Abstract
Spontaneous coronary artery dissection (SCAD) is an infrequent event that is most commonly associated with pregnant women or those in the postpartum period. Because of its rarity, the literature describing this condition is confined to sporadic case reports, with few reporting long-term follow-up, and no clear consensus exists on the optimal treatment strategy for these patients. The present article reports a single-centre experience with SCAD, highlighting the issues surrounding its management with a brief description of five cases of pregnancy-associated coronary dissection. The treatment used in these cases ranged from a conservative medical approach to surgical and percutaneous intervention, with one patient proceeding to transplantation. Four of the cases have long-term angiographic follow-up.
In addition, a comprehensive review of all previously published cases is presented, and temporal trends in the management strategy are highlighted. Possible pathophysiological mechanisms pertaining to this condition, and the complex diagnostic and therapeutic issues involved, which may affect both patient and fetus, are discussed. Finally, an optimal approach to patients with SCAD, informed by our experience and literature review, is described.
Keywords: Myocardial infarction, Peripartum, Pregnancy, Spontaneous coronary dissection
Spontaneous coronary artery dissection (SCAD) is an infrequent event, most commonly associated with pregnant women or those in the postpartum period (1). A United States population-based study (2) on all-cause acute myocardial infarction (AMI) in pregnancy estimated an incidence of one AMI in every 16,129 pregnancies, with a mortality rate reported at 5.1%. This compares with a mortality rate of 7% for the general population (3). Furthermore, a recent review of all published case reports on pregnancy-related AMI suggested that coronary artery dissection may account for 27%, somewhat higher than the 16% observed in an earlier review by the same authors (4,5). Atherosclerosis, therefore, remains the dominant pathology in this cohort.
Perhaps because of its rarity, the literature on pregnancy-related SCAD is confined to sporadic case reports, with little long-term follow-up data available. This lack of data has made conclusions regarding its etiology and treatment difficult to determine, and no guidelines have been established regarding the optimal management strategy for such patients. With the current trend of postponing pregnancy (6), and the advancement of technology allowing older women to conceive, the impact of SCAD (and indeed, atherosclerotic coronary artery disease associated with the more usual risk factors) can be expected to increase in this important population.
We present five interesting cases of pregnancy-related SCAD presenting to our service between 2002 and 2008, and in addition, we review all recently published cases of pregnancy-related SCAD. Our cases incorporate a range of treatment modalities, all with a successful outcome. All four invasively managed patients have angiographic follow-up. The present review will highlight the management challenges these patients represent in contemporary practice.
CASE PRESENTATIONS
Case 1 (Table 1)
TABLE 1.
Spontaneous coronary artery dissection related to pregnancy and the postpartum period
| Case number (reference) | Year | Age, years | Gravida/para | Risk factors | Event time | Presentation | Vessel involved | Treatment strategy | Status of patient, status of fetus (if pregnant) |
|---|---|---|---|---|---|---|---|---|---|
| Case series | |||||||||
| 1 | 2008 | 34 | 2/2 | None | 7 days PP | Anterior STEMI | LAD | PCI (BMS), repeat PCI (DES) | Alive |
| 2 | 2007 | 31 | 2/2 | None | 8 days PP | NSTEMI | RCA | PCI (BMS) | Alive |
| 3 | 2005 | 30 | 2/1 | †Cholesterol | 5 days PP | Anterior STEMI, shock | LAD | PCI, transplant | Alive |
| 4 | 2004 | 26 | 1/1 | None | 3 months PP | Anterior STEMI | LAD | Medical | Alive |
| 5 (7) | 2002 | 33 | 2/1 | None | 24-week gest | NSTEMI | LMS, LAD, Cx, RCA later | CABG | Alive, fetal loss |
| Published case reports | |||||||||
| 6 (38) | 1999 | 31 | Smoker | 4 days PP | Anterior STEMI | LAD | PCI (BMS) | Alive | |
| 7 (39) | 1999 | 29 | 1/1 | None | 23-week gest | Inferior STEMI | RCA, LAD | PCI (BMS) | Alive, cesarean section, status unknown |
| 8 (21) | 2008 | 34 | Hypertension, smoker, NIDDM | 34-week gest | Anterior STEMI | LAD, Cx | CABG on CPB | Alive, healthy baby | |
| 9 (40) | 2008 | 33 | None | 1st trimester | Inferior STEMI | RCA, LAD | Not discussed | Alive, therapeutic abortion | |
| 10 (30) | 2006 | 23 | Smoker | 2 months postabortion (14-week gest) | Anterior STEMI, shock | LMS, LAD and Cx. RCA at 18 months | PCI (BMS) | Alive | |
| 11 (41) | 2001 | 36 | None | 36-week gest | Inferior STEMI, shock | RCA | PCI (BMS) | Alive, healthy baby | |
| 12 (37) | 2007 | 36 | None | 36-week gest | Anterior STEMI, shock | LAD, Cx | CABG off pump | Alive, healthy baby | |
| 13 (31) | 2005 | 37 | 3/2 | None | 2 months PP | Anterior STEMI | LAD | Medical | Alive |
| 14 (42) | 2007 | 29 | None | 1 day PP | NSTEMI, shock | LMS | CABG on CPB | Alive | |
| 15 (43) | 2006 | 31 | 2/2 | None | 3 days PP | Anterior STEMI | LMS, LAD | PCI (DES) | Alive |
| 16 (44) | 2006 | 38 | 2/1 | †Cholesterol, family history | 34-week gest | Anterior STEMI | LAD | Medical | Alive, cesarean section, twins, healthy |
| 17 (22) | 2005 | 22 | 1/1 | Smoker | 10 days PP | NSTEMI | LAD | Medical | Alive |
| 18 (45) | 2004 | 31 | 5/2 | None | 8 days PP | Anterior STEMI | LAD | Medical | Alive |
| 19 (46) | 2002 | 36 | 3/3 | Family history, hypertension | 4 weeks PP | Anterior STEMI | LAD | Medical | Alive |
| 20 (1) | 2002 | 34 | Smoker | 5 days PP | Anterior STEMI | LAD | PCI (BMS) | Alive | |
| 21 (29) | 2001 | 37 | 2/1 | Smoker | 4 days PP | Anterior STEMI | LAD, Cx | PCI, then CABG on CPB | Alive |
| 22 (47) | 2006 | 38 | 2/2 | None | 2 weeks PP | NSTEMI | LAD | CABG on CPB | Alive |
| 23 (48) | 2006 | 23 | 2/2 | None | 8 days PP | Anterior STEMI | LMS | CABG on CPB | Alive |
| 24 (49) | 2005 | 36 | 4/1 | None | 34-week gest | Inferior STEMI | RCA | PCI (DES) | Alive, healthy baby |
| 25 (50) | 2007 | 34 | 4/3 | None | 32-week gest | Inferior STEMI | RCA | PCI (BMS) | Alive, healthy baby |
BMS Bare metal stents; CABG Coronary artery bypass graft; CPB Cardiopulmonary bypass; Cx Circumflex; DES Drug-eluting stents; gest Gestation; LAD Left anterior descending; LMS Left main stem; NIDDM Non-insulin-dependent diabetes mellitis; NSTEMI Non-ST elevation myocardial infarction; PCI Percutaneous coronary intervention; PP Postpartum; RCA Right coronary artery; STEMI ST elevation myocardial infarction;
A 34-year-old woman, gravida (G) 2 para (P) 2, experienced searing chest pain seven days following a normal vaginal delivery. An electrocardiogram (ECG) revealed anterior ST elevation, and her troponin I concentration was 0.97 μg/L. She received acetylsalicylic acid, clopidogrel and heparin, and was transferred to the Peter Munk Cardiac Centre (Toronto, Ontario) for coronary angiography.
She had no known cardiovascular risk factors. On examination, she appeared healthy and was hemodynamically stable. She was complaining of ongoing pain, although less severe. Angiography revealed a normal left main stem (LMS), dominant right coronary artery (RCA) and circumflex (Cx). The left anterior descending (LAD) artery was hazy and narrowed distally, although no clear dissection flap could be identified (Figure 1A). Left ventricular (LV) angiography demonstrated apical hypokinesis with grade 2 (40% to 59%) ventricular function. To reverse any possible spasm and optimize distal blood flow, she received large boluses of intracoronary adenosine and diltiazem, but no difference was seen on repeat angiography. Percutaneous coronary intervention (PCI) was performed with two overlapping bare metal stents (BMS) in the distal LAD. The distal vessel beyond, a wrap-around LAD, appeared very small with diffuse irregularity but there was thrombolysis in myocardial infarction (TIMI) 3 flow (Figure 1B). She was discharged home on acetylsalicylic acid, clopidogrel and an angiotensin-converting enzyme (ACE) inhibitor.
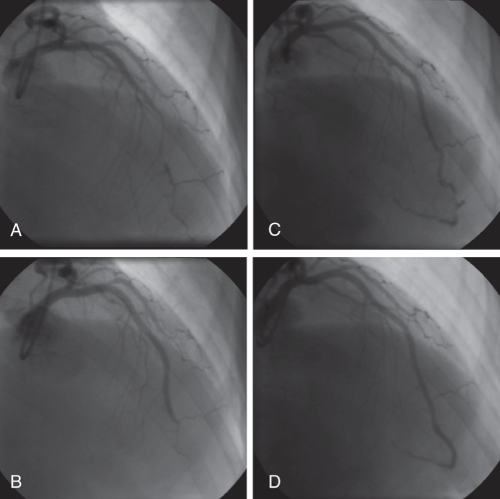
Figure 1).
Case 1. Angiography (right anterior oblique view) of the left anterior descending artery. A Initial angiogram demonstrating mid to distal vessel narrowing, no dissection plane identified. B Following percutaneous coronary intervention with bare metal stents, there is an excellent mid-vessel appearance with residual narrowing of the distal vessel. Thrombolysis in myocardial infarction 3 flow was demonstrated distally. C Second admission with chest pain, extension of dissection clearly evident in distal left anterior descending artery. The patient underwent further stenting with drug-eluting stents. D Repeat angiography demonstrated patent left anterior descending stents, with minor distal disease (unstented)
Three weeks later, she was readmitted with further chest pain. Troponin tests were negative and an ECG demonstrated deep T-wave inversion (TWI) anteriorly. An angiography revealed widely patent stents, but a long segment of dissection extending distally to the apex (Figure 1C). The remaining vessels were normal and repeat LV angiography showed some improvement in LV function; however, the inferoapical segment remained akinetic. She received two drug-eluting stents (DES) distal to the original BMS (Figure 1D).
She continued to have atypical chest pain. Repeat angiography demonstrated widely patent stents with diffuse disease beyond the apical portion of the LAD (Figure 1D). She has remained stable since being discharged on medical therapy, including acetylsalicylic acid, clopidogrel, ramipril, bisoprolol and amlodipine.
Case 2 (Table 1)
A 31-year-old woman, G2 P2, returned to hospital eight days after a normal vaginal delivery complaining of a 45 min episode of crushing chest pain radiating to the jaw. Her condition was associated with TWI inferiorly, an elevated troponin I concentration of 0.27 μg/L, and an elevated creatine kinase (CK) concentration of 211 U/L. An echocardiogram demonstrated inferior wall hypokinesis. She received acetylsalicylic acid and enoxaparin, and was transferred to our cardiac unit for angiography.
She had no known risk factors for atherosclerosis. Following the delivery of her first child, she had pulmonary edema and was diagnosed with peripartum dilated cardiomyopathy. She was treated with ACE inhibitors and her LV function improved to baseline after 12 months. On this admission, there was no evidence of failure and she was hemodynamically stable. Angiography revealed a normal left coronary system and a large spiral dissection extending the entire length of the RCA (Figure 2A). Inferior akinesia was confirmed. In view of the minimal troponin elevation, it was believed appropriate to attempt to revascularize the artery percutaneously. She received 600 mg of clopidogrel and, after securing a wire distally, integrellin was commenced. Initial balloon dilation resulted in poor distal flow, and multiple stents were required throughout the length of the vessel to restore flow. Five overlapping BMS were placed, and TIMI 3 flow was obtained with an excellent angiographic result (Figure 2B). She was discharged home on acetylsalicylic acid and clopidogrel, with only minimal inferior hypokinesia and a grade 1 (greater than 60% ejection fraction) ventricular function.
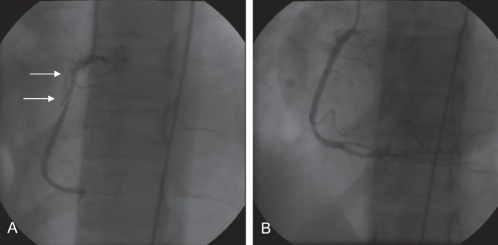
Figure 2).
Case 2. Angiography (left anterior oblique view) of the right coronary artery. A Initial angiogram showing the dissection plane in the right coronary artery (arrows). B Appearance after stenting with bare metal stents
Given the extensive nature of her procedure, a routine angiogram was scheduled at three months. This revealed minor focal in-stent restenosis in the posterior descending artery and no further intervention was required. At seven months post-procedure, a perfusion scan revealed no evidence of ischemia, and she remained entirely pain-free.
Case 3 (Table 1)
A 30-year-old woman, G2 P1, presented to the Peter Munk Cardiac Centre with a 1 h history of severe central chest pain five days after having a normal vaginal delivery. An ECG showed an anterolateral ST elevation MI (STEMI) and she received half-dose fibrinolysis before urgent transfer for angiography. Her CK concentration was 10,637 U/L and her troponin concentration was greater than 100 μg/L. On arrival, she was hypotensive with signs of pulmonary edema and had a ventricular fibrillation arrest from which she was successfully resuscitated, neurologically intact.
She had no known risk factors but was diagnosed with hypercholesterolemia on admission. An intra-aortic balloon pump was inserted. Angiography revealed a normal LMS, Cx and RCA, with a proximally dissected LAD, and grade 3 (20% to 39%) LV function with extensive apical and anterolateral akinesis. She proceeded to have PCI with three overlapping Cypher stents (Cordis Corporation, USA), which resulted in TIMI 2 flow.
Subsequently, she remained relatively hypotensive with New York Heart Association (NYHA) class II or III symptoms and had limited tolerance to medical management with beta-blockers and ACE inhibitors. Her ejection fraction remained less than 20%. An automatic implantable cardioverter defibrillator was implanted six months postprocedure, and at 18 months the patient received an appropriate discharge for monomorphic ventricular tachycardia associated with syncope. Repeat angiography demonstrated patent stents but poor distal run-off. Because of a continued low cardiac output state, she was assessed by a transplant service and, at 29 months after the initial presentation, following an emergency admission requiring inotropic support, the patient underwent successful transplantation. To date, she remains well with NYHA class I or II symptoms.
Case 4 (Table 1)
A 26-year-old woman, G1 P1, presented to another institution three months following a cesarean section. Three days before admission, she experienced nausea and vomiting associated with chest pain. An ECG demonstrated anterior ST elevation and cardiac enzymes were elevated (troponin I 23 μg/L), but she was treated conservatively. An echocardiogram demonstrated mild LV systolic dysfunction with apical akinesia.
She had no cardiac risk factors, and was hemodynamically stable and pain-free on transfer to our institution. Her ECG showed anterior Q waves. Angiography revealed a normal LMS, Cx and RCA with evidence of a long spiral dissection in the LAD and apical akinesia. Given that she was now remote from the ischemic episode, a conservative approach was adopted. She was discharged home on acetylsalicylic acid, metoprolol and captopril. A dipyridamole perfusion scan showed evidence of a large anterior infarct but no ischemia, and a reasonably preserved LV function despite the infarct size.
She remains under annual review, and is well with Canadian Cardiovascular Society class I and NYHA class I symptoms. A recent echocardiography suggests a normalized LV function, and repeat nuclear perfusion scans continue to show preserved LV function with no evidence of ischemia.
Case 5 (Table 1)
This case has been described elsewhere (7). Briefly, a 33-year-old woman, G2 P1, presented at 24 weeks gestation with chest pain radiating to her left arm. On the basis of elevated cardiac enzymes (troponin I 3.9 μg/L and CK 215 U/L) and anterior TWI, she was diagnosed with a non-STEMI. Angiography revealed a dissected LMS (Figure 3A), and she was transferred to the Peter Munk Cardiac Centre for further management. She was commenced on clopidogrel and integrellin, in addition to acetylsalicylic acid and heparin. Repeat angiography, however, revealed extension of the dissection to the LAD and Cx systems (Figure 3B), and she went emergently for coronary artery bypass graft (CABG) surgery, receiving four saphenous vein grafts to the LAD, diagonal, Cx and obtuse marginal branches. This procedure was performed under cardiopulmonary bypass (CPB). The procedure resulted in fetal demise.
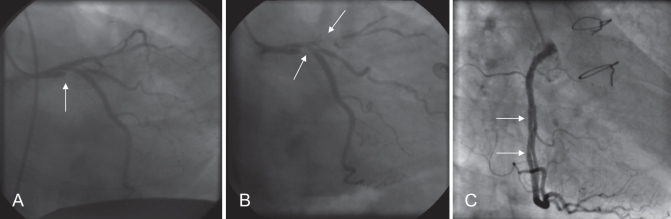
Figure 3).
Case 5. Angiography of the left main stem. A Initial angiogram (right anterior oblique caudal view) showing left main stem dissection (arrow). B The following day the dissection extended into the left anterior descending and circumflex arteries (arrows). C Angiogram five years after coronary artery bypass graft surgery. Dissection plane with false lumen seen in the right coronary artery (arrows) (left anterior oblique cranial view). The timing of this event is unknown
She re-presented recently with atypical left-sided chest pain, and angiography demonstrated patency of all four vein grafts. Interestingly, however, she appeared to have chronic dissection of the RCA (Figure 3C). No intervention was required. She remains well on current medical therapy (acetylsalicylic acid, atorvastatin and metoprolol).
DISCUSSION
SCAD associated with pregnancy and the postpartum period is infrequent but presents a unique challenge to the physician, occurring invariably in young, otherwise healthy women with few, if any, traditional risk factors for coronary artery disease, and often involving consideration of fetal and patient well-being. Its clinical severity depends on the rate and extent of dissection as well as the territory involved, and its presentation ranges across the spectrum of acute coronary syndromes, as in the cases reported here. Its occurrence poses significant risks for the mother and fetus. While short-term mortality is high (38% compared with 5.1% for all-cause AMI), survival through the initial presentation predicts an excellent long-term outcome (8).
A review of pregnancy-associated SCAD case reports, from 1952 to 1999, totalling 58 cases, by Koul et al (8) has been previously published. Pregnancy-associated SCAD is described as being more frequent in older (greater than 30 years of age) and multiparous pregnant women in the postpartum period, generally occurring in the LAD, with up to one-quarter of cases involving the LMS and one-third demonstrating an involvement of more than one vessel. We have undertaken a comprehensive review of the literature since 1999. PubMed was searched using the terms ‘acute myocardial infarction’, ‘pregnancy’ and ‘coronary dissection’, and then a manual search of the bibliographies of all selected reports was performed. An additional 25 pregnancy-associated SCAD cases, including those described here, were identified (Table 1).
Perhaps the most striking difference between our series and those of Koul et al (8) is the survival rate (100%). This difference likely reflects contemporary guidelines regarding the management of chest pain, advances in medical and interventional strategies for AMI, a higher index of suspicion by admitting physicians and a lower threshold for referral for urgent cardiac catheterization.
In the 1999 to 2008 cohort, SCAD occurred more commonly in the postpartum period (60%). The average age of the women at presentation was 32.6 years, the average parity when reported was 1.6, and presentation occurred between the first trimester and three months postpartum. The LAD was involved in 80% of cases, with seven cases of multivessel dissection (28%). LMS disease was evident in 20% of cases.
Etiology
The incidence of SCAD in the general population is between 0.28% (9) and 1.1% (10), an estimation derived from studies of consecutive patients with myocardial infarction (MI) undergoing coronary angiography. The incidence is in marked comparison with that in the pregnant population, where dissection accounts for 27% of all MI cases (4) and, coupled with a demonstration of multivessel involvement in one-quarter of cases, suggests a pathological role for pregnancy. The principle abnormality common to both populations is a plane of dissection within the outer one-third of the media, together with intramural hematoma, which causes compression of the true lumen and threatens distal blood flow. An intimal tear is not consistently identified, and some authors believe that the two mechanisms can exist independently (11).
It is established that pregnancy and the postpartum state are associated with several hormonal and hemodynamic changes (Table 2), changes that can persist for up to six months post-partum. No single unifying theory has been proposed that accounts for the association with SCAD, although it is suggested that morphological changes in the arterial wall coupled with hemodynamic stress may contribute. Although SCAD in the general population has been described in relation to excessive exercise (12), and pregnancy is associated with increased stroke volume (13), hemodynamic shear forces alone are unlikely to account for pregnancy-related dissection because the majority of cases occur in the postpartum period. It is postulated that weakening of the vessel wall occurs due to decreased collagen synthesis (14), which might also account for the preponderance of idiopathic dissection reported in patients with Marfan’s and Ehlers-Danlos syndromes. Sometimes eosinophilic infiltrates, which may further damage collagen, possibly as a result of the lytic action of proteases released, are observed. Borczuk et al (15) found that 13 of 24 pregnant patients (54%) diagnosed with dissection had an elevated eosinophil to lymphocyte ratio. Pregnancy is also associated with an excess of progesterone, which results in the loss of the normal corrugation of elastic fibres, increases fragmentation of reticular fibres and decreases the amount of acid mucopolysaccharides, further reducing wall strength (4). These changes are normalized within three months of delivery (8). Others have argued that fluctuations in estrogen may be attributable (16). Additionally, pregnancy induces alterations in the coagulation and fibrinolytic systems, which include decreased releasable tissue plasminogen activator, increased fast-acting tissue plasminogen activator inhibitor, altered levels of coagulation factors and reduced functional protein S levels (4), all of which may predispose to thrombus formation.
TABLE 2.
Pathophysiological changes in pregnancy and the postpartum period hypothesized to have an etiological role in spontaneous coronary artery dissection (SCAD)
| Change | Description | Reference |
|---|---|---|
| Increase in cardiac output | Due to increased blood volume (50%) and heart rate (10%). Cardiac output increases by 30% to 50% (higher during labour) and may contribute to increased arterial shear forces, predisposing to dissection | 4,51,52 |
| Excess progesterone | Excess levels of progesterone lead to the loss of normal corrugation of elastic fibres, a decrease in acid mucopolysaccharide ground substance and smooth muscle cell hyperplasia, hypothesized to weaken the tunica media, causing cystic medial necrosis. Multivessel involvement supports the hypothesis of generalized arterial wall changes under hormonal influence | 53,54 |
| Excess estrogen | Estrogen promotes release of matrix metalloproteinases resulting in increased medial breakdown and medial necrosis. Paradoxically, it is also believed to a have vasculoprotective effects | 16,55 |
| Relaxin | A pregnancy hormone that is believed to protect the vasculature from vasoconstriction by inhibiting the stimulation of endothelin-1, a potent vasoconstrictor. It also has potent chronotropic and inotropic effects mediated by interactions with specific relaxin receptors. The increase in cardiac chronotropy and inotropy could lead to increased risk for coronary dissection | 56,57 |
| Eosinophilia | Pathological reviews of pregnant patients demonstrate a periadventitial eosinophilic infiltrate in many. It is hypothesised that lytic action from proteases released by the infiltrate weakens the medial wall; contentious, because many suggest that this is reactive rather than causative | 15 |
| Elevated lipoprotein (a) and decreased plasma factor | Described in a single case, the authors concluded that a hemostatic imbalance caused by a lack of prostacyclin synthesis stimulating plasma factor and elevated lipoprotein (a) would lead to thromboembolic complications | 58 |
| Decreased collagen production | Impaired collagen synthesis due to changes in hormonal equilibrium in the postpartum state. Mechanism proposed to be similar to inborn defects such as Marfan’s syndrome | 14 |
| Disruption of vaso vasorum | Hypothesized mechanism for all types of SCAD in which an intimal tear is not identified, but in pregnant patients lack of atherosclerosis is believed to prevent pathological ‘stenting’ of the vessel, allowing extension throughout the vascular bed | 59,60 |
| Antiphospholipid antibodies | Single case, patient positive for anticardiolipin antibodies. Authors point to an association between antiphospholipid syndrome and carotid artery dissection | 45 |
| Alterations in the coagulation-fibrinolysis system | Decreased releasable tissue plasminogen activator, increased fast-acting tissue plasminogen activator inhibitor, change in the levels of coagulation factors and reduction in functional protein S levels, all leading to a pregnancy-related prothrombotic state | 3 |
Clinical presentation and angiographic diagnosis
Presentation of SCAD is variable and can range from asymptomatic pathology to MI and sudden cardiac death. In the series described here, 80% presented as a STEMI, with 20% being diagnosed with non-STEMI/acute coronary syndrome. This contrasts with the earlier case reports (1958 to 1999) in which 60% of patients had an AMI (this may, however, have been an underestimate of the true rate given that 36% of patients were diagnosed postmortem). Five patients presented in cardiogenic shock (20%), again highlighting the significant risks posed by coronary dissection.
Emergency catheterization in these patients serves to establish the diagnosis and to facilitate the choice of therapy. In pregnancy, care must be taken to minimize the risk of fetal exposure to radiation, necessitating judicious use of cine and fluoroscopy time (17), as well as measures such as abdominal lead draping. The average PCI procedure exposes the fetus to less than 1 rad of radiation, and termination of pregnancy is not generally considered for exposures below 10 rads (17). Radiation exposure can affect fetal organogenesis in the first trimester, and is postulated to lead to central nervous system damage and behavioural difficulties in the later stages of pregnancy (18). Given the radiation dose of a multislice computed tomography (0.7 rads to 2.1 rads), this imaging modality does not play a useful role in this population (19).
The typical appearance of dissection, along with an obvious false lumen and delayed clearance of contrast material, confirms the diagnosis. It is not always possible, however, to identify an intimal tear angiographically, and in this situation luminal narrowing may be the only feature, as in case 1 (Figure 1A), such that clinical suspicion must remain high in low-risk patients without features of atherosclerosis. Intravascular ultrasound (IVUS) may be required to further define the coronary anatomy (20).
Management strategies
The optimal management strategy for pregnancy-related SCAD takes into account the hemodynamic status of the patient, and the territory involved and the extent of the myocardium at risk, in addition to the well-being of the fetus. The importance of a multidisciplinary approach, particularly in prepartum patients, cannot be underestimated (21), and there are clear implications regarding both intervention and medication in this vulnerable group. With regard to pregnant patients, arrangements may need to be made for urgent delivery of a potentially viable fetus.
Medical therapy
Between 1999 and 2008, six of 25 patients (24%) were treated medically compared with 13 of 36 surviving patients (47%) between 1952 and 1999 (8). This difference reflects major changes in the treatment of AMI during the intervening period. However, even in contemporary practice, it might still be appropriate to treat the patients medically, particularly those who are stable, remote from the ischemic event and have demonstrated limited dissection on angiography. In postpartum patients, antiplatelet therapy with acetylsalicylic acid and clopidogrel (to reduce thrombus formation), together with ACE inhibitors (in the presence of LV dysfunction) and beta-blockers (aimed at reducing shear stress), is desirable. Caution is advised for lactating mothers in whom clopidogrel is not recommended. In pregnant patients, drug therapy is more complicated: the risk of clopidogrel has not been studied (Food and Drug Administration risk category B – no risk in humans is known) and ACE inhibitors are contraindicated (category C), although low-dose acetylsalicylic acid in the second and third trimesters, and selective beta-blockers are considered safe. A detailed discussion regarding drug safety in pregnant and lactating women has been previously published (4).
Spontaneous resolution of dissection has been reported in five of 10 medically treated cases of pregnancy-related SCAD in whom angiographic follow-up was available (mean 90 days, range 13 to 293 days) (22). It is pertinent to note that in many cases, including three described here, there is extension of dissection, which may have severe hemodynamic effects, while in other cases the dissection fails to resolve but causes no further ischemia. Thus, a very low threshold for performing repeat angiography is crucial in patients treated medically, and indeed, we would advocate an attempt at stabilization with coronary stenting in all but the lowest risk cases.
Thrombolysis
In Koul et al’s (8) review of SCAD cases reported between 1952 and 1999, six patients (10.3%) were given thrombolytic drugs compared with none being administered in cases published between 1999 and 2008. Thrombolytics are not believed to pose a direct risk to the fetus because placental transfer of the drug is too low (23–25) and teratogenicity has not been observed in animal models. The use of thrombolytics in this population, however, remains controversial, and there is anecdotal evidence to suggest that it may lead to a worse outcome, possibly by extension of hemorrhage into the dissection plane (26). Therefore, in the context of pregnancy-related SCAD, thrombolysis is best avoided, and pregnancy remains a relative contraindication to thrombolysis under any circumstance (27). When transfer to a centre with interventional capability is feasible, this must be the preferred option (28).
PCI
Between 1952 and 1999, the management of reported pregnancy-related SCAD cases was predominantly medical, with only three patients (5%) being treated by PCI (two patients received stents and one patient underwent balloon angioplasty only) (8). In this series (1999 to 2008), 13 patients (52%) underwent PCI, all with coronary stents. In one case (2001), stenting failed to maintain vessel patency and the patient proceeded emergently to surgery (29). With the advent of primary PCI, and more aggressive intervention in patients presenting with acute coronary syndromes, it is likely that more women with pregnancy-related SCAD will be referred urgently for angiography and consideration of percutaneous intervention.
PCI is the treatment of choice in patients with ongoing signs of ischemia and single-vessel disease, or in those in whom a large, viable myocardial territory is at risk, such as proximal LAD disease. It can also be considered in multivessel dissection with hemodynamic compromise, but we would advocate discussion with cardiothoracic surgical colleagues before pursuing this strategy. Heparin does not cross the placenta and can be used safely. The safety of glycoprotein IIb/IIIa inhibitor is unknown and indications for their use is based on isolated case reports only (risk category B: eptifibatide, tirofiban; risk category C: abciximab).
Stenting will effectively scaffold the vessel wall and might theoretically protect against further dissection. Paradoxically, in the non-pregnancy-related SCAD literature, there is a report of stent implantation causing propagation of the dissection/intramural hematoma such that more extensive stenting was required (9). An additional potential concern is the possibility of wiring a false lumen, although steps can be taken to avoid this such as the use of IVUS to guide wire placement. To date, all published reports of PCI in this context have had a successful outcome (except for one patient who was sent emergently for CABG [29]), and in those cases where follow-up angiography is available, stents remained patent ([22,30,31] cases 1 to 3 above). A study by James et al (2) reported PCI in 135 pregnant patients with all-cause AMI (stenting in 127 [15%]). No information was available, however, regarding outcome.
It is not clear whether the use of DES conveys any benefit above BMS in this population. Stenting a relatively young, otherwise healthy patient raises the possibility of in-stent restenosis, a risk that would be offset by the use of DES. While randomized trials have consistently shown the superiority of DES over BMS in reducing late repeat revascularization, the safety and effectiveness of DES have not been established in pregnant or lactating women and, in light of the absence of significant atherosclerosis in this group, restenosis might be a remote eventuality. As discussed earlier, clopidogrel is not recommended for lactating mothers and its safety in pregnancy is unknown, although its safe use has been reported in small numbers. In pregnant patients, the prospect of a cesarean section delivery might direct bare metal stenting to avoid long-term clopidogrel use.
CABG surgery
Surgery has been used successfully in SCAD involving the LMS, multivessel dissections, or in cases of medical or PCI failure. Postpartum women are generally good surgical candidates and their risk profile is expected to correspond with that of the age-matched general population undergoing emergency CABG surgery.
Clearly, pregnancy represents a challenge to both the surgeon and anesthetist. The maternal mortality rate of CPB during pregnancy varies in the literature from 1% to 5%, with an average of 2.5%, and appears not to differ from nonpregnant women with similar disease states (32). Surgical revascularization was reported in 51 pregnant women (6%) with all-cause AMI but no outcome data were described (2). The largest registry of CPB during pregnancy reported 133 cases with four maternal deaths (3%) and 25 fetal deaths (19%), but only four of these cases involved CABG, and in this group there were no maternal or fetal deaths (33). A more recent case series (32) of 74 pregnant women, including one patient operated on for coronary artery disease, quoted a maternal mortality rate of 8.6% – higher than average due to poor preoperative conditions – and a fetal mortality rate of 18.6%. The lowest risk for both mother and fetus occurred during the second trimester, with a trend toward fetal malformations in the first trimester, and preterm delivery, maternal hemodynamic changes and fetal mortality in the third trimester (32).
CPB induces changes in the coagulation profile and complement systems, predisposes to the release of vasoactive substances and emboli, and is a risk factor for hypothermia and hypotension, all of which may reduce placental blood flow and increase uterine contractility (34). The decision to put a pregnant patient on CPB should be made, if at all possible, in conjunction with an experienced obstetric team who may be required to perform emergency cesarean section on a viable fetus (17). It is preferable for the patient to be placed in a left lateral recumbent position, although this may be determined by the territory requiring grafting. Hypothermia must be avoided, and bypass time kept to a minimum. Equally important is fetal monitoring during and after surgery (34). Performance of coronary bypass without CPB (‘off-pump’ surgery) might be considered optimal because it is associated with reduced patient blood loss and theoretically poses less fetal risk. However, off-pump surgery is also associated with decreased graft patency at three months and one year, and should ideally be coupled with the use of arterial conduits. Additionally, it offers a technical compromise, particularly if mobilization of the heart is required, and is not well suited to RCA or Cx grafting (35). Minimal access surgery is similarly limited. The published pregnancy-related SCAD cases treated by surgical revascularization have been generally performed on CPB, with successful off-pump surgery described in just two cases (36,37).
CONCLUSION
Pregnancy-related SCAD is increasingly being recognized as an important cause of acute coronary syndromes in women with few or no conventional risk factors for atherosclerosis and coronary artery disease. A unifying pathophysiological process to explain its occurrence has not been identified. Clinically, it is associated with significant morbidity and mortality, and a high level of suspicion should be maintained to ensure timely and appropriate investigation and management. Urgent angiography is crucial to establish the diagnosis, but in cases where a dissection is not evident and the patient does not have obvious atherosclerotic disease, IVUS may prove useful.
No guidelines have been established regarding the management of SCAD, and given the small number of cases and the sporadic nature of the literature on this topic, an evidence-based approach is lacking and likely to remain so. Thrombolysis is best avoided in patients in whom dissection is suspected, and direct transfer to an interventional centre is recommended. For stable patients in whom the dissection is limited, and/or only a small area of myocardium is at risk, medical therapy is appropriate, and in many cases, resolution of the dissection can be anticipated. Equally, many cases demonstrate extension of the dissection within the same vessel, or the occurrence of multivessel involvement. Therefore, it is essential to maintain a low threshold for performing repeat angiography in these patients. For single-vessel dissections with ongoing ischemia, or in those in whom a large area of myocardium is at risk, regardless of status, we recommend PCI as the preferred strategy. CABG may be considered in multivessel dissection or LMS involvement. Although the majority of SCAD occurs in postpartum women, in pregnant patients the presence of a viable fetus mandates a multidisciplinary approach, requiring the cooperation of interventionists, cardiothoracic surgeons, anesthetists, obstetricians and neonatologists.
Footnotes
CONFLICT OF INTEREST: No conflict of interest exists for any of the authors.
REFERENCES
- 1.Kamineni R, Sadhu A, Alpert JS. Spontaneous coronary artery dissection: Report of two cases and a 50-year review of the literature. Cardiol Rev. 2002;10:279–84. doi: 10.1097/00045415-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 2.James AH, Jamison MG, Biswas MS, et al. Acute myocardial infarction in pregnancy: A United States population-based study. Circulation. 2006;113:1564–71. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 3.Steg PG, Goldberg JR, Gore JM, et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002;90:358–63. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 4.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52:171–80. doi: 10.1016/j.jacc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. Ann Intern Med. 1996;125:751–62. doi: 10.7326/0003-4819-125-9-199611010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Leridon H, Slama R. The impact of a decline in fecundity and of pregnancy postponement on final number of children and demand for assisted reproduction technology. Hum Reprod. 2008;23:1312–9. doi: 10.1093/humrep/den106. [DOI] [PubMed] [Google Scholar]
- 7.Shah P, Cusimano RJ, Sermer M, et al. Spontaneous dissection of left main coronary artery. Can J Cardiol. 224(20):815–8. [PubMed] [Google Scholar]
- 8.Koul AK, Hollander G, Moskovits N, Frankel R, Herrera L, Shani J. Coronary artery dissection during pregnancy and the postpartum period: Two case reports and review of literature. Catheter Cardiovasc Interv. 2001;52:88–94. doi: 10.1002/1522-726x(200101)52:1<88::aid-ccd1022>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa H, Nakanishi S, Nishiyama S, et al. Primary coronary artery dissection: Its incidence, mode of the onset and prognostic evaluation. J Cardiol Suppl. 1988:307–17. [PubMed] [Google Scholar]
- 10.Hering D, Piper C, Hohmann C, Schultheiss HP, Horstkotte D. [Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection.] Zeitschrift für Kardiologie. 1998;87:961–70. doi: 10.1007/s003920050253. [DOI] [PubMed] [Google Scholar]
- 11.Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol. 2002;89:466–8. doi: 10.1016/s0002-9149(01)02272-x. [DOI] [PubMed] [Google Scholar]
- 12.Choi JW, Davidson CJ. Spontaneous multivessel coronary artery dissection in a long-distance runner successfully treated with oral antiplatelet therapy: A case report and review of the literature. J Invasive Cardiol. 2002;14:675–8. [PubMed] [Google Scholar]
- 13.Elkayam U, Gleicher N. Hemodynamics and cardiac function during normal pregnancy and the puerperium. In: Elkayam U, Gleicher N, editors. Cardiac Problems in Pregnancy. 3rd edn. New York: Wiley-Liss; 1998. pp. 3–20. [Google Scholar]
- 14.Bonnet J, Aumailley M, Thomas D, et al. Spontaneous coronary artery dissection: Case report and evidence for a defect in collagen metabolism. Eur Heart J. 1986;7:904–9. doi: 10.1093/oxfordjournals.eurheartj.a061979. [DOI] [PubMed] [Google Scholar]
- 15.Borczuk AC, van Hoeven KH, Factor SM. Review and hypothesis: The eosinophil and peripartum heart disease (myocarditis and coronary artery dissection) – coincidence or pathogenetic significance? Cardiovasc Res. 1997;33:527–32. doi: 10.1016/s0008-6363(96)00257-x. [DOI] [PubMed] [Google Scholar]
- 16.Heefner WA. Dissecting hematoma of coronary artery. JAMA. 1973;223:550–1. [PubMed] [Google Scholar]
- 17.Colletti PM, Lee K. Cardiovascular imaging in the pregnant patient. In: Elkayam U, Gleicher N, editors. Cardiac Problems in Pregnancy. 3rd edn. New York: Wiley-Liss; 1998. pp. 33–6. [Google Scholar]
- 18.Weber MD, Halligan RE, Schumacher JA. Acute infarction, intracoronary thrombolysis, and primary PTCA in pregnancy. Cathet Cardiovasc Diagn. 1997;42:38–43. doi: 10.1002/(sici)1097-0304(199709)42:1<38::aid-ccd12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Morin RL, Gerber TC, McCollough CH. Radiation dose in computed tomography of the heart. Circulation. 2003;107:917–22. doi: 10.1161/01.cir.0000048965.56529.c2. [DOI] [PubMed] [Google Scholar]
- 20.Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol. 2002;89:466–8. doi: 10.1016/s0002-9149(01)02272-x. [DOI] [PubMed] [Google Scholar]
- 21.Sherif HMF, Nguyen HC, Sarter BH, et al. Spontaneous coronary dissection in late pregnancy: A multidisciplinary approach to management. Ann Thorac Surg. 2008;85:1793–4. doi: 10.1016/j.athoracsur.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Maeder M, Ammann P, Drack G, Rickli H. Pregnancy-associated spontaneous coronary artery dissection: Impact of medical treatment. Case report and systematic review. Z Kardiol. 2005;94:829–35. doi: 10.1007/s00392-005-0302-6. [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer GW. Distribution studies on placental transfer of 131I streptokinase during labor. Ann Med. 1970;19:17–8. doi: 10.1111/imj.1970.19.s1.17. [DOI] [PubMed] [Google Scholar]
- 24.Lecander I, Nilsson M, Astedt B. Depression of plasminogen activator activity during pregnancy by the placental inhibitor PAI 2. Fibrinolysis. 1988;2:165–7. [Google Scholar]
- 25.Leonhardt G, Gaul C, Nietsch HH, Buerke M, Schleussner E. Thromobolytic therapy in pregnancy. J Thromb Thrombolysis. 2006;21:271–6. doi: 10.1007/s11239-006-5709-z. [DOI] [PubMed] [Google Scholar]
- 26.Zupan I, Noc M, Trinkaus D, Popovic M. Double vessel extension of spontaneous left main coronary artery dissection in young women treated with thrombolytics. Catheter Cardiovasc Interv. 2001;52:226–30. doi: 10.1002/1522-726x(200102)52:2<226::aid-ccd1054>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Task Force on the Management of Cardiovascular Diseases During Pregnancy of the European Society of Cardiology. Expert consensus document on management of cardiovascular diseases during pregnancy. Eur Heart J. 2003;24:761–81. doi: 10.1016/s0195-668x(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 29.Lane JE, Cartledge RG, Johnson JH. Successful surgical treatment of spontaneous coronary artery dissection. Curr Surg. 2001;58:316–8. doi: 10.1016/s0149-7944(00)00456-6. [DOI] [PubMed] [Google Scholar]
- 30.Luceri S, Paolillo V, De Benedictis M, Scrocca I. Spontaneous dissection of the left coronary tree after an interruption of pregnancy treated with extensive stenting. J Invasive Cardiol. 2006;13:117–20. [PubMed] [Google Scholar]
- 31.Terrovitis JV, Kanakakis J, Nanas JN. Spontaneous coronary artery dissection as a cause of acute myocardial infarction in the postpartum period. Cardiol Rev. 2005;13:211–3. doi: 10.1097/01.crd.0000134862.82534.b3. [DOI] [PubMed] [Google Scholar]
- 32.Arnoni RT, Arnoni AS, Bonini RCA, et al. Risk factors associated with cardiac surgery during pregnancy. Ann Thorac Surg. 2003;76:1605–8. doi: 10.1016/s0003-4975(03)01188-3. [DOI] [PubMed] [Google Scholar]
- 33.Parry AJ, Westaby S. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. 1996;61:1865–9. doi: 10.1016/0003-4975(96)00150-6. [DOI] [PubMed] [Google Scholar]
- 34.Pomini F, Mercogliano D, Cavalletti C, Caruso A, Pomini P. Cardiopulmonary bypass in pregnancy. Ann Thorac Surg. 1996;61:259–68. doi: 10.1016/0003-4975(95)00818-7. [DOI] [PubMed] [Google Scholar]
- 35.Barner HB. Operative treatment of coronary atherosclerosis. Ann Thorac Surg. 2008;23:1473–82. doi: 10.1016/j.athoracsur.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Silberman S, Fink D, Berko RS, Mendzelevski, Bitran D. Coronary artery bypass surgery during pregnancy. Eur J Cardiothorac Surg. 1996;10:925–6. doi: 10.1016/s1010-7940(96)80325-3. [DOI] [PubMed] [Google Scholar]
- 37.Aliyary S, Mariani MA, Verhorst PMJ, Hartmann M, Stoel MG, von Birgelen C. Staged therapeutic approach in spontaneous coronary dissection. Ann Thorac Surg. 2007;83:1879–81. doi: 10.1016/j.athoracsur.2006.11.085. [DOI] [PubMed] [Google Scholar]
- 38.Porras MC, Ares MA, Gill JZ. Intracoronary stenting for postpartum coronary artery dissection. Ann Intern Med. 1998;128:873. doi: 10.7326/0003-4819-128-10-199805150-00020. [DOI] [PubMed] [Google Scholar]
- 39.Togni M, Amann FW, Follath F. Spontaneous multivessel coronary artery dissection in a pregnant woman treated successfully with stent implantation. Am J Med. 1999;107:407–8. doi: 10.1016/s0002-9343(99)00177-1. [DOI] [PubMed] [Google Scholar]
- 40.Iltumur K, Karahan Z, Ozmen S, Danis R, Toprak N. Spontaneous coronary artery dissection during hemodialysis in the post-abortion period. Int J Cardiol. 2008;127:e45–7. doi: 10.1016/j.ijcard.2007.01.105. [DOI] [PubMed] [Google Scholar]
- 41.McKechnie RS, Patel D, Eitzman DT, Rajagopalan S, Murthy TH. Spontaneous coronary artery dissection in a pregnant woman. Obstet Gynecol. 2001;98:899–902. doi: 10.1016/s0029-7844(01)01563-0. [DOI] [PubMed] [Google Scholar]
- 42.Goland S, Schwarz ER, Siegel RJ, Czer LSC. Pregnancy-associated spontaneous coronary artery dissection. Am J Obstet Gynecol. 2007;197:e11–3. doi: 10.1016/j.ajog.2007.08.054. [DOI] [PubMed] [Google Scholar]
- 43.Chabrot P, Motreff P, Boyer L. Postpartum spontaneous coronary artery dissection: A case of pseudoaneurysm evolution detected on MDCT. AJR Am J Roentgenol. 2006;187:W660. doi: 10.2214/AJR.06.0783. [DOI] [PubMed] [Google Scholar]
- 44.Phillips LM, Makaryus AN, Beldner S, Spatz A, Smith-Levitin M, Marchant D. Coronary artery dissection during pregnancy treated with medical therapy. Cardiol Rev. 2006;14:155–7. doi: 10.1097/01.crd.0000173944.99498.84. [DOI] [PubMed] [Google Scholar]
- 45.Krishnamurthy M, Desai R, Patel H. Spontaneous coronary artery dissection in the postpartum period: Association with antiphospholipid antibody. Heart. 2004;90:e53. doi: 10.1136/hrt.2004.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhawan R, Singh G, Fesniak H. Spontaneous coronary artery dissection: The clinical spectrum. Angiology. 2002;53:89–93. doi: 10.1177/000331970205300112. [DOI] [PubMed] [Google Scholar]
- 47.Frey BW, Grant RJ. Pregnancy-associated coronary artery dissection: A case report. J Emerg Med. 2006;30:307–10. doi: 10.1016/j.jemermed.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Rogers IS, Rinaldi MJ, Humphrey CB, Boden WE, Dougherty JE. Postpartum dissection of the left main coronary artery. Clin Cardiol. 2006;29:175–8. doi: 10.1002/clc.4960290410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nallamothu BK, Saint M, Saint S, Mukherjee D. Double jeopardy. N Engl J Med. 2005;353:75–80. doi: 10.1056/NEJMcps050117. [DOI] [PubMed] [Google Scholar]
- 50.Schif JH, Ehehalt R, Elsaesser M, Katus HA, Meyer FJ. A pregnant woman with acute myocardial infarction due to coronary artery dissection: Pre-hospital and in-hospital management. Resuscitation. 2007;73:467–74. doi: 10.1016/j.resuscitation.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Clark SL, Cotton DB, Lee W, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439–42. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 52.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060–5. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- 53.Madu EC, Kosinski DJ, Wilson WR, Burket MW, Fraker TD, Jr, Ansel GM. Two-vessel coronary artery dissection in the peripartum period. Case report and literature review. Angiology. 1994;45:809–16. doi: 10.1177/000331979404500909. [DOI] [PubMed] [Google Scholar]
- 54.Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol. 1967:336–4. [PubMed] [Google Scholar]
- 55.Wingrove CS, Godslad IF, Stevenson JC. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. 1998;1406:169–74. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 56.Dschietzig T, Bartsch C, Richter C, et al. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist. Circ Res. 2003;92:32–40. doi: 10.1161/01.res.0000051884.27117.7e. [DOI] [PubMed] [Google Scholar]
- 57.Sherwood OD. Relaxin’s physiological roles and other diverse actions. Endocr Rev. 2004;25:205–34. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 58.Ulm MR, Obwegeser R, Ploeckinger B, Nowotny C, Pidlich J, Sinzinger H. A case of myocardial infarction complicating pregnancy – a role for prostacyclin synthesis stimulating plasma factor and lipoprotein (a)? Thromb Res. 1996;83:237–42. doi: 10.1016/0049-3848(96)00132-6. [DOI] [PubMed] [Google Scholar]
- 59.Thayer J, Healy R, Maggs P. Spontaneous coronary artery dissection. Ann Thorac Surg. 1987;44:97–102. doi: 10.1016/s0003-4975(10)62372-7. [DOI] [PubMed] [Google Scholar]
- 60.Bulkley B, Roberts W. Isolated coronary arterial dissection. J Thorac Cardiovasc Surg. 1978;67:148–51. [PubMed] [Google Scholar]