Abstract
Mesenchymal stem cells (MSCs) are defined by their ability to self-renew and differentiate into the cells that form mesodermal tissues such as bone and fat. Low magnitude mechanical signals (LMMS) have been shown to be anabolic to bone and have been recently reported to suppress the development of fat in normal animals fed a regular diet. Using male C57BL/6J mice, the ability of LMMS (0.2g, 90-Hz signal applied for 15 min/d, 5 d/wk) to simultaneously promote bone formation and prevent diet-induced obesity was correlated to mechanical influences on the molecular environment of the bone marrow, as indicated by the population dynamics and lineage commitment of MSCs. Six weeks of LMMS increased the overall marrow-based stem cell population by 37% and the number of MSCs by 46%. Concomitant with the increase in stem cell number, the differentiation potential of MSCs in the bone marrow was biased toward osteoblastic and against adipogenic differentiation, as reflected by upregulation of the transcription factor Runx2 by 72% and downregulation of PPARγ by 27%. The phenotypic impact of LMMS on MSC lineage determination was evident at 14 wk, where visceral adipose tissue formation was suppressed by 28%, whereas trabecular bone volume fraction in the tibia was increased by 11%. Translating this to the clinic, a 1-yr trial in young women (15–20 yr; n = 48) with osteopenia showed that LMMS increased trabecular bone in the spine and kept visceral fat at baseline levels, whereas control subjects showed no change in BMD, yet an increase in visceral fat. Mechanical modulation of stem cell proliferation and differentiation indicates a unique therapeutic target to aid in tissue regeneration and repair and may represent the basis of a nonpharmacologic strategy to simultaneously prevent obesity and osteoporosis.
Key words: mesenchymal stem cells, osteoblasts, osteoporosis, mechanical loading, adipocytes, obesity, adipogenesis, osteoblastogenesis
INTRODUCTION
The relationship between bone and fat is complex and highly contingent on a multitude of factors including genetic, nutritional, biochemical, metabolic, and physical inputs that coordinate the overall interaction and resulting phenotype. At the clinical level, the control of bone and fat formation is critically relevant to the increasing prevalence of, and costs associated with, osteoporosis and obesity. Predominantly, treatments are designed to treat obesity and osteoporosis once symptoms have already manifested, and such strategies, pharmacologic in particular, are primarily designed to target the extant fat cells (obesity) or bone cells (osteoporosis) to modify their activity, whether to reduce the lipid accumulation in adipocytes or to suppress activation of osteoclasts.
With the ever-increasing knowledge of the etiology of these diseases, new targets for primary prevention are being considered. Rather than target the resident cell population in bone and fat, strategies are being designed to control the fate of their progenitors, mesenchymal stem cells (MSCs), that have yet to commit and differentiate into the mature cell types.(1,2) Pluripotent MSCs are considered ideal therapeutic targets for regenerative medicine and strategies intended to mitigate disease pathogenesis, because they hold the capacity to differentiate into osteoblasts, adipocytes, fibroblasts, chondrocytes, and myocytes. In the case of obesity and osteoporosis, biasing the fate of these precursors would help control the phenotypic outcomes of bone and fat mass, yet the difficulty in identifying the key environmental cues that regulate the lineage selection of MSCs has made it difficult to translate this to the clinic.(3–5)
Although the interest in MSCs and their application to regenerative therapies and molecular therapeutics has been intense, the understanding of these cells remains in the nascent stages. Even gaining consensus on the criteria that serve to identify the MSC population has proven difficult, because stem cells and their neighboring cells within tissues are difficult to delineate and distinguish based on current histological methods. Illustrating the inherent difficulty of high-fidelity cellular identification, a combination of markers used to distinguish another stem cell type, hematopoietic stem cells (HSCs), from 99.9% of the other cells in the bone marrow yields a population estimated at only 3% purity.(6) The expression of surface markers is not well characterized, and to date, those that can be used to exclusively define MSCs have not been identified. Cell populations obtained in current isolation methods inevitably include heterogeneous mixtures of several cell types.(7) Although a consensus has not yet been reached, there are several markers such as stem cell antigen (Sca-1) that are commonly used for identification of stem cells in murine models.(8,9) Sca-1 is an antigen previously associated with hematopoietic cells, but more recently cells expressing Sca-1 have been shown to exhibit adipogenic, chondrogenic, and osteogenic potential.(8) Pref-1 (also known as fetal antigen 1 [FA1] and delta-like 1 [Dlk-1]) of the epidermal growth factor (EGF)-like protein family, has been identified as promoting the maintenance of a bipotential stem cell population.(10) In addition, the molecule is robustly expressed on pre-adipocytes and regulates the ability of this precursor cell to achieve the mature adipocyte phenotype.(10)
When the bone marrow stem cell population is driven to differentiate toward one cell fate, the establishment of another cell type is inherently suppressed. More specifically, MSCs express small amounts of both adipogenic and osteogenic factors, which cross-regulate to retain the cell in an undifferentiated state.(11) When oncostatin, a member of the interleukin (IL)-6 family, is used to promote osteogenesis, it simultaneously inhibits adipogenesis.(12) Conversely, anti-diabetic thiazolidinediones such as rosiglitazone, a potent activator of peroxisome proliferator-activated receptor γ (PPARγ), promotes adipogenesis while suppressing osteogenesis.(13–15) This inverse relationship is also evident in the physical realm; hyperphysiologic levels of tensile strain will increase proliferation of bone marrow cells(16) and downregulate PPARγ in the local bone marrow, thus favoring osteoblastogenesis over adipogenesis.(2) Furthermore, the ability of different mechanical signals, including fluid flow and compression, to alter the differentiation patterns of MSCs has been explored and used by tissue engineers to promote the growth of both bone and cartilage.(17–20)
The potential to harness MSCs as a means of prevention and treatment of disease is dependent on an improved understanding of the means by which exogenous signals regulate cell activity and the ability of these stimuli to influence either/both proliferation and differentiation.(21) Pharmacologic enhancement of stem cell proliferation has recently been shown in vivo,(22) whereas extremely low magnitude mechanical signals (LMMS) have been shown to promote bone formation(23) and suppress adipogenesis in the growing animal without the use of drugs.(24)
MATERIALS AND METHODS
Animal model to prevent diet-induced obesity
All animal procedures were reviewed and approved by the Stony Brook University Animal Care and Use Committee. The overall experimental design consisted of two similar protocols, differing in the duration of treatment to assess mechanistic responses of cells to LMMS (6 wk of LMMS compared with sham control, n = 8 per group) or to characterize the phenotypic effects (14 wk of LMMS compared with sham control). Furthermore, two models of diet-induced obesity (DIO) were used: (1) to examine the ability of LMMS to prevent obesity, a high-fat diet condition (n = 12 each, LMMS and CON) was evaluated, where LMMS and DIO were initiated simultaneously, and (2) to examine the ability of LMMS to reverse obesity, an obese condition (n = 8 each, LMMS and CON) was established, whereby LMMS treatment started 3 wk after the induction of DIO and was compared with sham controls who were fed a high-fat diet of this same extended time period.
Mechanical enhancement of stem cell proliferation and differentiation in DIO
Beginning at 7 wk of age, C57BL/6J male mice were given free access to a high-fat diet (45% kcal fat, 58V8; Research Diet, Richmond, IN, USA). The mice were randomized into two groups, defined as LMMS (5 d/wk of 15 min/d of a 90-Hz, 0.2g peak acceleration mechanical signal, where 1.0g is earth's gravitational field) and placebo sham controls (CON). The LMMS protocol(24) provides low-magnitude, high-frequency mechanical signals by a vertically oscillating platform,(25) and for this acceleration and frequency generates less than five microstrain in bone tissue, several orders of magnitude below peak strains generated during strenuous activity.(23,26) Animal mass and food consumption were monitored weekly.
Status of MSC pool by flow cytometry
Cellular and molecular changes in the bone marrow resulting from 6-wk LMMS (n = 8 animals per group, CON or LMMS) were determined at death from bone marrow harvested from the right tibia and femur (animals at 13 wk of age). Red blood cells in the bone marrow aspirate were removed by room temperature incubation with Pharmlyse (BD Bioscience) for 15 min. Single cell suspensions were prepared in 1% sodium azide in PBS, stained with the appropriate primary and (when indicated) secondary antibodies, and fixed at a final concentration of 1% formalin in PBS. Phycoerythrin (PE) conjugated rat anti-mouse Sca-1 antibody and isotype control were purchased from BD Pharmingen and used at 1:100. Rabbit anti-mouse Pref-1 antibody and FITC-conjugated secondary antibody were purchased from Abcam (Cambridge, MA, USA) and used at 1:100 dilutions. Flow cytometry data were collected using a Becton Dickinson FACScaliber flow cytometer (San Jose, CA, USA).
RNA extraction and real-time RT-PCR
At death, the left tibia and femur were removed and marrow was flushed into RNAlater solution (Ambion, Foster City, CA, USA). Total RNA was harvested from the bone marrow as described previously.(27) Briefly, TRIzol reagent (Life Technologies, Gaithersburg, MD, USA) was added to the total bone marrow cell suspension, and the solution was homogenized. Phases were separated with chloroform under centrifugation. RNA was precipitated by ethanol addition and applied directly to an RNeasy Total RNA isolation kit (Qiagen, Valencia, CA, USA). DNA contamination was removed on column with RNase free DNase. Total RNA was quantified on a Nanodrop spectrophotometer and RNA integrity monitored by agarose electrophoresis. Expression levels of candidate genes was quantified using a real-time RT-PCR cycler (Lightcycler; Roche) relative to the expression levels of samples spiked with exogenous cDNA.(28) A “one-step” kit (Qiagen) was used to perform both the reverse transcription and amplification steps in one reaction tube.
Body habitus established by in vivo μCT
Phenotypic effects of DIO, for both the prevention and reversal of obesity test conditions, were defined at both 12 and 14 wk of LMMS. At 12 wk, in vivo μCT scans were used to establish fat, lean, and bone volume of the torso (VivaCT 40; Scanco). Scan data were collected at an isotropic voxel size of 76 μm (45 kV, 133 μA, 300-ms integration time) and analyzed from the neck to the distal tibia for each animal. Threshold parameters were defined during analysis to segregate and quantify fat and bone volumes. Detailed CT scanning protocol and analysis techniques are reported elsewhere.(29,30) At 14 wk, after death, the epididymal fat pads and a subcutaneous fat pad from the mesenteric region were harvested and used to validate CT volume data generated at 12 wk. Correlations between ex vivo and in vivo fat measurements (reported elsewhere) were high (R 2 > 0.90).(29)
Bone phenotype established by ex vivo μCT
Trabecular bone morphology of the proximal left tibia of each mouse was established by μCT at 12-μm resolution (MicroCT 40; Scanco). The metaphyseal region spanned 600 μm, beginning 300 μm distal to the growth plate. Bone volume fraction (BV/TV), connectivity density (Conn.D), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and the structural model index (SMI) were determined.(31)
Serum and tissue biochemistry
Blood collection was performed after overnight fast by cardiac puncture with the animal under deep anesthesia. Serum was harvested by centrifugation. Mice were killed by cervical dislocation, and the different tissues (i.e., epididymal fat pad and subcutaneous fat pads from the lower torso, liver, and heart) were excised, weighed, frozen in liquid nitrogen, and stored at −80°C. Total lipids from white adipose tissue (epididymal fat pad) and liver were extracted and purified based on a chloroform–methanol extraction. Total triglyceride (TG) and nonesterified free fatty acid (NEFA) were measured on serum (n = 10 per group) and lipid extracts from adipose tissue (n = 5 or 6 per group) and liver (n = 10 per group) using enzymatic colorimetric kits (TG Kit; Sigma, St Louis, MO, USA; and NEFA C from Wako Chemicals, Richmond, VA, USA). ELISA assays were used to determine serum concentrations of leptin, adiponectin, resistin (all from Millipore, Chicago, IL, USA), osteopontin (R&D Systems, Minneapolis, MN, USA), and osteocalcin (Biomedical Technologies, Stoughton, MA, USA), using a sample size of n = 10 per group.
Human trial to examine inverse relationship of adipogenesis and osteoblastogenesis
A trial designed to evaluate if 12 mo of LMMS could promote BMD in the spine and hip of women with low BMD relative to controls(32) was evaluated retrospectively to examine whether there were concurrent changes in visceral fat volume. All procedures were reviewed and approved by the Childrens Hospital of Los Angeles Committee on Research in Human Subjects.
Forty-eight healthy young women (15–20 yr of age) were randomly assigned into either LMMS or CON groups (n = 24 in each group). The LMMS group underwent brief (10 min requested) daily treatment with LMMS (30-Hz, 0.3g signal) for 1 yr. CT scans were performed at baseline and at 1 yr, with the same scanner (model CT-T 9800; General Electric, Milwaukee, WI, USA), the same reference phantom for simultaneous calibration, and specially designed software for fat and muscle measurements. Identification of the abdominal site to be scanned was performed with a lateral scout view, followed by a cross-sectional image obtained from the midportion of the third lumbar vertebrae at 80 kVp, 70 mA, and 2S.
Cancellous bone of the first, second, and third lumbar vertebrae was established as measures of BMD in milligrams per cubic centimeter (mg/cm3). Visceral fat area (cm2) was defined at the midportion of the third lumbar vertebrae (L3) as the intra-abdominal adipose tissue surrounded by the rectus abdominus, external oblique, quadratus lumborum, and psoas muscles and the lumbar spine, and consisted mainly of perirenal, pararenal, retroperitoneal, and mesenteric fat. The average area of paraspinous musculature (cm2) was defined as the summed area of the erector spinae, psoas major, and quadratus lumborum muscles at the midportion of L3.(32) All analyses of BMD and muscle and fat area were performed by an operator blinded as to subject enrollment.
Statistical analyses
All data are shown as mean ± SD, unless noted. To determine significant differences between LMMS and CON groups, two-tailed t-tests were used throughout. Ex vivo trabecular bone data were analyzed with body mass of the animals as a covariate. Animal outliers were determined based on animal mass at baseline (before the start of any treatment) as animals falling outside of 2 SD from the total population or in each respective group at the end of 6- or 14-wk LMMS (or sham CON) by failure of the Weisberg one-tailed t-test (α = 0.01). The Weisberg test has been shown as an objective tool for showing consistency within small data sets.(33) No outliers were identified in the 6-wk CON and LMMS groups. Two outliers per group (CON and LMMS) were identified in the high-fat diet model (14-wk study) and removed. Data from these animals were not included in any analyses, resulting in a sample size of n = 10 per group for all data, unless otherwise noted. No outliers were identified in the 14-wk obese model (n = 8). Data presented from the human trial are based on the intent to treat data set (all subjects included in the evaluation). The significance of absolute changes in BMD, muscle, and visceral fat area, relative to baseline, were determined for LMMS and CON subjects using a two-tailed t-test. Statistical significance was considered at the 5% level.
RESULTS
Bone marrow stem cell population is promoted by LMMS
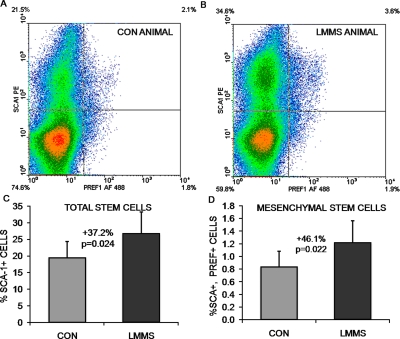
Flow cytometric measurements using antibodies against Sca-1 indicated that, in animals in the prevention DIO group, 6 wk of LMMS treatment significantly increased the overall stem cell population (including hematopoietic and mesenchymal stem cells) relative to controls. Analysis focused on the primitive population of cells with low forward (FSC) and side scatter (SSC), indicating the highest Sca-1 staining for all cell populations. Cells in this region showed a 37.2% (p = 0.024) increase in LMMS stem cell numbers relative to sham CON animals. MSCs as represented by cells positive for both Sca-1 and Pref-1(3) represented a much smaller percentage of the total cells. Identified in this manner, LMMS-treated animals had a 46.1% (p = 0.022) increase in specifically MSCs relative to CON (Fig. 1).
FIG. 1.
Representative density dot plots from flow cytometry experiments indicate the ability of LMMS to increase the number of stem cells in general (Sca-1 single positive, top quadrants), and MSCs specifically (both Sca-1 and Pref-1 positive, top right quadrant). Red, high cell density; blue, low cell density. Compared with control animals (A), LLMSs increase the number of stem cells in the bone marrow of LMMS animals (B). The actual increase in total bone marrow–derived stem cell number (C) and MSC number (D) was calculated as percent positive cells/total cells for the cell fraction showing highest intensity staining.
LMMS biases marrow environment and lineage commitment toward osteogenesis
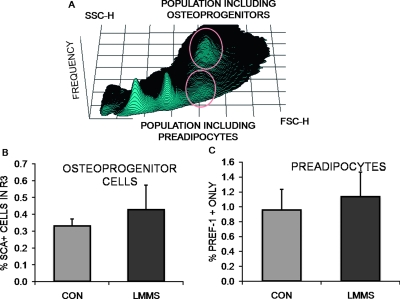
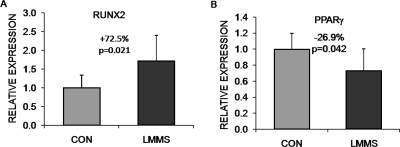
After 6 wk, cells expressing only the Pref-1 label, considered committed pre-adipocytes, were elevated by 18.5% (p = 0.25) in LMMS-treated animals relative to CON (Fig. 2). Osteoprogenitor cells in the bone marrow population, identified as Sca-1 positive with high FSC and SSC, were 29.9% (p = 0.23) greater in the LMMS group. This cell population can synthesize alkaline phosphatase, collagen, and osteocalcin and form a mineralized matrix in culture.(34) This trend indicating that differentiation in the marrow space of LMMS animals had shifted toward osteogenesis was confirmed by gene expression data, which showed that transcription of Runx2 in total bone marrow isolated from LMMS animals was upregulated 72.5% (p = 0.021) relative to CON. In these same LMMS animals, expression of PPARγ was downregulated by 26.9% (p = 0.042) relative to CON (Fig. 3).
FIG. 2.
LMMS influence on stem cells was focused on the distinct cell populations identified in flow cytometry (A), with stem cells being identified as low forward (FSC) and side (SSC) scatter. Osteoprogenitor cells were identified as Sca-1+ cells, residing in the region highlighted as high FSC and SSC, and were 29.9% (p = 0.23) more abundant in the bone marrow of LMMS-treated animals (B). The pre-adipocyte population, identified as Pref-1+, Sca-1−, showed a trend (+18.5%; p = 0.25) toward an increase in LMMS relative to CON animals (C).
FIG. 3.
Relative to CON, LMMS biases the bone marrow environment toward osteogenesis and away from adipogenesis. Real-time RT-PCR analysis of bone marrow samples harvested from animals subject to 6-wk LMMS treatment or sham control indicated a significant upregulation of the osteogenic gene Runx2 (A) and downregulation of the adipogenic gene PPARγ (B). Data are shown as expression levels relative to values for sham handled CON animals (represented as 1.0).
LMMS enhancement of bone quantity and quality
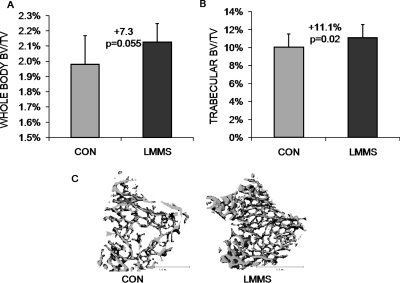
The ability of LMMS-induced changes in proliferation and differentiation of MSCs to elicit phenotypic changes in the skeleton was first measured at 12 wk by in vivo μCT scanning of the whole mouse (neck to distal tibia). Animals subject to LMMS showed a 7.3% (p = 0.055) increase in bone volume fraction of the axial and appendicular skeleton (BV/TV) over sham CON. After death, μCT scans of the isolated proximal tibia of the LMMS animals showed 11.1% (p = 0.024) greater bone volume fraction than CON (Fig. 4). The microarchitectural properties were also enhanced in LMMS compared with CON, as evidenced by a 23.7% greater connectivity density (p = 0.037), a 10.4% higher trabecular number (p = 0.022), a 11.1% smaller separation of trabeculae (p = 0.017), and a 4.9% lower structural model index (SMI, p = 0.021; Table 1).
FIG. 4.
Bone volume fraction, as measured in vivo by low-resolution μCT, indicated that LMMS increased bone volume fraction across the entire torso of the animal (A). After death, high-resolution CT of the proximal tibia indicated a significant increase in trabecular bone density (B). Body mass of the animal at death was used as a covariate in the statistical analysis. Compared with controls (C), representative μCT reconstructions of the proximal tibia indicate the enhanced morphological properties of LMMS animals.
Table 1.
Microarchitectural Parameters of Trabecular Bone in High-Fat Diet Animals Measured At 14 wk (Mean ± SD, N = 10) Show Enhanced Structural Quality of Bone in the Proximal Tibia of LMMS-Treated Animals Compared With Controls
| CON | LMMS | % diff | p | |
| Conn.D (1/mm3) | 105.3 ± 34.2 | 130.3 ± 28.9 | 23.7 | 0.037 |
| Tb.N (1/mm) | 3.06 ± 0.45 | 3.38 ± 0.37 | 10.4 | 0.022 |
| Tb.Th (mm) | 0.029 ± 0.001 | 0.030 ± 0.001 | 1.0 | 0.398 |
| Tb.Sp (mm) | 0.304 ± 0.046 | 0.270 ± 0.035 | −11.1 | 0.017 |
| SMI | 2.93 ± 0.22 | 2.78 ± 0.14 | −4.9 | 0.021 |
Prevention of obesity by LMMS
At 12 wk, neither body mass gains nor the average weekly food intake differed significantly between the LMMS or CON groups (Table 2). At this point, (19 wk of age), CON weighed 32.9 ± 4.2 g, whereas LMMS mice were 6.8% lighter at 30.7 ± 2.1 g (p = 0.15). CON were 15.0% heavier than mice of the same strain, sex, and age that were fed a regular chow diet,(24) and the increase in body mass caused by the high-fat diet was comparable to previously reported values.(35) Adipose volume from the abdominal region (defined as the area encompassed by lumbar vertebrae L1–L5) was segregated as either subcutaneous or visceral adipose tissue (SAT or VAT, respectively). LMMS animals had 28.5% (p = 0.021) less VAT by volume and 19.0% (p = 0.016) less SAT by volume. The epididymal fat pad weight was 24.5% (p = 0.032) less in LMMS than CON, and the subcutaneous fat pad from the lower back region weighed 26.1% (p = 0.018) less in LMMS animals (Table 2).
Table 2.
Despite Similar Body Mass and Weekly Food Consumption, Phenotypic Parameters of the High-Fat Diet Animals After 12 wk of LMMS or at Death (14 wk, Mean ± SD, N = 10) Show a Leaner Body Habitus, Because the Adipose Burden (Visceral and Subcutaneous Fat) Is Significantly Lower in the LMMS Animals
| CON | LMMS | % diff | p | |
| Animal weight at 12 wk (g) | 32.9 ± 4.12 | 30.7 ± 2.74 | −6.8 | 0.152 |
| Weekly food consumption (g) | 18.9 ± 1.57 | 18.5 ± 1.47 | −2.5 | 0.406 |
| VAT (cm3) | 2.3 ± 0.72 | 1.6 ± 0.34 | −28.5 | 0.021 |
| SAT (cm3) | 0.84 ± 0.16 | 0.68 ± 0.08 | −19.0 | 0.016 |
| Epididymal fat pad (g) | 1.85 ± 0.52 | 1.40 ± 0.32 | −24.5 | 0.032 |
| Subcutaneous fat pad (g) | 0.67 ± 0.17 | 0.50 ± 0.12 | −26.1 | 0.018 |
| Liver (g) | 0.99 ± 0.16 | 0.94 ± 0.07 | −4.9 | 0.399 |
LMMS prevents increased biochemical indices of obesity
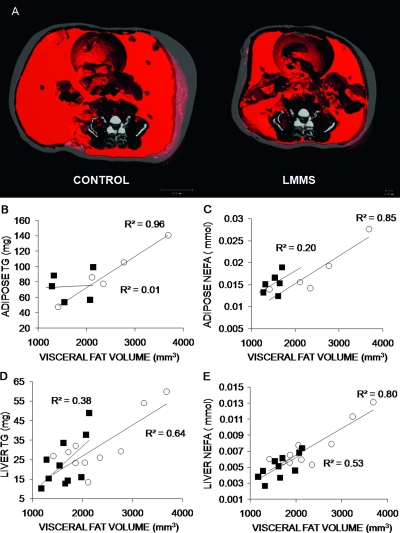
TG and NEFA levels measured in plasma, epididymal adipose tissue, and liver were all lower in LMMS compared with CON (Table 3). Liver TG levels decreased by 25.6% (p = 0.19) in LMMS animals, paralleled by a 33.0% (p = 0.022) decrease in NEFA levels. Linear regressions of adipose and liver TG and NEFA values to μCT visceral volume (VAT) showed strong positive correlations for CON animals, with R 2 = 0.96 (p = 0.002) for adipose TG, R 2 = 0.85 (p = 0.027) for adipose NEFA, R 2 = 0.64 (p = 0.006) for liver TG, and R 2 = 0.80 (p = 0.003) for liver NEFA (Fig. 5). LMMS resulted in weaker correlations between all TG and NEFA levels to increases in VAT.
Table 3.
Biochemical Parameters of the High-Fat Diet Animals (Mean ± SD, N = 10) Highlight Lower Level of TG, NEFA, and Circulating Adipokines After 14 wk of LMMS Stimulation Compared With Controls
| CON | LMMS | % diff | p | |
| TG liver (total (mg) | 31.8 ± 14.3 | 23.6 ± 12.7 | −25.6 | 0.195 |
| NEFA liver (total mol) | 7.5 ± 2.7 | 5.0 ± 1.5 | −33.0 | 0.022 |
| TG adipose (total mg) | 91.6 ± 34.6 (n = 5) | 72.9 ± 18.1 (n = 6) | −20.4 | 0.321 |
| NEFA adipose (total mmol) | 18.1 ± 5.8 (n = 5) | 15.3 ± 2.4 (n = 6) | −15.8 | 0.345 |
| TG serum (mg/dl) | 46.2 ± 17.0 | 47.0 ± 18.4 | 1.6 | 0.928 |
| NEFA serum (mM) | 0.68 ± 0.10 | 0.64 ± 0.14 | −5.3 | 0.526 |
| Leptin serum (ng/ml) | 15.9 ± 7.2 | 10.1 ± 4.7 | −37.6 | 0.049 |
| Resistin serum (ng/ml) | 4.3 ± 1.2 | 3.6 ± 1.0 | −15.8 | 0.200 |
| Adiponetin serum (μg/ml) | 9.2 ± 1.7 | 7.0 ± 1.4 | −23.5 | <0.01 |
| Osteopontin serum (ng/ml) | 197.8 ± 22.8 | 183.0 ± 39.6 | −7.5 | 0.409 |
| Osteocalcin serum (ng/ml) | 55.7 ± 17.2 | 47.6 ± 7.8 | −14.6 | 0.218 |
FIG. 5.
Representative in vivo μCT images used to discriminate visceral and subcutaneous adiposity in the abdominal region of a CON and LMMS animal. Visceral fat is shown in red; subcutaneous fat in gray (A). Linear regressions of calculated VAT volume against adipose and liver biochemistry values showed strong positive correlations in CON, and weak correlations in LMMS groups, as well as generally lower levels for all LMMS biochemical values (n = 6 for adipose, n = 10 for liver). Regressions for adipose TG (p = 0.002, B), adipose NEFA (p = 0.03, C), liver TG (p = 0.006, D), and liver NEFA (p = 0.003, E) were significant for CON animals, but only liver NEFA (p = 0.02) was significant for LMMS. Overall, LMMS mice exhibited lower, nonsignificant correlations in liver TG (p = 0.06), adipose TG (p = 0.19), and adipose NEFA (p = 0.37) to increases in visceral adiposity. ○, CON; ■, LMMS.
At death, fasting serum levels of adipokines were lower in LMMS compared with CON. Circulating levels of leptin were decreased by 35.3% (p = 0.05), adiponectin by 21.8% (p = 0.009), and resistin by 15.8% (p = 0.20) compared with CON (Table 3). Circulating serum osteopontin (−7.5%, p = 0.41) and osteocalcin (−14.6%, p = 0.22) levels were not significantly affected by the mechanical signals.
LMMS fails to reduce existing adiposity
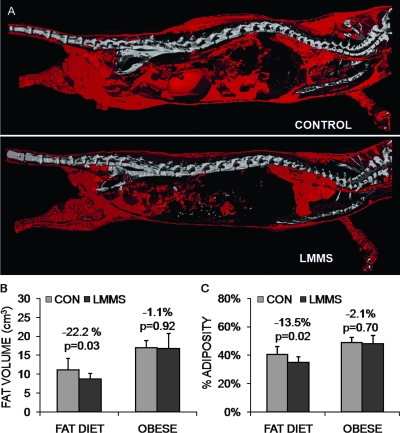
In the reversal model of obesity, 4-wk-old animals were started on a high-fat diet for 3 wk before beginning the LMMS protocol at 7 wk of age. These “obese” animals were on average 3.7 g heavier (p < 0.001) than the age-matched “prevention” animals at the start of the protocol. The early-adolescent obesity in these obese animals manifested into adulthood, such that by the end of the 12-wk protocol, they weighed 21% more than the CON animals who begun the high-fat diet at 7 wk of age (p < 0.001). In stark contrast to the prevention animals, where LMMS realized a 22.2% (p = 0.03) lower overall adipose volume relative to CON (neck to distal tibia), no differences were seen for fat (−1.1%, p = 0.92) or bone volume (−0.2%, p = 0.94) between LMMS and CON groups after 12 wk of LMMS for these already obese mice (Fig. 6).
FIG. 6.
Suppression of the obese phenotype was achieved to a degree by stem cells preferentially diverting from an adipogenic lineage. Reconstructed in vivo μCT images of total body fat (red; A) indicate that, after 12 wk, animals that began LMMS at the time that the high-fat diet was introduced exhibited 22.2% less fat volume compared with controls. In contrast, animals allowed a high-fat diet for 4 wk before LMMS failed to show any reduction of fat volume (B). Shown as a relative percentage of fat to total animal volume, LMMS reduced the percent animal adiposity by 13.5% (p = 0.017), whereas the lack of a response in the already obese animals reinforces a conclusion that the mechanical signal works primarily at the stem cell development level, because existing fat is not metabolized by LMMS stimulation.
LMMS promotes bone and muscle and suppresses visceral fat in humans
To determine whether the ability of LMMS to suppress adiposity and increase osteogenesis in mice can translate to humans, young women with low BMD were subject to daily exposure to LMMS for 12 mo. The study cohort ranged from 15 to 20 yr old and was originally designed to evaluate if LMMS could enhance the musculoskeletal system. Detailed descriptions of this study population are provided elsewhere,(32) and the results reported here represent a retrospective evaluation of the data to assess visceral adiposity.
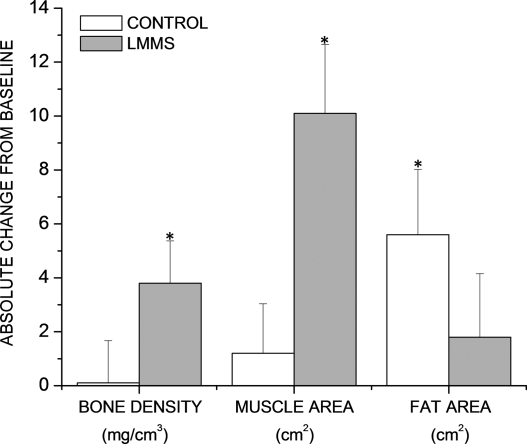
At the end the of the 1-yr study, women (n = 24) in the CON group had no significant change in cancellous BMD of the spine (0.1 ± 1.5 [SE] mg/cm3; p = 0.93) compared with a 3.8 ± 1.6 mg/cm3 increase in the spine of the LMMS-treated cohort (p = 0.025; Fig. 7). Furthermore, at the level of the umbilicus, the average area of paraspinous muscle failed to change in CON (1.2 ± 1.9 cm2; p = 0.43) but was sharply elevated in the LMMS women (10.1 ± 2.5 cm2; p < 0.001). Visceral fat area measured at the lumbosacral region of CON subjects increased significantly from baseline (5.6 ± 2.4 cm2, p = 0.03). In contrast, visceral fat area in LMMS subjects saw a small, nonsignificant increase from baseline (1.8 ± 2.3 cm2, p = 0.45).
FIG. 7.
As measured by CT scans in the lumbar region of the spine, a group of young osteopenic women subject to LMMS for 12 mo (n = 24; gray bars ± SE) increased both BMD (p = 0.025 relative to baseline; mg/cm3) and muscle area (p < 0.001; cm2), changes that were paralleled by a nonsignificant increase in visceral fat formation (p = 0.45; cm2). Conversely, women in the CON group (n = 24; white bars ± SE), while failing to increase either BMD (p = 0.93) or muscle area (p = 0.43), realized a significant increase in visceral fat formation (p = 0.03). *Changes that are significantly different from baseline.
DISCUSSION
The interaction between bone and fat formation is influenced by a multitude of factors including genetic, metabolic, and physical inputs to coordinate an appropriate adaptive response. The experiments reported here focus on the mechanical contribution to this environment and indicate that extremely LMMS, well below those generated during locomotion, can promote the number of stem cells residing in the marrow. Furthermore, these subtle mechanical signals biased the differentiation of the MSC population toward osteoblastogenesis over adipogenesis, such that obesity was prevented while bone formation was promoted. Translating the ability of LMMS to bias bone formation over fat formation to a group of young osteopenic women, LMMS promoted bone and muscle mass and concurrently suppressed the accumulation of visceral fat in the treated group, whereas controls failed to gain either bone or muscle over the course of the year and showed a significant increase in visceral fat.
These data provide support for the growing body of evidence of an inversely coupled relationship between pre-osteoblasts and pre-adipocytes in the marrow cavity and osteogenesis and adipogenesis overall.(36) It has been reported that overweight individuals tend to have higher BMD than normal weight individuals and are less prone to osteoporosis(37) because the load-bearing challenges inherent to obesity should have a beneficial impact on the skeleton. Conflicting evidence, however, indicates this increase in bone quantity is not proportional to the increase in weight. In a review of risk factors for fractures in normally active children and adolescents, it was seen that obesity increased the incidence of fracture from 15.5% in normal weight children to 33.3% in obese children.(38,39)
Sca-1 was used to give a general indication of the status of the bone marrow–derived stem cell population, representing both HSCs and MSCs, because the marker does not distinguish between the two cell types. The relative increase in the overall bone marrow stem cell population induced by LMMS reflected an enhancement of stem cells of both hematopoietic and mesenchymal lineages. The method used for bone marrow harvesting (flushing of bones) does not remove the significant portion (33%) of HSCs that resides in proximity to the endosteal surface of the bone,(40) and thus the influence of LMMS on HSCs is likely underestimated in this model. MSCs have been reported as expressing a series of surface markers, including both Sca-1 and Pref-1.(3) The use of both markers should be more specific in identifying MSCs, and although accordingly these cells occur less frequently, this population showed a larger increase in response to LMMS than that measured in the overall stem cell population. In all, these data indicate that the stem cell pool has been positively influenced by mechanical signals, resulting in an increase in total number of cells.
At the molecular level, LMMS induced a clear shift in the biological balance of the key osteogenic and adipogenic factors, with a marked increase in the expression level of Runx2 and a significant decrease in the level of PPARγ. Together, this change in balance may conspire to bias stem cells in the undifferentiated state preferentially toward the formation of bone and away from fat.(36) Reduced levels of PPARγ are permissive to osteoblastogenesis and can lead to higher trabecular bone volume(41) by promoting the osteoblastic lineage decision of MSCs.(42)
Ultimately, it will be important to perform in vitro assays to more comprehensively validate the commitment of stem cells to a given lineage, despite the inherent limitations of using culture systems to define stem cell development.(43) The plasticity of MSCs and their ability to transdifferentiate make attempts at in vitro functional characterization difficult,(44) and subtle shifts in differentiation potential such as we have observed in these studies may be difficult to detect. Of course, even given the many advantages of a culture system, an inherent limitation is that the “system” level interactions of stem cells, bone marrow, blood flow, mechanical signals, the hormonal milieu, etc., cannot serve as an agent of change. Although not statistically significant, the increased percentage of cells in the osteoprogenitor and pre-adipocyte populations in bone marrow from the LMMS animals showed in vivo trends that support a conclusion that lineage selection of cells has been altered by the mechanical signal. There is evidence suggesting that pre-adipocytes, through expression of the plasma membrane protein Pref-1, are responsive to differentiation signals from the extracellular environment,(45) with Pref-1 expression being inhibitory of adipogenesis and terminal adipocyte differentiation.(46) The ultimate fate of marrow pre-adipocytes and their ability to migrate to other adipose tissue depots has not been definitively addressed and ultimately may highlight the inherent difficulty in harnessing stem cell plasticity as a therapeutic endpoint.(47,48)
Whereas the impact of the LMMS signal was evaluated in bone marrow–derived stem cells, the systemically delivered stimulus is certain to influence other cell populations as well. As such, the phenotypic changes measured in fat and bone are likely not the exclusive result of regulating the bone marrow stem cell population, and more realistically result from influencing many interacting cell populations, including bone and fat cells. What is apparent, however, is that LMMS increases the size of the precursor pool in the bone marrow and biases them away from adipogenesis and toward higher-order connective tissues. We believe these data support a conclusion that the mechanical biasing of MSC lineage selection toward osteoblastogenesis inherently suppresses adipogenesis because the stem cell ultimately can only make a “single pathway” commitment. Indeed, even though adipose tissue mass was >20% greater in the controls relative to the LMMS animals, animal weights differed by <7%, because the reduced fat mass of the LMMS animals was, to a degree, compensated by the increase in bone mass, and further emphasized the binary nature of the differentiation process.
Whereas these data indicate a role of development in the etiology of obesity, they do not preclude a critical contribution of the overall metabolic state of the organism, because increased adiposity alters the systemic physiology by changing the endocrine and metabolic state of the fat tissue,(49) as well as susceptibility to diseases. The amount of VAT is an important risk factor to metabolic complications that afflict the obese, with adiposity positively correlating with fasting plasma insulin, triglyceride, low-density lipoprotein, and apolipoprotein B levels, as well as the cholesterol/high-density lipoprotein ratio.(50) Increased abdominal adiposity is also known to be a significant risk factor for type 2 diabetes.(51) Control animals presented with these same positive correlations of TG and NEFA levels in adipose tissue and the liver to visceral adipose accumulation, whereas adipose gains in LMMS-treated animals did not translate into proportional increases, suggesting that suppression of adipogenesis and/or adiposity could ultimately inhibit associated sequelae. While also of significance, specimen limitations prevented a full characterization of marrow adiposity, specifically, for DIO and/or LMMS-treated animals. Ultimately, whereas histology-based determinations of adipose infiltration of the marrow space may provide qualitative data in this regard, various imaging techniques under development, such as high-field μMRI, may provide a more accurate quantification of marrow adiposity in a small animal model such as the one used here.(52)
Serum levels of osteopontin were measured to assay systemic changes in bone tissue, because osteopontin is secreted by osteoblasts and acts to activate osteoclasts in the normal process of bone remodeling. Additionally, osteopontin has been reported as a potent constraining factor on HSC proliferation.(53) That osteopontin is unaffected by LMMS treatment highlights the general promotion of stem cell proliferation (HSC and MSC), but the specificity of the proposed mechanism to MSC differentiation as biasing the formation of osteoprogenitors did not examine if osteoclasts, which derive from HSCs, were either elevated or activated. Circulating levels of the key adipokines leptin, adiponectin, and resistin are known to be elevated under conditions of DIO, because these molecules are secreted by white adipose tissue.(35,54) These molecules each exhibit pleiotropic effects, with implications for inflammatory and immune responses.(55) In light of the similar food intake between groups, the markedly reduced levels of circulating adipokines in LMMS animals may best reflect the reduced adipose burden in these mice.
In evaluating the ability of LMMS to prevent obesity, these data indicate that these mechanical signals were effective at the molecular, cellular, and tissue level, as indicated by a distinct bias toward osteogenesis after 6 wk, translating at 12 and 14 wk into clear phenotypic differences in bone and fat volume. In contrast, mice allowed to become obese (4 wk of a high-fat diet) before being subject to LMMS indicated not only the inability of these mechanical signals to reverse obesity in animals that were already fat, it also failed to influence their bone mass, despite receiving the same mechanical signal as the prevention group. This could either mean that the stem cell population in the “pre-obese” mice was already committed toward adipogenesis and away from bone by the time the mechanical signal was introduced or that a saturated, adipogenic environment catalyzed by a high-fat diet persists and supersedes the ability of other mechanical signals to drive MSCs toward specific lineages. Certainly, the inability to reduce the volume of adipose tissue in an already obese animal emphasizes the starkly different challenges of a developmental strategy to prevent obesity versus the metabolic realities of reversing it.
A similar scenario may well be evident in preventing versus reversing age-related bone loss with mechanical signals, because the deterioration of the marrow-based stem cell population that parallels aging may undermine the ability of any intervention to harness the potential of stem cells to treat disease.(56) Aging animals show a significant reduction in their stem cell population and their regenerative capacity,(57) while simultaneously predisposing this environment toward adipogenesis in the remaining MSCs.(58) Extending this “aging”-related deterioration to disuse, inactivity, and microgravity markedly reduce osteoblastogenesis in the MSC pool,(59) while the actual number of osteoprogenitor cells is also severely compromised.(60) Thus, both age and activity are determinants of the viability of the stem cell population, and independently or together may conspire toward a reduced regenerative capacity. This deterioration can be somewhat mitigated by replenishment of the bone marrow stem cell population, either directly or through exposure to a “young” environment, showing promise as a intervention to restore musculoskeletal health.(61,62) Considering this in the context of the data presented here in osteopenic women, where LMMS increased bone and muscle mass while concurrently suppressing visceral adiposity, suggests that susceptibility to diseases such as obesity and osteoporosis may be more closely linked than previously thought, caused, potentially, by a failure to drive stem cells toward the “right” fate and provide some early support that early prevention for both diseases is ultimately easier than treating either.
It has been estimated that 80% of obese adolescents develop into obese adults,(63) contributing to the conclusion by the American Heart Association that primary prevention is the key to constraining the societal impact,(64) because treatments once an individual is obese are limited. The differential response to LMMS in the two animal models presented herein further highlights this disparity, in that prevention of obesity through developmental control was achievable but that reversal of an obese state, once cell fate had been predetermined, was not realized. Rather than a metabolic pathway, these data indicate a developmentally mediated mechanism by which the suppression of fat and the enhancement of bone are coupled, as linked to mechanical influences on stem cell populations. Indeed, the mechanically mediated increase in the number of progenitor cells, taken together with the ability of these mechanical signals to drive commitment choices, indicates a viable means to enhance an organism's regenerative capacity and reduce susceptibility to disease, achieved by exploiting stem cell sensitivity to physical signals. Similar to bone, recent findings definitively showed that adipocytes do indeed turn over in humans, with ∼10% of fat cells renewed annually in adults, by a balance of adipocyte death and generation.(65) Based on the ability of LMMS to slow and deter the development of stem cells into fat, perhaps even adult obesity could be slowly repressed through capitalizing on the normal process of adipocyte death, by encouraging stem cells to orient toward osteoblastogenesis over adipogenesis.
ACKNOWLEDGMENTS
The authors thank S Lublinsky, E Ozcivici, B Adler, and J Pangilinan for help with animal imaging and tissue processing. Assistance from the laboratory of Dr M Hadjiargyrou and the Stony Brook University Medical Center Flow Cytometry Facility is gratefully acknowledged. This work was supported by National Institutes of Health Grants AR 43498, AR 45433, and DK33823; National Aeronautics and Space Administration Grant NAG 9-1499; The Goldman Foundation; and a W. H. Coulter Translational Research Award.
Footnotes
Drs Luu, Pessin, Judex, and Rubin have submitted a series of provisional patents to the U.S. Patent and Trademark Office regarding the method and application of the technology. Dr Rubin is a founder of and consultant for Juvent Medical and is an inventor of the technology investigated herein, and both he and the company may benefit from the results of this research. No other authors state that they have conflicts of interest.
REFERENCES
- 1.Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–45977. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 2.David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 3.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: Implications for tissue engineering. Adv Drug Deliv Rev. 2007;59:1309–1318. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment-Implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassem M. Stem cells: Potential therapy for age-related diseases. Ann NY Acad Sci. 2006;1067:436–442. doi: 10.1196/annals.1354.062. [DOI] [PubMed] [Google Scholar]
- 8.Hachisuka H, Mochizuki Y, Yasunaga Y, Natsu K, Sharman P, Shinomiya R, Ochi M. Flow cytometric discrimination of mesenchymal progenitor cells from bone marrow-adherent cell populations using CD34/44/45(-) and Sca-1(+) markers. J Orthop Sci. 2007;12:161–169. doi: 10.1007/s00776-006-1098-6. [DOI] [PubMed] [Google Scholar]
- 9.Wong SH, Lowes KN, Bertoncello I, Quigley AF, Simmons PJ, Cook MJ, Kornberg AJ, Kapsa RM. Evaluation of Sca-1 and c-Kit as selective markers for muscle remodelling by nonhemopoietic bone marrow cells. Stem Cells. 2007;25:1364–1374. doi: 10.1634/stemcells.2006-0194. [DOI] [PubMed] [Google Scholar]
- 10.Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–852. doi: 10.1359/JBMR.040118. [DOI] [PubMed] [Google Scholar]
- 11.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 12.Song HY, Jeon ES, Kim JI, Jung JS, Kim JH. Oncostatin M promotes osteogenesis and suppresses adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem. 2007;101:1238–1251. doi: 10.1002/jcb.21245. [DOI] [PubMed] [Google Scholar]
- 13.De CP, Milan G, Scarda A, Boldrin L, Centobene C, Piccoli M, Pozzobon M, Pilon C, Pagano C, Gamba P, Vettor R. Rosiglitazone modifies the adipogenic potential of human muscle satellite cells. Diabetologia. 2006;49:1962–1973. doi: 10.1007/s00125-006-0304-6. [DOI] [PubMed] [Google Scholar]
- 14.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crossno JT, Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song G, Ju Y, Shen X, Luo Q, Shi Y, Qin J. Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces. 2007;58:1507–1514. doi: 10.1016/j.colsurfb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Duty AO, Oest ME, Guldberg RE. Cyclic mechanical compression increases mineralization of cell-seeded polymer scaffolds in vivo. J Biomech Eng. 2007;129:531–539. doi: 10.1115/1.2746375. [DOI] [PubMed] [Google Scholar]
- 18.Emans PJ, Pieper J, Hulsbosch MM, Koenders M, Kreijveld E, Surtel DA, van Blitterswijk CA, Bulstra SK, Kuijer R, Riesle J. Differential cell viability of chondrocytes and progenitor cells in tissue-engineered constructs following implantation into osteochondral defects. Tissue Eng. 2006;12:1699–1709. doi: 10.1089/ten.2006.12.1699. [DOI] [PubMed] [Google Scholar]
- 19.LeBaron RG, Athanasiou KA. Ex vivo synthesis of articular cartilage. Biomaterials. 2000;21:2575–2587. doi: 10.1016/s0142-9612(00)00125-3. [DOI] [PubMed] [Google Scholar]
- 20.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32:407–417. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 21.Dazzi F, Horwood NJ. Potential of mesenchymal stem cell therapy. Curr Opin Oncol. 2007;19:650–655. doi: 10.1097/CCO.0b013e3282f0e116. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118:491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritton JC, Rubin CT, Qin YX, McLeod KJ. Whole-body vibration in the skeleton: Development of a resonance-based testing device. Ann Biomed Eng. 1997;25:831–839. doi: 10.1007/BF02684167. [DOI] [PubMed] [Google Scholar]
- 26.Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol. 1984;107:321–327. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- 27.Judex S, Zhong N, Squire ME, Ye K, Donahue LR, Hadjiargyrou M, Rubin CT. Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J Cell Biochem. 2005;94:982–994. doi: 10.1002/jcb.20363. [DOI] [PubMed] [Google Scholar]
- 28.Gilsbach R, Kouta M, Bonisch H, Bruss M. Comparison of in vitro and in vivo reference genes for internal standardization of real-time PCR data. Biotechniques. 2006;40:173–177. doi: 10.2144/000112052. [DOI] [PubMed] [Google Scholar]
- 29.Luu YK, Lublinsky S, Ozcivici E, Capilla E, Pessin JE, Rubin CT, Judex S. In vivo quantification of subcutaneous and visceral adiposity by micro-computed tomography in a small animal model. Med Eng Phys. 2008 doi: 10.1016/j.medengphy.2008.03.006. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lublinsky S, Luu YK, Rubin CT, Judex S. Automated separation of visceral and subcutaneous adiposity in In vivo microcomputed tomographies of mice. J Digit Imaging. 2008 doi: 10.1007/s10278-008-9152-x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 33.RJ Seely, L Munyakazi, TF Curry, H Simmerman, WH Rushing, J Haury. Demonstrating the consistency of small data sets: Application of the weisberg t-test for outliers. Biopharm Int. 2003;16:36–42. [Google Scholar]
- 34.Van VP, Falla N, Snoeck H, Mathieu E. Characterization and purification of osteogenic cells from murine bone marrow by two-color cell sorting using anti-Sca-1 monoclonal antibody and wheat germ agglutinin. Blood. 1994;84:753–763. [PubMed] [Google Scholar]
- 35.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 36.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 37.Hoiberg M, Nielsen TL, Wraae K, Abrahamsen B, Hagen C, Andersen M, Brixen K. Population-based reference values for bone mineral density in young men. Osteoporos Int. 2007;18:1507–1514. doi: 10.1007/s00198-007-0399-8. [DOI] [PubMed] [Google Scholar]
- 38.Goulding A. Risk factors for fractures in normally active children and adolescents. Med Sport Sci. 2007;51:102–120. doi: 10.1159/000103007. [DOI] [PubMed] [Google Scholar]
- 39.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20:2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 40.Haylock DN, Williams B, Johnston HM, Liu MC, Rutherford KE, Whitty GA, Simmons PJ, Bertoncello I, Nilsson SK. Hemopoietic stem cells with higher hemopoietic potential reside at the bone marrow endosteum. Stem Cells. 2007;25:1062–1069. doi: 10.1634/stemcells.2006-0528. [DOI] [PubMed] [Google Scholar]
- 41.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takada I, Suzawa M, Matsumoto K, Kato S. Suppression of PPAR transactivation switches cell fate of bone marrow stem cells from adipocytes into osteoblasts. Ann N Y Acad Sci. 2007;1116:182–195. doi: 10.1196/annals.1402.034. [DOI] [PubMed] [Google Scholar]
- 43.Joseph NM, Morrison SJ. Toward an understanding of the physiological function of Mammalian stem cells. Dev Cell. 2005;9:173–183. doi: 10.1016/j.devcel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 45.Sul HS, Smas C, Mei B, Zhou L. Function of pref-1 as an inhibitor of adipocyte differentiation. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S15–S19. doi: 10.1038/sj.ijo.0801494. [DOI] [PubMed] [Google Scholar]
- 46.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 47.Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scadden DT. The weight of cell identity. J Clin Invest. 2007;117:3653–3655. doi: 10.1172/JCI34181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Despres JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6:51–59. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- 51.Haffner SM. Abdominal adiposity and cardiometabolic risk: Do we have all the answers. Am J Med. 2007;120:S10–S16. doi: 10.1016/j.amjmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Wehrli FW, Song HK, Saha PK, Wright AC. Quantitative MRI for the assessment of bone structure and function. NMR Biomed. 2006;19:731–764. doi: 10.1002/nbm.1066. [DOI] [PubMed] [Google Scholar]
- 53.Haylock DN, Nilsson SK. Osteopontin: A bridge between bone and blood. Br J Haematol. 2006;134:467–474. doi: 10.1111/j.1365-2141.2006.06218.x. [DOI] [PubMed] [Google Scholar]
- 54.Gregoire FM. Adipocyte differentiation: From fibroblast to endocrine cell. Exp Biol Med (Maywood) 2001;226:997–1002. doi: 10.1177/153537020122601106. [DOI] [PubMed] [Google Scholar]
- 55.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 56.Duque G. Will reducing adopogenesis in bone increase bone mass?: PPARgamma2 as a key target in the treatment of age-related bone loss. Drug News Perspect. 2003;16:341–346. doi: 10.1358/dnp.2003.16.6.829305. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 58.Astudillo P, Rios S, Pastenes L, Pino AM, Rodriguez JP. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J Cell Biochem. 2007;103:1054–1065. doi: 10.1002/jcb.21516. [DOI] [PubMed] [Google Scholar]
- 59.Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 60.Basso N, Bellows CG, Heersche JN. Effect of simulated weightlessness on osteoprogenitor cell number and proliferation in young and adult rats. Bone. 2005;36:173–183. doi: 10.1016/j.bone.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 61.Takada K, Inaba M, Ichioka N, Ueda Y, Taira M, Baba S, Mizokami T, Wang X, Hisha H, Iida H, Ikehara S. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells. 2006;24:399–405. doi: 10.1634/stemcells.2005-0068. [DOI] [PubMed] [Google Scholar]
- 62.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 63.Schonfeld-Warden N, Warden CH. Pediatric obesity. An overview of etiology and treatment. Pediatr Clin North Am. 1997;44:339–361. doi: 10.1016/s0031-3955(05)70480-6. [DOI] [PubMed] [Google Scholar]
- 64.Eckel RH, Krauss RM. American Heart Association call to action: Obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation. 1998;97:2099–2100. doi: 10.1161/01.cir.97.21.2099. [DOI] [PubMed] [Google Scholar]
- 65.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]