Abstract
Background
Allogeneic human skin removed from cadaveric donors is the covering of choice for deep burns, since it accelerates the re-epithelialisation of autologous skin. In this study we evaluated the cellular viability of cryopreserved skin at the regional tissue bank of Verona (Italy).
Methods
From 1st June 2007 to 30th September 2007, tests of cutaneous cell viability were carried out on 21 consecutive skin donors using the MTT (tetrazolium salt) method on samples prior to freezing and on thawed samples after a period of cryopreservation.
Results
The mean percentage viability was 45.1% (±20.1%), which is similar to results obtained in other tissue banks. It was noted that viability decreased with increasing age of the donor.
Conclusions
The results of the evaluation of cutaneous cell viability document the validity of the skin cryopreservation procedure in use at the tissue bank in Verona.
Keywords: skin, tissue bank, cryopreservation, transplantation
Introduction
Viable allogeneic human skin removed from cadaveric donors is the covering of choice for wide, deep burns, providing temporary protection of the burnt area from infections, preventing the loss of water, proteins and electrolytes and significantly accelerating the re-epithelialisation of the autologous skin. It is, therefore, essential to ensure that the viability of the skin tissue undergoing cryopreservation is and remains good.
In this study we present the results of an evaluation of pre-freezing and post-thawing viability of cutaneous tissue stored at the regional tissue bank in Verona.
The evaluation was conducted using the MTT test (tetrazolium salts [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]; Sigma-Aldrich, Milan, Italy), in accordance with national guidelines1,2.
Materials and methods
Between the June 1, 2007 and September 30, 2007, at the regional tissue bank in Verona (a multi-tissue bank that collects, processes, validates and distributes musculoskeletal and cutaneous tissue), samples from 21 consecutive skin donors were analysed. The donors’ mean age was 47.8 years ± 16.2 (range, 16 to 74 years). Tests of viability were carried out for each donor at baseline and after 10 days of cryopreservation of the tissue.
a) The MTT test
The viability test was carried out as part of an evaluation of the quality of the method used to cryopreserve skin tissue. The test is based on the MTT method, which evaluates the reduction of tetrazolium salts (MTT) by enzymes of viable cells and is carried out on fresh skin (within 72 hours of collection), to determine baseline viability, and after 10 days of cryopreservation (using the cryopreservant dimethylsulphoxide [DMSO]) to determine the index of viability of the cryopreserved skin. In order to carry out the test, flaps of skin 4 × 4 cm of homogenous thickness are prepared such that 12 biopsies, each with a diameter of about 6 mm, can be obtained.
The MTT assay is used to evaluate cell viability, exploiting a chromogenic oxidizing agent (MTT bromide), corresponding to a polycyclic system (C18H16BrN5S) with a tetrazolic ring that is readily reduced by mitochondrial dehydrogenases or other electron transport systems, forming, through the opening of the tetrazolic ring, a nitrogenous chromogenic compound called formazan, whose characteristic functional group is R1NH-N=CR2-N=NR3. Within cells, formazan forms purplish-red, water-insoluble crystals to which the cell membrane is impermeable; the molecule can, therefore, enter cells, but the product is unable to leave the cell if it has been correctly metabolised, that is, if the electron transport chain is still metabolically active (able to reduce the molecule)3–4.
The impermeability of the cell membrane to MTT and other findings have suggested that this salt is transported into the cell through endocytosis and that the formazan accumulates within the endosomal and lysosomal compartments of the cell to then be transported out of it by exocytosis5.
The transformation of MTT is accompanied by a change in colour from yellow to dark blue-purple. In order to release the formazan salts from the intracellular compartments of the cells, they must be extracted using 2-methoxyethanol solvent (Sigma-Aldrich, Milan, Italy). The molecular formula of this compound is CH3OCH2CH2OH and its action consists of dissolving the cell membranes, allowing the formazan salts to exit from the cells and dissolve, forming a purple solution. The optical density of this solution is then measured using a spectrophotometer (Eppendorf, Hamburg, Germany) at a wavelength of 570 nm. The optical density gives an estimate of the number of active mitochrondria and, therefore, of viable cells present in the sample.
b. Determination of cell viability
The skin flap chosen for the determination of cell viability is divided into three equal parts: one part for the baseline determination, the second part for determination of cell viability after 10 days of cryopreservation at −80°C and the third part for the determination of cell viability after 6 months at −80°C (this test was not carried out in the present study). Once washed, the part of the skin flap to be used for the evaluation of baseline cell viability is placed within a Petri dish and 12 biopsies, of as similar weight as possible, are taken.
The biopsies are weighed and then placed in the wells of a 12-well cell culture plate (Corning Incorporated, New York, USA). Of the 12 biopsies, the first two are used as negative controls and the other ten are the samples for testing. The negative controls are placed in microtubes (Eppendorf, Hamburg, Germany) and then heated in order to kill the cells. Next, 1 mL of a previously prepared solution of MTT tetrazolium salts is added to each well of the culture plate and the plate is then placed in a incubator in a CO2 environment for at least 3 hours.
After 3 hours of incubation the MTT solution is removed, by aspiration, from the wells, and 1 mL of 2-methoxyethanol is added to each well. The plate is then incubated for another 3 hours in CO2 at 37°C. During this period the 2-methoxyethanol dissolves the cell membranes and allows the formazan salts within the cell to escape into the surroundings. The colourless solution in the wells therefore becomes purple, to a degree proportional to the amount of formazan salts produced.
Once the incubation is completed, the coloured solution is transferred into microcuvettes that are then introduced into the spectrophotometer for reading of the light absorption of the coloured solution at a wavelength of 570 nm, which is the wavelength of the peak absorbance of reduced formazan salts. The absorption and weight (in milligrams) of each biopsy are recorded. The following formula is then used to calculate the index of viability:
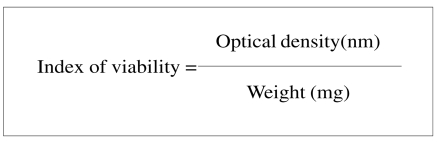 |
In order to determine cell viability after 10 days of cryopreservation at −80 °C, the skin flaps are thawed, placed in Petri dishes and then subjected to all the passages previously described for the determination of the viability index.
Using the formula reported above, the index of viability of each sample after 10 days of cryopreservation is obtained. The percentage viability maintained by the cryopreserved samples (x) is calculated as follows:
 |
c) Statistical analysis
Spearman’s coefficient (rs) was used to determine whether there were correlations between the variables analysed (percentage cell viability maintained after cryopreservation, baseline index of viability, index of viability after 10 days of cryopreservation, age of donor); p values <0.05 were considered statistically significant. Spearman’s rank correlation coefficient is a non-parametric measure of correlation - that is, it assesses how well an arbitrary monotonic function could describe the relationship between two variables, without making any assumptions about the frequency distribution of the variables.
Results
With the purpose of evaluating the quality of skin donation and banking at the regional tissue bank of Verona, cell viability was tested using the MTT method, in accordance with national guidelines.
The indices and percentages of viability of cutaneous tissue from the 21 donors recruited in the 4 months from June to September 2007 are illustrated in the following Figures 1a and 1b.
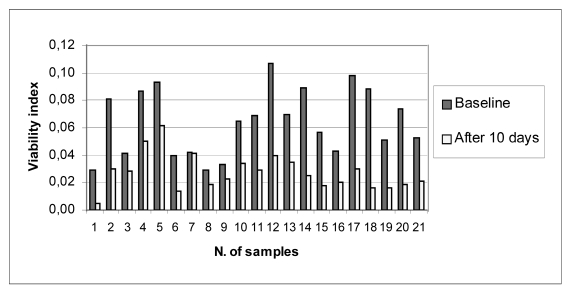
Figure 1.a.
Indices of cell viability (pre-freezing and after 10 days of cryopreservation) of the 21 samples analysed
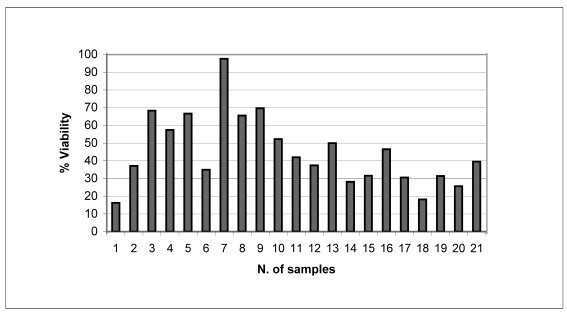
Figure 1.b.
Percentages of cell viability maintained after cryopreservation in the 21 samples analysed
The mean baseline cell viability index was 0.064 ± 0.024 (range, 0.024 – 0.107), and the mean viability index after 10 days of cryopreservation was 0.027 ± 0.013 (range 0.005 – 0.062).
The percentage cell viability in the 21 samples analysed was 45.09 ± 20.11 percent (range, 16.21 – 97.62 %).
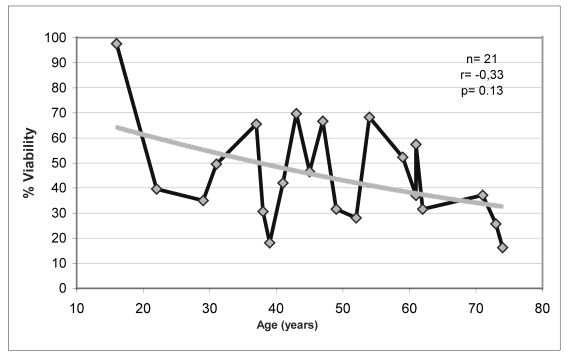
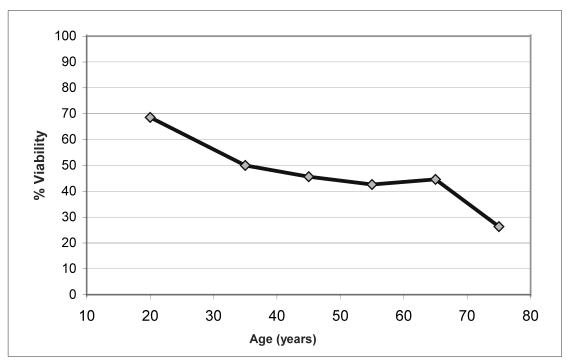
With the aim of identifying possible factors influencing cell viability, the indices of viability (at baseline and after 10 days of cryopreservation) and the percentage of cell viability maintained after cryopreservation were correlated with the donors’ age (Figure 2 a, b, c, d).
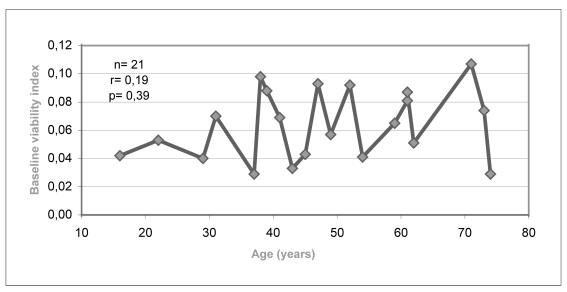
Figure 2.a.
Relationship between baseline viability index and donors’ age
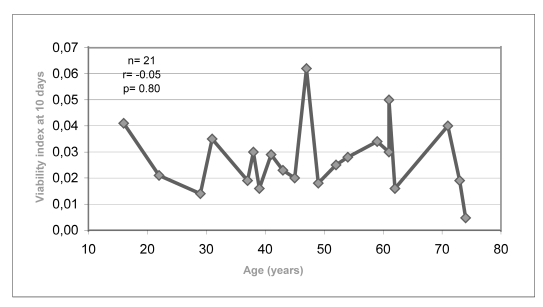
Figure 2.b.
Relationship between indices of cell viability after 10 days of cryopreservation and donors’ age
Figure 2.c.
Relationship between percentage of cell viability and donors’ age
Figure 2.d.
Relationship between percentage of cell viability and age (by decades) of the donors
Although statistically significant changes were not found in the variables analysed, there was a trend towards a decrease in percentage cell viability with increasing age of the donors (Figure 2.c). This decrease was more obvious when the percentage of viability was analysed in relation to decades of age of the donors (Figure 2.d).
Other variables were also analysed but, although interesting results were obtained, further information from a greater number of samples is necessary.
These additional evaluations showed a clear tendency for the percentage cell viability maintained to be lower in samples processed more than 24 hours after collection compared to that in samples processed within 24 hours.
Indeed, the mean percentage of cell viability of these latter samples was 44.65 ± 14.38 percent (range, 16.21 – 66.67%), while the mean percentage of the former was 55.31 ± 29.37 percent (range, 18.18 – 97.62%) (p = 0.07). The results of the evaluation of percentage cell viability maintained in tissues from female and male donors were also interesting.
The mean percentage for tissues from male donors was 49.04 ± 22.35 percent (range, 16.21 – 97.62%), while the mean percentage for tissues from female donors was 34.88 ± 7.24 percent (range, 25.68 – 46.51%) (p = 0.07).
Discussion
The good quality and safety of cyropreserved skin tissue are fundamental objectives of the tissue bank at Verona, which has a strategic role not only at a regional level (being the only skin bank in the Region of Veneto), but also at a national level. In fact, this bank is part of a network of banks of skin tissue, co-ordinated by the CNT, which interact with the aim of ensuring national self-sufficiency in allogeneic skin and to deal with any emergencies.
Despite the fact that evaluation of cell viability has been performed at the tissue bank for only a few months and the number of samples analysed is still limited, the results obtained so far do allow some interesting observations. In particular, analysing the correlation between cell viability and donors’ age, it was noted that the percentage cell viability maintained after cryopreservation tended to decrease progressively with increasing age of the donor. Although this was only a trend, it is thought that a statistically significant correlation will emerge as more samples are tested. If these results are confirmed, it would be sensible to lower the maximum age of donors of viable skin, currently 75 years old in our protocol, to 65 years, as has already been done in other national facilities6,7. One possible explanation for this phenomenon could lie in the changing characteristics of cutaneous tissue with age. Indeed, it is know that in the elderly the skin loses some of its elasticity and its capacity for cell replication, defence and repair of genetic material following physical, chemical or biological damage8,9. It can, therefore, be hypothesised that the capacity for the skin to maintain its viability during the thermal shock induced by freezing the tissue at −80 °C decreases with advancing age of the donor.
The percentage cell viability maintained after 10 days of cryopreservation of cutaneous tissue in the tissue bank in Verona appears to be in line with published data, although slightly lower4. This is presumably due to the limited number of samples analysed and to the variability in results obtained from the earliest samples because of difficulties in standardisation always occurring in the initial period of introducing a new method. There is a trend, however, towards progressive, constant standardisation of all the various stages of the method and, therefore, to optimisation and reproducibility of the results.
Conclusions
Viable homologous skin grafting has a fundamental role in the treatment of patients with severe burns, for whom it can be considered a life-saving procedure10,11. However, the efficacy of skin grafting is also evaluated on the basis of its capacity to improve the quality of life of patients. Consequently, it is fundamental that skin banks not only to supply a product that is safe from an infectious point of view, but also guarantee the viability of the cryopreserved skin that should be grafted.
The method described here for evaluating the cell viability of cyropreserved skin is easy and quick to perform, and has good reproducibility after suitable training of the laboratory staff. It can, therefore be considered an excellent instrument available for the Tissue Bank for the validation of cryopreservation procedures.
References
- 1.Documento Tecnico della Consulta Permanente e del Centro Nazionale per i Trapianti (CNT): Linee guida per il prelievo, la conservazione e l’utilizzo della cute a scopo di trapianto. Approvato il 15 luglio 2004.
- 2.Documento Tecnico della Consulta Permanente e del Centro Nazionale per i Trapianti (CNT): Linee guida per il prelievo, la processazione e la distribuzione dei tessuti a scopo di trapianto. Approvato il 19 giugno 2007.
- 3.Castagnoli C, Alotto D, Cambieri I, et al. Evaluation of donor skin viability: fresh and cryopreserved skin using tetrazolium salt assay. Burns. 2003;29:759–67. doi: 10.1016/j.burns.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997;69:581–93. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 5.Bravo D, Rigley TH, Gibran N, Strong DM, Newman-Gage H. Effect of storage and preservation methods on viability in transplantable human skin allografts. Burns. 2000;26:367–78. doi: 10.1016/s0305-4179(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 6.Pianigiani E, Ierardi F, Cherubini Di Simplicio F, Andreassi A. Skin bank organization. Clin Dermatol. 2005;23:353–6. doi: 10.1016/j.clindermatol.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Kalter ESJ, de By TMMH. Tissue banking programmes in Europe. British Medical Bulletin. 1997;53:798–816. doi: 10.1093/oxfordjournals.bmb.a011649. [DOI] [PubMed] [Google Scholar]
- 8.Fisher GJ, Kang S, Varani J, et al. Mechanism of photoaging and chronogical skin aging. Arch Dermatol. 2002;138:1462–70. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 9.Sauvaigo S, Bonnet-Duquennoy M, Odin F, et al. DNA repair capacities of cutaneous fibroblasts: effect of sun exposure, age and smoking on response to an acute oxidative stress. Br J Dermatol. 2007;157:26–32. doi: 10.1111/j.1365-2133.2007.07890.x. [DOI] [PubMed] [Google Scholar]
- 10.Alexander JW, McMillan BG, Law E, Kittur DS. Treatment of severe burns with widely meshed skin autografts and meshed skin allograft overlay. J Trauma. 1981;21:434. [PubMed] [Google Scholar]
- 11.Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet. 1986;1:1123–4. doi: 10.1016/s0140-6736(86)91838-6. [DOI] [PubMed] [Google Scholar]