or BM cells were injected intravenously into the tail vein of lethally irradiated (900 cGy) wild-type RAG2p-GFP−/− adults (7-8 weeks old). Six to 7 weeks later, CD4+ LN cells were purified and sorted for RTEs as described in “Preparation of cells from neonatal and adult RAG2p-GFP+/− mice.” The cells were then activated with PBαCD3 and αCD28 for 48 hours to determine cytokine production by ELISA. In parallel, some cells were stimulated with 10 ng/mL of rIL-7, and proliferation was assessed by 3H-thymidine incorporation.
Statistical analysis
Statistical analysis was performed using SAS for Windows (Version 9.2; SAS Institute, Cary, NC). Comparisons between groups were based on the exact nonparametric tests: the Kruskal-Wallis test was used when there were more than 2 groups, and the Wilcoxon score test was used when there were 2 groups. A P value less than or equal to .05 was considered significant.
Results
RTEs are more abundant in neonates and are phenotypically distinct from adult RTEs
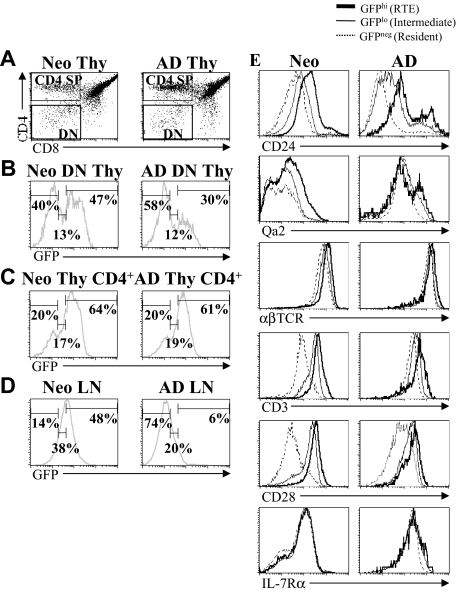
Using the RAG2p-GFP transgenic mouse model, Boursalian et al3 demonstrated that adult CD4+ splenic RTEs undergo a phenotypic postthymic maturation as they enter the resident cell pool. However, it is presently unknown whether the expression of maturation markers on neonatal and adult RTEs is equivalent. As Boursalian et al demonstrated that phenotypic differences were associated with functional differences in adults,3 differences in the phenotype of neonatal and adult RTEs may be indicative of functional differences. To address this issue, the expression of several markers was compared on neonatal and adult CD4+ LN cells. To optimally compare with the work reported by Boursalian et al3 using adult RAG2p-GFP+/− mice, we identified GFPhi RTEs, GFPlo intermediate, and GFP− resident cell populations in neonatal and adult mice by gating on double-negative (DN) thymocytes and determining the expression of GFP (Figure 1A,B). These gates were then used to determine the percentage of RTEs, intermediate, and resident cells among CD4+ single-positive (SP) thymocytes and LN cells from both age groups (Figure 1C,D). The percentage of GFPhi cells among CD4+ SP thymocytes was similar in neonates and adults but differed markedly among CD4+ LN cells. Similar to previous reports on splenic RTEs,3,20 lymph node RTEs were more abundant (∼ 8 times) in neonates, and there was a significant GFPlo intermediate cell population. RTEs and intermediate cells composed approximately 85% of the total CD4+ LN cells in neonates, compared with only approximately 30% in adults. We then determined phenotypic marker expression on CD4+ RTEs, intermediate, and resident LN cells (Figure 1E). The pattern of CD24, αβTCR, CD3, CD28, and IL-7Rα expression was similar on neonatal and adult cells, decreasing as GFP expression was lost (Figure 1E). However, all subsets of neonatal cells, and in particular RTEs, expressed significantly higher levels of CD24 than their respective adult subset (Table 1). Neonatal RTEs also expressed higher levels of CD3 and CD28 than adult RTEs. In contrast, the expression of Qa2 and αβTCR was significantly lower on all subsets of neonatal CD4+ LN cells, most notably on RTEs (Figure 1E; Table 1). These data demonstrate that neonatal and adult RTEs are phenotypically distinct. High-level CD24 expression and low-level Qa2 expression are indicative of immaturity in adult T cells.5,14,15 Therefore, these results led to the prediction that neonatal RTEs will show immature functional properties, compared with adult RTEs.
Figure 1.
RTEs are more abundant in neonates than adults. Thymocytes from neonatal (7-day-old) and adult (6- to 8-week-old) RAG2p-GFP+/− mice were stained for CD4 and CD8. (A) Gates were set on DN and CD4+ SP cells. (B) GFP expression within the gated DN thymocyte population was then used to define GFPhi RTEs, GFPlo intermediates, and GFP− resident cells. The percentage of RTEs, intermediate, and resident cells among CD4+ SP thymocytes (C) and CD4+ LN cells (D) was then determined based on the gates set on DN thymocytes (B). (E) Using the GFP gates defined in panel B, the expression of CD24, Qa2, αβTCR, CD3, CD28, and IL-7Rα was determined on CD4+ RTEs, intermediate, and resident LN cells from neonates and adults. Histograms shown are representative of staining profiles from 6 to 14 individual neonates and 6 to 9 individual adults. For IL-7Rα, data represent 2 independent experiments, using a pool of LN cells from 14 or 15 neonates and 2 adults per experiment.
Table 1.
Differences in phenotypic marker expression by neonatal and adult RTE
| CD24 | Qa2 | αβTCR | CD3 | CD28 | IL-7Rα | |
|---|---|---|---|---|---|---|
| Neonatal CD4+ | ||||||
| GFPhi RTE | 148 ± 58* | 49 ± 8* | 1158 ± 99* | 478 ± 24* | 355 ± 35* | 114 ± 11 |
| GFPlo intermediate | 89 ± 40* | 29 ± 5* | 893 ± 71* | 286 ± 17 | 208 ± 20* | 113 ± 6 |
| GFP− resident | 62 ± 34* | 25 ± 3* | 717 ± 61* | 114 ± 10* | 53 ± 6 | 103 ± 5 |
| Adult CD4+ | ||||||
| GFPhi RTE | 74 ± 5 | 76 ± 2 | 1379 ± 79 | 316 ± 91 | 133 ± 76 | 301 ± 7 |
| GFPlo intermediate | 30 ± 1 | 86 ± 2 | 1175 ± 43 | 226 ± 58 | 76 ± 53 | 262 ± 14 |
| GFP− resident | 13 ± 1 | 87 ± 4 | 1047 ± 45 | 168 ± 56 | 44 ± 26 | 197 ± 8 |
The average mean fluorescence intensity (MFI) of the cell-surface markers shown in Figure 1E was calculated for neonatal and adult LN cells, and is shown as the mean plus or minus SD. Where 2 peaks were evident (CD24 and Qa2), the MFI was determined using the larger (main) peak. For all markers except IL-7Rα, data represent mean plus or minus SD of MFI from individual animals. CD24: n = 11 neonates and 9 adults. Qa2: n = 14 neonates and 6 adults. αβTCR, CD3, and CD28: n = 10 neonates and 8 adults. For IL-7Rα, data represent 2 independent experiments using a pool of LN cells from 14 or 15 neonates and 2 adults per experiment.
P < .005, neonatal cell subset versus respective adult cell subset.
Neonatal RTEs produce higher levels of Th effector cytokines than adult RTEs
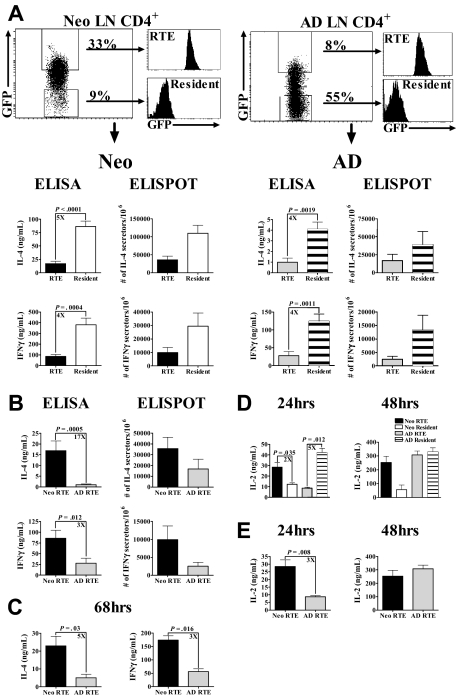
To directly assess the function of RTEs from both age groups, CD4+ LN RTEs and resident cells from neonatal and adult RAG2p-GFP+/− mice were sorted, activated with PBαCD3 and αCD28 for 48 hours, and effector cytokine production was measured. RTEs from both age groups exhibited similar patterns of Th1/Th2 cytokine production compared with the resident cell population, with RTEs producing significantly less IL-4 and IFN-γ than resident cells (Figure 2A). This was accompanied by a 2- to 5-fold decrease in the frequency of cytokine producing cells. However, contrary to our expectation from the phenotypic analyses, neonatal RTEs produced significantly more of both IL-4 and IFN-γ and had a higher frequency of cytokine-secreting cells than adult RTEs (Figure 2B). Similar differences in effector cytokine production by neonatal and adult RTEs were observed after 68 hours of activation (Figure 2C). In contrast to Th1/Th2 cytokine production, neonatal RTEs produced significantly more IL-2 than resident cells (Figure 2D) after 24 hours of polyclonal activation. As previously reported,3 adult RTEs produced significantly less IL-2 than resident cells at this time point (Figure 2D). However, when compared, neonatal RTEs produced significantly more IL-2 at 24 hours than adult RTEs (Figure 2E). Together, these findings are the first demonstration that neonatal and adult RTEs are functionally distinct.
Figure 2.
Neonatal RTEs produce higher levels of IL-2 and Th1/Th2 cytokines than adult RTEs in response to PBαCD3 and αCD28 stimulation. (A) GFPhi RTEs and GFP− resident cells were sorted from purified neonatal and adult LN CD4+ cells, as described in “Preparation of cells from neonatal and adult RAG2p-GFP+/− mice.” Neonatal (left) and adult (right) RTEs and resident cells were activated for 48 hours with 0.5 μg/well PBαCD3 and 0.5 μg/mL soluble αCD28 (lower concentrations of PBαCD3, 0.005 or 0.05 μg/well, elicited only low-level cytokine production). Cytokine production and the number of cytokine-secreting cells were then determined. Graphs depict pooled data from 8 to 11 ELISA experiments and 3 ELISPOT experiments and are shown as the mean ± SEM; n = 35 to 60 neonates and 4 to 6 adults per experiment. (B) The data from panel A were regraphed to show a direct comparison of neonatal and adult RTEs. (C) Neonatal and adult RTEs were stimulated with PBαCD3 and αCD28 for 68 hours. Cytokine production was determined by ELISA. Graphs depict pooled data from 4 experiments; n = 18 to 35 neonates and 2 to 17 adults per experiment. (D) Sorted RTEs and resident cells from neonates and adults were stimulated with PBαCD3 and soluble αCD28 as described in panel A for 24 and 48 hours. Supernatants were harvested at each time point and assayed for IL-2 by ELISA. Graphs depict pooled data from 4 or 5 experiments; n = 18 to 25 neonates and 2 adults per experiment. (E) The data from panel D were regraphed to show a comparison of neonatal and adult RTEs.
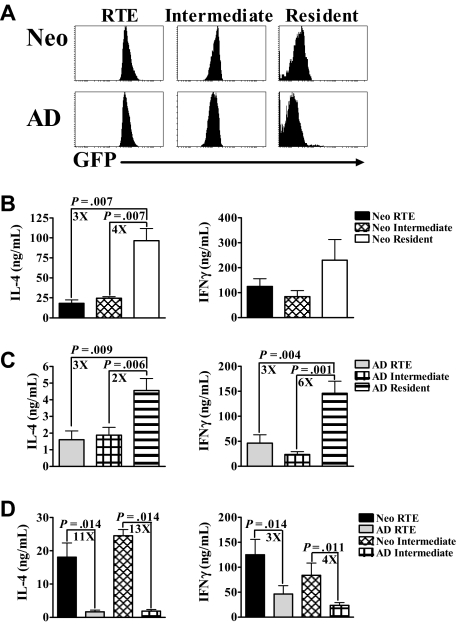
Neonatal intermediate cells also produce higher levels of Th effector cytokines than adult intermediate cells
As discussed in “RTE are more abundant in neonates and are phenotypically distinct from adult RTE,” previous work using adult RAG2p-GFP mice demonstrated the presence of a GFPlo intermediate population, which may be a functional intermediate between RTEs and resident cells.3 However, the functional abilities of these cells were not reported. This intermediate population constitutes 30% to 35% of neonatal CD4+ LN cells (Figure 1D) and is therefore potentially of greater overall functional significance in neonates. To determine their ability to produce Th effector cytokines, we activated sorted RTEs, intermediate, and resident LN cells (Figure 3A) from neonates and adults with PBαCD3 and αCD28. As observed with RTEs, intermediate cells from both age groups produced significantly reduced levels of Th cytokines compared with resident cell populations (Figure 3B,C). Therefore, although the intermediate population is phenotypically distinct, these cells do not appear to be a functional intermediate in either neonates or adults. Nonetheless, similar to neonatal RTEs, intermediate cells from neonates produced significantly higher amounts of both IL-4 and IFN-γ than their adult counterparts (Figure 3D).
Figure 3.
Intermediate (GFPlo) cells from neonates also produce greater levels of cytokine compared with intermediate (GFPlo) cells from adults. (A) CD4+ LN cells from RAG2p-GFP+/− neonates and adults were sorted into RTEs (GFPhi), intermediate (GFPlo), and resident (GFP−) populations. These purified neonatal (B) and adult (C) cells were then activated with PBαCD3 and αCD28 for 48 hours, and supernatants were harvested for cytokine-specific ELISA. (D) The data from panels B and C were regraphed to show a comparison of neonatal and adult RTEs and intermediate cells. Graphs represent pooled data from 4 or 5 independent experiments and are shown as the mean ± SEM; n = 35 to 60 neonates and 6 adults per experiment.
The relative Th effector cytokine response of RTEs and resident cells is dependent on the conditions of activation
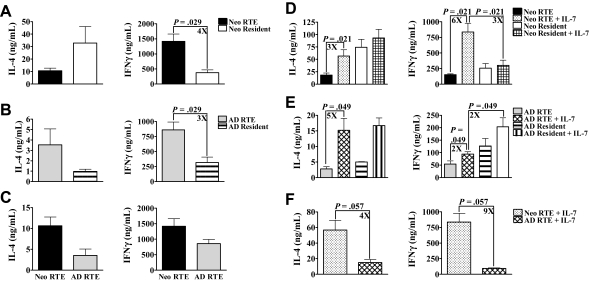
Bendelac et al previously reported in adult mice that RTEs produce higher levels of Th effector cytokines than resident cells.5 In their study, RTEs were activated with PBαTCR and dendritic cell (DC)–enriched APCs; consequently, we hypothesized that additional costimulatory signals provided by APC may boost cytokine production by RTEs. Accordingly, we activated sorted RTEs and resident cells from both age groups with PBαCD3 and adult APC. Under these conditions, adult RTEs produced higher levels of IL-4 and IFN-γ than resident cells (Figure 4B), as previously reported.5 In contrast, neonatal RTEs produced higher levels of IFN-γ, but not IL-4, than resident cells (Figure 4A). Nevertheless, as observed with PBαCD3 and αCD28, neonatal RTEs activated with PBαCD3 and APC produced higher levels of both IL-4 and IFN-γ than adult RTEs (Figure 4C). This demonstrates that, even under different activation conditions, neonatal and adult RTEs are functionally different.
Figure 4.
Relative response of RTEs and resident cells is dependent on the conditions of activation. (A-C) Sorted CD4+ LN RTEs and resident cells from RAG2p-GFP+/− neonates (A) and adults (B) were activated with PBαCD3 and APC for 48 hours. Supernatants were then harvested for cytokine-specific ELISA. (C) The data from panels A and B were regraphed to show a comparison of neonatal and adult RTEs activated with PBαCD3 and APC. Graphs represent pooled data from 4 independent experiments and are shown as the mean ± SEM; n = 34 to 44 neonates and 6 adults per experiment. (D-F) Sorted CD4+ LN RTEs and resident cells from neonatal (D) and adult (E) RAG2p-GFP+/− were activated with PBαCD3 and αCD28 in the presence or absence of 10 ng/mL IL-7 for 48 hours. Supernatants were then harvested for cytokine-specific ELISA. Graphs represent pooled data from 4 independent experiments and are shown as the mean ± SEM. (F) The data from panels D and E were regraphed to show a comparison of neonatal and adult RTEs activated in the presence of IL-7; n = 25 to 35 neonates; n = 4 to 6 adults per experiment.
IL-7, which can be produced by DC and LN stromal cells,21–23 is thought to provide significant costimulation to T cells.24,25 It seemed possible that IL-7 produced by APC may contribute to the increased cytokine production by RTEs under these conditions. We therefore determined whether activation with PBαCD3 and αCD28 in the presence of IL-7 would also affect the relative cytokine responses of RTEs and resident cells. Activation in the presence of IL-7 resulted in a significant increase in the production of IL-4 by both neonatal and adult RTEs to levels that were similar to resident cell production (Figure 4D,E). IFN-γ production by adult RTEs was increased in the presence of IL-7 but did not reach the level of adult resident cells (Figure 4E). In contrast, neonatal RTEs produced significantly more IFN-γ when activated in the presence of IL-7 (Figure 4D) to levels significantly higher than those of neonatal resident cells. Importantly, neonatal RTEs again produced greater levels of both IL-4 and IFN-γ than adult RTEs (Figure 4F).
Neonatal RTEs proliferate in response to IL-7 in the absence of stimulation through the TCR
Another potential functional difference between neonatal and adult RTEs was first described in humans by Hassan and Reen,10 who reported that naive CB CD4+ cells, but not naive adult CD4+ peripheral blood cells, proliferated in response to IL-7 in the absence of stimulation through the TCR. However, the percentage of RTEs in the adult naive CD4+ population is probably small; therefore, any proliferation of adult RTEs may have been too small for detection. Thus, we used the RAG2p-GFP transgenic mouse model to determine whether proliferative responses to IL-7 were similar or distinct among neonatal and adult RTEs. When activated with PBαCD3 and αCD28, RTEs and intermediate cells from both age groups displayed similar proliferative responses (Figure 5A,B). However, none of the sorted adult subsets proliferated in response to IL-7 at this early time point, even at 100 ng/mL IL-7 (Figure 5A and data not shown). In contrast to adults, RTEs and intermediate cells from neonates proliferated after 48 hours of stimulation with IL-7 (Figure 5B). However, the frequency of proliferating cells was low (∼1%-2%) at this early time point (data not shown). After 72 hours of IL-7 exposure, approximately 3% of RTEs and intermediate cells were proliferating (Figure 5C). Interestingly, this frequency is similar to the frequency of cells that undergo homeostatic proliferation in neonates.26 These data suggest that the proliferative responsiveness of RTEs and intermediate cells to IL-7 may represent a mechanism for homeostatic proliferation in neonates. These results also demonstrate an additional functional difference between RTEs from neonates and adults.
Figure 5.
Neonatal RTEs, but not adult RTEs, proliferate in response to IL-7. (A) CD4+ RTEs, intermediate, and resident LN cells were sorted from adults and cultured with increasing concentrations of IL-7 or PBαCD3 and αCD28 for 48 hours. 3H-thymidine was added during the last 20 hours of culture. (B) Neonatal RTEs and intermediate cells were sorted and cultured in complete media, 10 ng/mL IL-7, or activated with PBαCD3 and αCD28 for 48 hours. 3H-thymidine was added for the last 20 hours of culture. Graphs represent pooled data from 3 or 4 independent experiments; n = 6 to 8 adults and 28 to 44 neonates per experiment. All data are shown as the mean ± SEM. (C) CD4+ RTEs and intermediate cells were sorted from neonatal LN and then cultured in complete media, 10 ng/mL IL-7, or activated with PBαCD3 and αCD28 for 72 hours. The percentage of proliferating cells was determined by propidium iodide (PI) staining, as described in “Measurement of proliferation and cell cycle.” Graphs represent pooled data from 3 independent experiments; n = 23 to 56 neonates per experiment. All data are shown as the mean ± SEM.
Neonatal RTEs proliferative responses to IL-7 are associated with rapid down-regulation of IL-7Rα and higher levels of pSTAT5
IL-7 signals are propagated through the IL-7 receptor (IL-7R). Ligation of the IL-7R leads to the loss of IL-7Rα expression on the cell surface27 and the activation of both survival and proliferative pathways.28 As neonatal, but not adult, RTEs proliferate in response to IL-7, we hypothesized that this functional difference was the result of differential signaling through the IL-7R. We first hypothesized that neonatal RTEs expressed higher levels of IL-7Rα, which could lead to greater transduction of IL-7 signals. Contrary to this expectation, when CD4+ LN RTEs were stained directly ex vivo, neonatal RTEs exhibited lower levels of IL-7Rα than adult RTEs (Figures 1E, 6A). However, because IL-7Rα levels may be decreased because of exposure to IL-7 in vivo, we cultured the cells overnight in complete media to allow for full up-regulation of this receptor subunit.27 Both neonatal and adult RTEs up-regulated IL-7Rα, although expression on neonatal RTEs remained lower than that of adult RTEs (Figure 6A). These results demonstrate that the proliferative responses of neonatal RTEs are not the result of increased levels of IL-7Rα.
Figure 6.
IL-7Rα is down-regulated more rapidly on neonatal RTEs than on adult RTEs after exposure to IL-7 and is associated with higher levels of pSTAT5. (A) Purified CD4+ lymph node cells from RAG2p-GFP+/− neonates and adults were either stained directly ex vivo with anti–IL-7Rα (left panel) or were cultured overnight in complete media before staining (right panel). IL-7Rα expression on RTEs was determined by gating on CD4+GFPhi cells. (B) Purified CD4+ LN cells from neonatal and adult RAG2p-GFP+/− mice were either stained directly ex vivo (0 minutes) or cultured with 10 ng/mL IL-7 for 30 minutes, 60 minutes, or 6 hours, and then stained with anti–IL-7Rα. IL-7Rα expression on RTEs was determined by gating on CD4+GFPhi cells. IL-7Rα mean fluorescence intensity (MFI) for neonatal (in black) and adult (in gray) RTEs are shown in each histogram. Histograms represent data from 2 independent experiments; n = 2 adults and 15 neonates per experiment. In panels A and B, curves represent 800 to 2000 gated CD4+GFPhi RTEs for adults and 7000 to 13 000 gated CD4+GFPhi RTEs for neonates. (C) Sorted neonatal and adult RTEs were cultured overnight in complete media. The cells were then stimulated with IL-7 (10 ng/mL) for 5 to 60 minutes, fixed and permeabilized, and stained intracellularly for pSTAT5. The specificity of the staining was confirmed by competition with an excess of unlabeled pSTAT5 antibody (data not shown). Points represent pSTAT5 MFI data from 2 independent experiments; n = 40 to 49 neonates and 4 adults per experiment. ΔMFI was calculated by subtracting the MFI of pSTAT5 in the absence of IL-7 from the pSTAT5 MFI at each time point.
We next hypothesized that signaling through the IL-7R was prolonged in neonatal RTEs because of a longer retention of the receptor on the cell surface. However, we observed that IL-7Rα was down-regulated on neonatal RTEs within 30 minutes of exposure to IL-7 but was not lost on adult RTEs until 6 hours (Figure 6B). These data suggest that the rapid down-regulation of IL-7Rα on exposure to IL-7 may lead to faster and/or stronger initiation of downstream proliferative signaling pathways in neonatal RTEs. In support of this idea, apoptosis of neonatal RTEs is very low (< 1%) after 72 hours of exposure to IL-7 (data not shown). Moreover, pSTAT5 levels were consistently higher among neonatal than adult RTEs (Figure 6C). This may lead to differential signaling through downstream pathways and contribute to the different proliferative responses observed between neonatal and adult RTEs.
Functional properties of neonatal RTEs do not appear to be regulated exclusively by the developmental age of the T-cell precursors
The use of CB cells as a source of stem cells (SCs) for transplantation is relatively recent. Using CB cells instead of adult BM or SCs is advantageous, as it allows for greater mismatch between donor and recipient and results in fewer incidences of severe graft-versus-host disease.29 However, there are few published data on the immune status of CB transplantation recipients compared with adult BM or SC recipients shortly after immune reconstitution.30 Reconstitution of the peripheral lymphoid compartment after SC transplantation appears to be at least partially dependent on thymic output and the generation of RTEs.31,32 The data presented here suggest that the function of RTEs derived from a fetal/neonatal SC source (CB) may be quite different from those derived from an adult SC source (BM). As neonatal RTEs may be largely descended from fetal SC, we adoptively transferred FL and adult BM cells from RAG2p-GFP+/− mice into lethally irradiated wild-type RAG2p-GFP−/− adults to test whether the functional properties of neonatal RTEs are dependent on their developmental origin. Six to 7 weeks later, we compared the production of effector cytokines and proliferative responses to IL-7. Thymic and peripheral reconstitution was similar in mice transplanted with either FL or BM cells (Figure 7A). There was no significant difference in the production of IL-4 or IFN-γ by RTEs derived from FL SCs or BM SCs (Figure 7B). Finally, there was no difference in IL-7 responsiveness between RTEs from either group (Figure 7C). Therefore, the properties of neonatal RTEs do not appear to be the result of intrinsic properties of the fetal SC precursors.
Figure 7.
Functional characteristics of neonatal RTEs are not solely the result of the developmental age of the hematopoietic stem cell. Day 14 FL cells or adult BM cells from RAG2p-GFP+/− mice were transplanted intravenously into lethally irradiated wild-type RAG2p-GFP−/− adults. Six to 7 weeks later, LN CD4+ cells were purified and then sorted to obtain GFPhi RTEs. (A) Thymic reconstitution was similar in mice who received either FL (left panel) or BM cells (right panel). Both groups also possessed similar levels of GFPhi RTEs in the peripheral LN CD4+ compartment. Purity of sorted RTEs was > 99%. (B) Sorted RTEs were activated with PBαCD3 and αCD28 for 48 hours. Supernatants were then harvested for cytokine-specific ELISA. Data represent pooled data from 3 independent experiments and are shown as the mean ± SEM; n = 5 or 6 mice per group (FL and BM recipients). (C) Sorted RTEs were cultured in complete media or stimulated with either 10 ng/mL IL-7 or PBαCD3 and αCD28 for 48 hours. 3H-thymidine was added for the last 24 hours of culture to measure proliferation. Graphs represent pooled data from 3 independent experiments; n = 5 or 6 mice per group. All data are shown as the mean ± SEM.
Discussion
The data presented in this study provide the first direct comparison of purified RTE populations from neonates and adults. Using the RAG2p-GFP transgenic mouse model, we have demonstrated that neonatal and adult RTEs are phenotypically and functionally distinct. Neonatal and adult RTEs differed in the expression of most markers examined, including CD24, Qa2, αβTCR, and CD28. Neonatal RTEs produced higher levels of IL-2 and Th1/Th2 effector cytokines than adult RTEs. Moreover, neonatal, but not adult, RTEs proliferated in response to IL-7 alone. Because of their greater presence in neonatal peripheral lymphoid organs, these data suggest that neonatal RTEs may contribute more to neonatal immune responses than adult RTEs, particularly in antigenic systems where neonatal IFN-γ production is greatly enhanced.33–38
Neonatal and adult RTEs responses to IL-7 are functionally distinct
Recently, neonatal CD4+ CB cells, but not naive adult CD4+ peripheral blood mononuclear cells (PBMCs), were shown to proliferate in response to IL-7 in the absence of stimulation through the TCR.10 Although it was also demonstrated that neonatal CB cells are enriched in RTEs, the lack of phenotypic RTEs markers in humans prevented the direct comparison of neonatal and adult RTEs responses to IL-7 in that study. As RTEs are a rare cell type in adults, it is possible that any proliferative response of adult RTEs may have been lost among the total CD4+ PMBC population. However, on direct comparison, we have found that neonatal, but not adult, RTEs proliferate in response to IL-7 within 72 hours of exposure (Figure 5).
The proliferative response of neonatal RTEs was not the result of increased levels of IL-7Rα. However, the kinetics of IL-7Rα down-regulation in response to IL-7 was different among neonatal and adult RTEs. Neonatal RTEs rapidly down-regulated IL-7Rα on exposure to IL-7 (30 minutes), but adult RTEs did not do so until after 6 hours of stimulation, similar to a previous report using total adult CD4+ cells.27 This suggested that signaling through the IL-7R was either stronger or faster in neonatal RTEs. Indeed, levels of pSTAT5 were consistently higher in neonatal RTEs, compared with adult RTEs (Figure 6C). Evidence from human neonates supports the hypothesis that increased levels of pSTAT5 may result in different functional outcomes. Neonatal NKT cells exhibited higher levels of pSTAT5 than T cells on exposure to IL-7, which was associated with increased production of both IFN-γ and IL-4.39 Therefore, the differences in pSTAT5 activation observed between neonatal and adult RTEs may be responsible for the differences in IL-7–induced proliferation. Importantly, the observation that murine, like human, neonatal RTEs proliferate to IL-7 signals alone adds to the growing list of similarities between murine and human neonatal T cells, further substantiating the murine system as a faithful model of pediatric immunity.
Proliferative responses of neonatal RTEs to IL-7 may be a mechanism for homeostatic proliferation in neonates
IL-7 is a homeostatic cytokine, and along with self-major histocompatibility complex, has been shown to be important in the slow cycling of naive T cells in the periphery.40–42 It is also important for driving homeostatic proliferation in lymphopenic conditions (ie, after BM transplantation regimens).40,43 It is thought that IL-7 is constitutively expressed by stromal cells within the peripheral lymphoid organs44 and to be limiting in lympho-replete animals.45 Moreover, in humans, lymphopenia is usually accompanied by increased levels of circulating IL-7.46 Significantly, murine neonates are considered to be lymphopenic.26 Therefore, it is conceivable that IL-7 levels may be elevated in neonatal mice, which may then drive the homeostatic proliferation that occurs in normal neonates.26 In early life, neonatal peripheral lymphoid organs contain reduced numbers of T cells and are essentially devoid of antigen-specific memory cells.1 As neonates are exposed to a large number of antigens shortly after birth, the lack of antigen-experienced memory cells is a distinct disadvantage. The process of homeostatic proliferation in neonates may at least partially overcome this deficit by producing memory-like CD4+ T cells26 that are capable of rapidly producing effector cytokines on activation.47 Because RTEs are a major population in neonates, their ability to proliferate in response to IL-7 stimulation may provide an explanation for the increased percentage of neonatal cells that spontaneously undergo homeostatic proliferation.26 In support of this idea, the percentage of neonatal RTEs proliferating to IL-7 in vitro (Figure 5C) was similar to the percentage of neonatal CD4+ cells undergoing homeostatic proliferation in vivo.26 Moreover, our finding that adult RTEs do not proliferate at early time points to IL-7 is consistent with Min et al,48 who demonstrated that the proliferation of adult cells adoptively transferred into the lymphopenic neonatal environment is largely IL-7 independent. Therefore, the signals regulating homeostatic proliferation may differ for neonatal and adult T cells.
RTEs functional responsiveness can be increased to mature levels under selected activation conditions
Similar to previous reports on adult RTEs,3,6 we found that cytokine production by both neonatal and adult RTEs was deficient compared with resident cells when activated with PBαCD3 and αCD28 (Figure 2). However, these data conflict with reports that RTEs are functionally mature.5,7 Notably, these studies used different activation conditions, which may explain the varied responses of RTEs compared with resident cells. We first hypothesized that, under activation conditions similar to those used by Bendelac et al (PBαTCR and DC-enriched APC),5 the relative response of RTEs and resident cells could be altered. Indeed, in our system, when adult RTEs were activated with PBαCD3 and APC, they produced higher levels of both IL-4 and IFN-γ than resident cells (Figure 4B). We also used other activation conditions to determine whether the relative responses of RTEs and resident cells could be changed. Polyclonal activation of RTEs in the presence of IL-7 resulted in increased IL-4 production by both neonatal and adult RTEs to levels equal to that of resident cells (Figure 4D,E). In addition, IFN-γ production by neonatal RTEs was greatly increased by the presence of IL-7. Therefore, the reduced cytokine production by RTEs when activated with PBαCD3 and αCD28 is not the result of a functional deficiency. Rather, it appears that the quantity and quality of neonatal and adult RTEs responses are highly dependent on the conditions of activation. Under the right conditions, the functional responses of both neonatal and adult RTEs can be enhanced to those of the resident cells. Importantly, these data provide a potential explanation for the conflicting reports in the literature on the function of adult RTEs.
Potential impact of functional differences between RTE populations on transplantation
Although neonatal and adult RTEs from intact animals are functionally distinct, the properties of neonatal RTEs do not appear to be the result of intrinsic properties of fetal/neonatal SC. When transplanted into adult mice, RTEs derived from FL cells produced levels of effector cytokines similar to those produced by BM-derived RTEs (Figure 7B). In addition, there was no difference in proliferation in response to IL-7 stimulation (Figure 7C). Therefore, these data suggest that, in adult transplantation settings, once immune reconstitution has been established and thymic output is restored, RTEs derived from CB cells may be functionally similar to adult BM-derived RTEs. However, it is possible that different outcomes may be observed in neonatal/pediatric patients in whom a neonatal environment may persist. In these young patients, vaccination and treatment regimens of persons that receive CB transplants may need to be altered to establish protective immunity after transplant.
Acknowledgments
The authors thank Patricia Guevara for excellent technical assistance and Jim Phillips for assistance with cell sorting.
This work was supported by National Institute of Allergy and Infectious Diseases grant AI44923-02 (B.A.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
Presented in abstract form at the 94th annual meeting of the American Association of Immunologists, Miami Beach, FL, May 20, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.J.O. designed and performed research, collected and analyzed data, and wrote the manuscript; T.K.-S. performed the statistical analysis; and B.A. was responsible for the overall study, designed research, and contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Becky Adkins, Department of Microbiology and Immunology, University of Miami Miller School of Medicine, 1600 NW 10th Ave, R-138, Miami, FL 33136; e-mail: radkins@med.miami.edu.
References
- 1.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 2.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration: quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 3.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 4.Luettig B, Sponholz A, Heerwagen C, Bode U, Westermann J. Recent thymic emigrants (CD4+) continuously migrate through lymphoid organs: within the tissue they alter surface molecule expression. Scand J Immunol. 2001;53:563–571. doi: 10.1046/j.1365-3083.2001.00897.x. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. J Exp Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JF, Thomas CA, 3rd, Kung JT. Induction of high level IL-2 production in CD4+8- T helper lymphocytes requires post-thymic development. J Immunol. 1991;147:851–859. [PubMed] [Google Scholar]
- 7.Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- 8.Hosseinzadeh H, Goldschneider I. Recent thymic emigrants in the rat express a unique antigenic phenotype and undergo post-thymic maturation in peripheral lymphoid tissues. J Immunol. 1993;150:1670–1679. [PubMed] [Google Scholar]
- 9.Yang CP, Bell EB. Functional maturation of recent thymic emigrants in the periphery: development of alloreactivity correlates with the cyclic expression of CD45RC isoforms. Eur J Immunol. 1992;22:2261–2269. doi: 10.1002/eji.1830220913. [DOI] [PubMed] [Google Scholar]
- 10.Hassan J, Reen DJ. Human recent thymic emigrants: identification, expansion, and survival characteristics. J Immunol. 2001;167:1970–1976. doi: 10.4049/jimmunol.167.4.1970. [DOI] [PubMed] [Google Scholar]
- 11.Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood. 2007;109:1034–1042. doi: 10.1182/blood-2006-06-027912. [DOI] [PubMed] [Google Scholar]
- 12.Scollay R, Chen WF, Shortman K. The functional capabilities of cells leaving the thymus. J Immunol. 1984;132:25–30. [PubMed] [Google Scholar]
- 13.Scollay R. Thymus cell migration: cells migrating from thymus to peripheral lymphoid organs have a “mature” phenotype. J Immunol. 1982;128:1566–1570. [PubMed] [Google Scholar]
- 14.Kelly KA, Scollay R. Analysis of recent thymic emigrants with subset- and maturity-related markers. Int Immunol. 1990;2:419–425. doi: 10.1093/intimm/2.5.419. [DOI] [PubMed] [Google Scholar]
- 15.Gabor MJ, Godfrey DI, Scollay R. Recent thymic emigrants are distinct from most medullary thymocytes. Eur J Immunol. 1997;27:2010–2015. doi: 10.1002/eji.1830270827. [DOI] [PubMed] [Google Scholar]
- 16.Yu W, Nagaoka H, Jankovic M, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 17.Adkins B, Hamilton K. Freshly isolated, murine neonatal T cells produce IL-4 in response to anti-CD3 stimulation. J Immunol. 1992;149:3448–3455. [PubMed] [Google Scholar]
- 18.Adkins B, Bu Y, Cepero E, Perez R. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J Immunol. 2000;164:2347–2353. doi: 10.4049/jimmunol.164.5.2347. [DOI] [PubMed] [Google Scholar]
- 19.Adkins B, Williamson T, Guevara P, Bu Y. Murine neonatal lymphocytes show rapid early cell cycle entry and cell division. J Immunol. 2003;170:4548–4556. doi: 10.4049/jimmunol.170.9.4548. [DOI] [PubMed] [Google Scholar]
- 20.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link A, Vogt TK, Favre S, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 22.Kroncke R, Loppnow H, Flad HD, Gerdes J. Human follicular dendritic cells and vascular cells produce interleukin-7: a potential role for interleukin-7 in the germinal center reaction. Eur J Immunol. 1996;26:2541–2544. doi: 10.1002/eji.1830261040. [DOI] [PubMed] [Google Scholar]
- 23.Sorg RV, McLellan AD, Hock BD, Fearnley DB, Hart DN. Human dendritic cells express functional interleukin-7. Immunobiology. 1998;198:514–526. doi: 10.1016/S0171-2985(98)80075-2. [DOI] [PubMed] [Google Scholar]
- 24.Gringhuis SI, de Leij LF, Verschuren EW, Borger P, Vellenga E. Interleukin-7 upregulates the interleukin-2-gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both nuclear factor of activated T cells and activator protein-1. Blood. 1997;90:2690–2700. [PubMed] [Google Scholar]
- 25.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Campion A, Bourgeois C, Lambolez F, et al. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proc Natl Acad Sci U S A. 2002;99:4538–4543. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JH, Yu Q, Erman B, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Hofmeister R, Khaled AR, Benbernou N, Rajnavolgyi E, Muegge K, Durum SK. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 1999;10:41–60. doi: 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 29.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 30.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roux E, Dumont-Girard F, Starobinski M, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000;96:2299–2303. [PubMed] [Google Scholar]
- 32.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 33.Opiela SJ, Levy RB, Adkins B. Murine neonates develop vigorous in vivo cytotoxic and Th1/Th2 responses upon exposure to low doses of NIMA-like alloantigens. Blood. 2008;112:1530–1538. doi: 10.1182/blood-2007-08-106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vekemans J, Ota MO, Sillah J, et al. Immune responses to mycobacterial antigens in the Gambian population: implications for vaccines and immunodiagnostic test design. Infect Immun. 2004;72:381–388. doi: 10.1128/IAI.72.1.381-388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31:1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Kovarik J, Bozzotti P, Love-Homan L, et al. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J Immunol. 1999;162:1611–1617. [PubMed] [Google Scholar]
- 37.Martinez X, Brandt C, Saddallah F, et al. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci U S A. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovarik J, Gaillard M, Martinez X, et al. Induction of adult-like antibody, Th1, and CTL responses to measles hemagglutinin by early life murine immunization with an attenuated vaccinia-derived NYVAC(K1L) viral vector. Virology. 2001;285:12–20. doi: 10.1006/viro.2001.0945. [DOI] [PubMed] [Google Scholar]
- 39.de Lalla C, Festuccia N, Albrecht I, et al. Innate-like effector differentiation of human invariant NKT cells driven by IL-7. J Immunol. 2008;180:4415–4424. doi: 10.4049/jimmunol.180.7.4415. [DOI] [PubMed] [Google Scholar]
- 40.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seddon B, Zamoyska R. TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J Immunol. 2002;169:3752–3759. doi: 10.4049/jimmunol.169.7.3752. [DOI] [PubMed] [Google Scholar]
- 44.Zamisch M, Moore-Scott B, Su DM, Lucas PJ, Manley N, Richie ER. Ontogeny and regulation of IL-7-expressing thymic epithelial cells. 2005;174:60–67. doi: 10.4049/jimmunol.174.1.60. [DOI] [PubMed] [Google Scholar]
- 45.Mahajan VS, Leskov IB, Chen JZ. Homeostasis of T cell diversity. Cell Mol Immunol. 2005;2:1–10. [PubMed] [Google Scholar]
- 46.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23:783–788. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 47.Adkins B, Guevara P, S Rose. Thymic and extrathymic contributions to T helper cell function in murine neonates. Haematol Rep. 2006;2:9–13. [PMC free article] [PubMed] [Google Scholar]
- 48.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]