Abstract
Background
It is not rare to observe in blood donors a level of haematocrit (Hct) above or close to the highest normal limit. In the case of blood donors the diagnosis and clinical evaluation of this alteration may be complicated by regular blood donations that can mask an underlying disease such as polycythaemia vera. Recently a single acquired mutation in the Janus kinase 2 gene (JAK2) on chromosome 9 was identified and it was found that the incidence of this mutation was high in patients with polycythaemia vera.
Material and Methods
From the January 1, 2006 to December 31, 2006 all consecutive donors with a Hct above 50% if males (n=84) and 46% if females (n=19) underwent JAK2 mutation analysis. Seventy-nine donors (59 males and 20 females) whose Hct was normal at their last blood donation were randomly selected and used as controls.
Results
Among the group of blood donors with a high Hct, we identified one donor who was positive for the JAK2 mutation. This man had a Hct of 50.6% at his last donation, while his average Hct in the preceding year was 51.7%. The prevalence of the JAK2 mutation could be estimated to be 1%, 0.6% or 0.02% in the three different populations considered: donors with a Hct level above the upper limit of normal, all tested donors or the entire donor cohort attending our transfusion service, respectively.
Conclusions
The present study suggests that apparently healthy subjects with repeatedly high levels of Hct may have the acquired mutation in JAK2. Laboratory screening tests for JAK2 may be offered to blood donors at transfusion services with expertise in molecular genetics.
Keywords: JAK2 mutations, haematocrit, polycythaemia vera, myeloproliferative disorder, blood donors
Introduction
Blood donors have their haematocrit (Hct) and haemoglobin (Hb) values tested regularly to exclude anaemia, which is the most frequent cause for not being accepted as a donor1. However, it is not uncommon to observe a Hb or Hct value above or close to the upper limit of normal and in some studies such values have been investigated to exclude or confirm the presence of secondary causes of erythrocytosis as well as a primary myeloproliferative disorder (MPD) such as polycythaemia vera (PV) 2. Among the cohort of blood donors, the diagnosis and clinical evaluation of high Hct and Hb values may be complicated by numerous, regular blood donations that can mask an underlying disease because of the steady decrement of blood mass caused by the donations.
MPD is the name given to a family of diseases in which blood cells are overproduced; based on the prevalent single lineage, different types of disorders can be distinguished, including idiopathic myelofibrosis, essential thrombocythaemia and PV3,4. Polycythaemia vera is characterized by an increased production of red blood cells and it is the most common chronic MPD with a reported annual incidence of about 2 per 100,000 persons. The male to female ratio is 1.2, the average age at diagnosis is approximately 60 years and the most frequent causes of death are thrombosis and haematological malignancies5–8. It is currently diagnosed according to the PV Study Group (PVSG) criteria, which are based on the findings of a physical examination plus laboratory examination of blood and bone marrow cells3,4.
The cause of PV was unknown until 2005 when four independent research groups identified a single acquired mutation in the Janus kinase 2 gene (JAK2) on chromosome 9 and it was found that the incidence of this mutation was high in patients with PV9–12. The JAK2 protein functions as an intermediate between membrane receptors and signalling molecules. The point mutation in exon 12 of JAK2, leading to a valine-to-phenylalanine substitution (V617F) due to replacement of G by T in nucleotide sequences (1848G>T), causes constitutive activation of the tyrosine kinase that is believed to confer erythropoietin hypersensitivity and erythropoietin-independent survival to the myeloid stem cell13. In recent studies the JAK2 mutation has been found in 65–97% of patients with PV, but also in other MPD such as hypereosinophilic syndrome, chronic neutrophilic leukaemia, chronic idiopathic myelofibrosis and essential thrombocythaemia 14,15. On the basis of these recent findings, a new diagnostic algorithm for PV has been suggested in which the first step in the diagnosis of an erythrocytosis is to determine whether JAK2V617F is present in the granulocytes15–19.
In keeping with the important aim of preventive medicine offered by transfusion services to blood donors, all consecutive donors at our centre with a Hct value >50% for males and >46% for females were screened for this mutation. Concurrently, blood samples from a group of donors with normal Hct values were collected to act as controls. The objective was not only to estimate the incidence of this mutation in blood donors with Hct values above or near the upper limit of normal, but also to evaluate whether or not blood banks might be able to contribute to screening for undiagnosed patients with PV.
Materials and methods
Study population
In our transfusion centre nearly 6,000 donors are provide a total number of approximately 12,000 donations per year, including whole blood, plasma, platelets and multi-component products (plasma-platelets, erythrocytes-plasma, etc.). Prior to each donation all data, including red blood cell, white blood cell and platelet counts, haematocrit and haemoglobin concentration, are routinely recorded in a database. Between January 1, 2006 and December 31, 2006 there were 11,240 donations from 5,636 regular blood donors (total donation index 1.99 per year). From these, 84 males and 19 females were identified as having a high Hct, defined as above 50% for men and above 46% for women. In this cohort, during a 1,846-year donation period, the average number of donations per male was 22.1 and per female 11.4. Seventy-nine donors (59 male and 20 female) whose Hct had been normal at their last blood donation were randomly selected and used as controls (Table I).
Table I.
General characteristics of subjects with high haematocrit and normal values
| Males | Females | |||||
|---|---|---|---|---|---|---|
| ↑ Hct (N=84) mean ± SD | Normal Hct (N=59) mean ± SD | P* | ↑ Hct (N=19) mean ± SD | Normal Hct (N=209) mean ± SD | P* | |
| Hct (%) | 49.74 ± 1.25 | 43.8 ± 1.87 | < 0.001 | 45.23 ± 1.35 | 40.8 ± 1.77 | < 0.001 |
| Hb (g/dL) | 16.85 ± 0.56 | 15.1 ± 0.6 | < 0.001 | 15.2 ± 0.56 | 13.9 ± 0.72 | < 0.001 |
| WBC (×109/L) | 6.456 ± 1.278 | 5.555 ± 1.202 | < 0.001 | 6.355 ± 1.012 | 5.888 ± 0.978 | NS |
| PLTs (×109/L) | 236 ± 51.7 | 226 ± 52.0 | NS | 244 ± 53.2 | 277 ± 49.6 | NS |
| Donations (n.) | 22.1 ± 17.8 | 19.98 ± 12.4 | NS | 11.42 ± 11.4 | 6.90 ± 11.1 | NS |
| Age (yrs) | 44.5 ± 11.3 | 42.0 ± 8.60 | NS | 43.8 ± 14.0 | 39.3 ± 11.8 | NS |
| Years since first donation | 11.1 ± 8.14 | 10.9 ± 6.8 | NS | 8.20 ± 7.04 | 7.0 ± 7.6 | NS |
Legend- Ht: haematocrit; Hb: haemoglobin; PLTs: platelet count; WBC: white blood cell count; SD: standard deviation; NS: not significant
T test for independent samples and homogeneous variance
JAK2 mutation screening
All analyses were carried out in our laboratory which is specialised in molecular medicine, mainly as a national referral centre for haemophilia genetics. In order to identify the V617F (1848G>T) mutation within JAK2 we used the amplification refractory mutation system (ARMS) which, under ideal polymerase chain reaction (PCR) conditions, is an extremely efficient detection method for the single base change20.
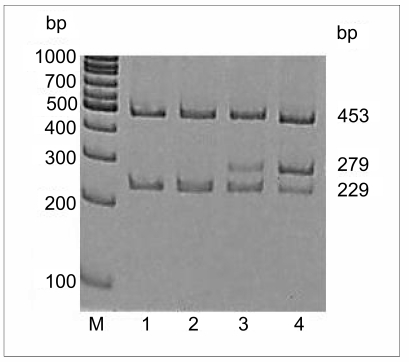
Peripheral blood was collected into Vacutainer tubes containing EDTA and DNA was extracted as previously reported18.. The presence of the V617F (1848G>T) mutation was identified by PCR, using four primers: forward outer (FO), 5′ TCC TCA GAA CGT TGA TGG CAG 3′, reverse outer (RO), 5′ ATT GCT TTC CTT TTT CAC AAG AT 3′, forward wild-type-specific (Fwt), 5′ CAT TTG GTT TTA AAT TAT GGA GTA TAT G 3′ and reverse mutant specific (Rmt) 5′ GTT TTA CTT ACT CTC GTC TCC ACA AAA 3′. The FO and RO primers flank the JAK2 exon 12 and amplify a 453 bp product control in all cases. The Fwt and RO primers amplify a 229 bp wild-type-specific band (1848G) while FO and Rmt amplify a 279 bp mutant-specific band (1848T).
The PCR were performed using AmpliTaq Gold PCR Master Mix 2X 250U/5mL (Appleare), 1.5 mM MgCl2, 150 ng of blood donor’s DNA and 1 μM and 0.5 μM of the outer primers (FO and RO) and the mutant/wild-type-specific inner primers (Fwt and Rmt), respectively. The amplification conditions consisted of a starting denaturation step of one cycle at 94°C for 5 min followed by 35 cycles of denaturation (95°C, 30 sec), annealing (60°C, 30 sec) and extension (72°C for 60 sec). These cycles were followed by a final extension step of one cycle at 72°C for 5 min. Products were resolved on 6% acrylamide gel and examined after staining with ethidium bromide (Figure 1).
Figure 1.
Pattern of the JAK2 1848G>T mutation detection in polyacrylamide gel. Lane M, 100 bp ladder; lane 1, negative control; lane 2, normal genotype sample; lane 3, heterozygous blood donor; lane 4, positive heterozygous control. FO and RO primers generate a control 453 bp band in all cases. Fwt and RO primers generate a 229 bp wild-type G-specific product and FO and Rmt primers generate a 279 bp mutant T-specific product.
Results
The mean haematological values in the two different populations over the entire donation life period are reported in table I. There were statistically significant differences between the two groups for Hct and Hb in both males and females (t-test for independent samples and homogeneous variance), as expected, but also for white blood cell count in the subgroup of males but not in the females (Table I). The mean Hct value of the last donations from the 103 donors with high Hct levels was 51.02% for males and 46.9% for females, while the mean value of the control cohort with a normal Hct at their last donation was 42% and 39% for males and females, respectively (data not shown).
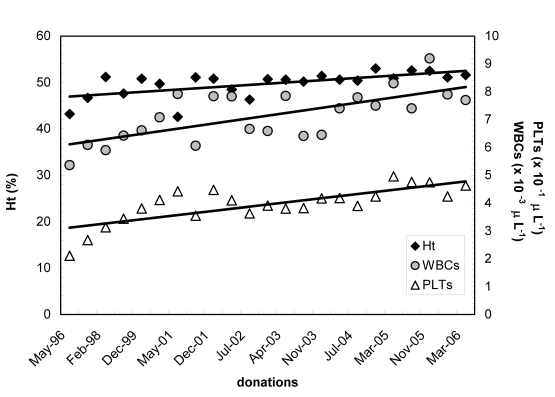
Among the 84 men with a high Hct we identified one positive for the JAK2 mutation. He had a Hct of 50.6% during the last donation while his average Hct in the last year was 51.7%. As the patient’s Hb concentration was above 18.5 g/dL he met both the major criteria of the new WHO proposal for the diagnosis of PV16. The entire donation history of this patient is shown in figure 2.
Figure 2.
Haematocrit (Ht), white blood cell count (WBCs) and platelet count (PLTs) in the JAK2-positive 43-year old male blood donor over the 10-year period he had donated blood. All three lines show an increasing trend, in accordance with the natural history of PV
These results account for a total prevalence of the JAK2 mutation of 1% in the blood donor population with elevated Hct (1 out of 103). None of the 79 blood donors (male and female) with a normal Hct in their last donation had the JAK2 mutation.
Discussion
The recent proposal of a new diagnostic flow procedure for the diagnosis of PV15–19 prompted us to perform a study on blood donors with high Hct levels to ascertain whether any of them had an underlying disease. For this purpose we looked for the JAK2 mutation in a cohort of 182 donors, a proportion of whom had what we defined as a high Hct (>50% for males, >46% for females) at their last donation. In this subgroup with high Hct, we found one male with the JAK2 mutation and after further haematological investigation PV was diagnosed according to the criteria of the PVSG.
The estimated prevalence of PV is considered to be 1–5 cases/million per year although from a large study performed in Vicenza21 the prevalence in an unselected population seems much higher (around 300/million annually).
This is also confirmed by another Italian study which reported 8 cases of MPD among a population of 90,000 blood donors22. On the other hand, in a pre-selected blood donor cohort of 81 consecutive donors with Hct levels >49%, Zanella et al. reported a 3.7% prevalence of PV, diagnosed according to the PVSG criteria2.
Our results seem to be in keeping with those of the few studies evaluating the supposed ‘normal’ population of blood donors2,22 or general population21. In these studies the prevalence of MPD is around 1–3% which is similar to the prevalence that we found, but very different from that in a hospitalised population. None of our blood donors had a history of present or past thrombosis, but this is very probably due to the presence of low vascular risk and presumably related to the young age of this cohort.
An interesting question is whether the prolonged myeloid ‘stimulus’ from phlebotomies can induce inhibition of the feed-back control of cells committed to becoming red cells; it could also be suspected that blood donations may trigger the onset of a pre-existing, latent, primary MPD. However, among the family of haematological malignancies, only PV has been reported to be more frequent in blood donors than in the general population and this might be due to early detection because of frequent blood tests carried out in such subjects22,23.
In a hospital setting, in which blood samples were randomly collected, surprisingly 37 out of a total of 3,935 were found to be positive for the JAK2 mutation. However, only one of these samples had blood results indicative of possible PV but several had non-haematological diseases. On average, samples with the mutation had normal red cell counts but significantly higher white blood cell and platelet counts, although most were within the normal range. These data suggest that the JAK2V617F mutation is much more common than MPD and its occurrence might be a prelude to whole blood cell abnormalities and other diseases, but it cannot by itself be used to diagnose MPD24. Passamonti et al. reported that they did not find the mutation in any of 75 healthy individuals investigated25.
The present study suggests that subjects with repeatedly high Hct levels may have the acquired mutation in JAK2. This finding should be confirmed in a larger donor population. The follow-up of the negative donors could, however, also be useful in the light of the possible identification of an acquired mutation, which would provide additional information for understanding the natural course of the development of PV.
This could be of particular importance if super-sensitive assays able to detect small amounts of JAK2V617F in healthy individuals were used, in a situation analogous to that of BCR/ABL26. Finally, the overall objectivity and accessibility of JAK2 mutation screening using peripheral blood enables a practical, modern approach to studying patients suspected of having PV that does not require red cell mass measurements and reduces the number of bone marrow examinations27,28.
The possibility of bypassing a bone marrow examination if peripheral blood mutation screening reveals the presence of JAK2V617F could be argued. However, such a policy is currently considered premature and would lead to the loss of useful baseline information from bone marrow histology and cytogenetic analysis. Nevertheless, the remarkably close association between PV and JAK2V617F has further undermined the already limited value of red cell mass measurements and specialised biological assays for the diagnosis of PV such as erythroid colony formation, megakaryocyte thrombopoietin receptor expression and platelet-rich plasma serotonin level. This news is to be welcomed because each of these assays entails a certain level of expertise that is not widely available clinically. Obviously, for routine clinical practice the feasibility of such a process depends on the availability of laboratory screening tests for JAK2V617F which may be offered also at a transfusion service with particular expertise in molecular genetics.
Acknowledgments
This study was supported in part by Avis Castellana, Borsa di studio Giorgio Lago and Associazione progresso ematologico, Castelfranco Veneto (TV), Italy.
We thank Mrs. Gabrielle Clarke for editing this manuscript.
References
- 1.Davey RJ. The blood centre as a community health resource. Vox Sang. 2006;91:206–13. doi: 10.1111/j.1423-0410.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Zanella A, Silvani C, Banfi P, et al. Screening and evaluation of blood donors with upper-limit hematocrit levels. Transfusion. 1987;27:485–7. doi: 10.1046/j.1537-2995.1987.27688071701.x. [DOI] [PubMed] [Google Scholar]
- 3.Thiele J, Kvasnicka HM. A critical reappraisal of the WHO classification of the chronic myeloproliferative disorders. Leuk Lymphoma. 2006;47:381–96. doi: 10.1080/10428190500331329. [DOI] [PubMed] [Google Scholar]
- 4.Thiele J, Kvasnicka HM. Chronic myeloproliferative disorders with thrombocythemia: a comparative study of two classification systems (PVSG, WHO) on 839 patients. Ann Hematol. 2003;82:148–52. doi: 10.1007/s00277-002-0604-y. [DOI] [PubMed] [Google Scholar]
- 5.Finazzi G. A prospective analysis of thrombotic events in the European collaboration study on low-dose aspirin in polycythemia (ECLAP) Pathol Biol. 2004;52:285–90. doi: 10.1016/j.patbio.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–32. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 7.Stuart BJ, Viera AJ. Polycythemia vera. Am Fam Physician. 2004;69:2139–44. [PubMed] [Google Scholar]
- 8.Tefferi A, Spivak JL. Polycythemia vera: scientific advances and current practice. Semin Hematol. 2005;42:206–20. doi: 10.1053/j.seminhematol.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Baxter RJ, Scott LM, Campbell PJ, et al. Cancer Genome Project Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 10.James C, Ugo V, Le Couedic SP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 11.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 12.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Zhao ZJ, Vainchenker W, Krantz SB, et al. Role of tyrosine kinases and phosphatases in polycythemia vera. Semin Hematol. 2005;42:221–9. doi: 10.1053/j.seminhematol.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Tefferi A, Gilliland DG. The JAK2V617F tyrosine kinase mutation in myeloproliferative disorders: status report and immediate implications for disease classification and diagnosis. Mayo Clin Proc. 2005;80:947–58. doi: 10.4065/80.7.947. [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A. Classification, diagnosis and management of myeloproliferative disorders in the JAK2V617F era. Hematology Am Soc Hematol Educ Program. 2006;34:240–5. doi: 10.1182/asheducation-2006.1.240. [DOI] [PubMed] [Google Scholar]
- 16.Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel Blood 2007. 151101092–7. [DOI] [PubMed] [Google Scholar]
- 17.Chang CC. BCR/ABL-negative chronic myeloproliferative disorders: JAK2 mutation and beyond. Arch Path Lab Med. 2006;130:1123–5. doi: 10.5858/2006-130-1123-ACMD. [DOI] [PubMed] [Google Scholar]
- 18.James C, Delhommeau F, Marzac C, et al. Detection of JAK2 V617F as a first intention diagnostic test for erythrocytosis. Leukemia. 2006;20:350–3. doi: 10.1038/sj.leu.2404069. [DOI] [PubMed] [Google Scholar]
- 19.Michiels JJ, De Raeve H, Berneman Z, et al. The 2001 World Health Organization and updated European clinical and pathological criteria for the diagnosis, classification, and staging of the Philadelphia chromosome-negative chronic myeloproliferative disorders. Semin Thromb Hemost. 2006;32:307–40. doi: 10.1055/s-2006-942754. [DOI] [PubMed] [Google Scholar]
- 20.Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri M, Tosetto A, Frezzato M, Rodeghiero F. The rate of progression to polycythemia vera or essential thrombocythemia in patients with erythrocytosis or thrombocytosis. Ann Intern Med. 2003;139:470–5. doi: 10.7326/0003-4819-139-6-200309160-00009. [DOI] [PubMed] [Google Scholar]
- 22.Randi ML, Rossi C, Barbone E, et al. Myeloproliferative disease in patients with a history of multiple blood donations: a report of 8 cases. Haematologica. 1994;79:137–40. [PubMed] [Google Scholar]
- 23.Merk K, Mattsson A, Holm G, et al. The incidence of cancer among blood donors. Int J Epidemiol. 1990;19:505–9. doi: 10.1093/ije/19.3.505. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Zhang Q, Luo J, et al. JAK2(V617F): Prevalence in a large Chinese hospital population. Blood. 2007;109:339–42. doi: 10.1182/blood-2006-03-009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passamonti F, Rumi E, Pietra D, et al. JAK2 (V617F) mutation in healthy individuals. Br J Haematol. 2007;136:678–9. doi: 10.1111/j.1365-2141.2006.06483.x. [DOI] [PubMed] [Google Scholar]
- 26.Bose S, Deininger M, Gora-Tybor J, et al. The presence of typical and atypical BCR-ABL fusion genes in leukocytes of normal individuals: biologic significance and implications for the assessment of minimal residual disease. Blood. 1998;92:3362–7. [PubMed] [Google Scholar]
- 27.Tefferi A. The rise and fall of red cell mass measurement in polycythemia vera. Curr Hematol Rep. 2005;4:213–7. [PubMed] [Google Scholar]
- 28.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]