Abstract
Apolipoprotein A5 [APOA5] is expressed primarily in the liver and modulates plasma triglyceride levels in mice and humans. Mice overexpressing APOA5 exhibit reduced plasma triglyceride levels. Since there is a tight association between plasma triglyceride concentration and traits of the metabolic syndrome, we utilized transgenic mice overexpressing human APOA5 to test the concept that these mice would be protected from diet-induced obesity and insulin resistance. Male and female transgenic and wild-type mice on the FVB/N genetic background were fed standard rodent chow or a diet rich in fat and sucrose for 18 weeks during which time clinical phenotypes associated with obesity and glucose homeostasis were measured. We found that APOA5 transgenic (A5tg) mice were resistant to diet induced changes in plasma triglyceride but not total cholesterol levels. Body weights were similar between the genotypes for females and males, although male A5tg mice showed a modest but significant increase in the relative size of inguinal fat pads. Although male A5tg mice showed a significantly increased ratio of plasma insulin to glucose, profiles of glucose clearance as evaluated following injections of glucose or insulin failed to reveal any differences between genotypes. Overall, our data showed that there was no advantage to responses to diet-induced obesity with chronic reduction of plasma triglyceride levels as mediated by overexpression of APOA5.
Keywords: APOA5, mice, obesity, triglyceride, glucose tolerance
INTRODUCTION
Hypertriglyceridemia is a feature of the metabolic syndrome (1, 2) and is frequently associated with obesity, insulin resistance (2–4) and atherosclerosis (5). The transition from insulin sensitivity to insulin resistance is progressive and is thought to be due in large part to increased fatty acid uptake and accumulation into specific tissues such as muscle, adipose and liver which result in aberrant whole body glucose homeostasis. Further, elevations in circulating triglyceride levels have been directly related to leptin resistance in brain suggesting that triglyceride levels have a direct bearing on appetite control (6). Thus, modulating circulating triglyceride levels is a potential therapeutic avenue that could be used to ameliorate features of the metabolic syndrome.
Apolipoprotein A5 [APOA5] is secreted primarily from the liver as a 366 amino acid protein associated with HDL. APOA5 was originally identified as a new apolipoprotein (7) and as an important factor required for liver regeneration (8). Postprandially, APOA5 also associates with VLDL (9). Evidence establishing that APOA5 is an important determinant of plasma triglyceride levels is two-fold: [1] epidemiological studies show a strong association between alleles of APOA5 and plasma triglyceride levels (10–15) and [2] altering the levels of APOA5 protein using genetic engineering or adenoviral expression leads to significant changes in plasma lipid levels (7–9, 16–18). Mice and humans (19) deficient in APOA5 have markedly elevated triglyceride levels, and overexpression of APOA5 leads to marked decreases in plasma triglyceride concentrations. APOA5 can reduce triglyceride levels by decreasing hepatic production of VLDL triglyceride (9, 17, 18, 20), increasing lipoprotein lipase [LPL] lipolysis of lipoprotein triglyceride (16–18), and increasing lipoprotein uptake by the liver (16). Thus, APOA5 contributes to whole body flux of free fatty acids.
In humans, specific alleles of APOA5 not only alter plasma lipid levels but exhibit significant interactions with diet which are related to body mass index and the risk for obesity (4). Studying participants of the Framingham Offspring Study, a strong gene-diet interaction was observed between the APOA5-1131T>C polymorphism and dietary fat which was predictive of the extent of obesity. Data were significant even in a multivariate model which included adjustments for sex and plasma triglyceride levels. These data suggest that APOAV plays an important role in modulating body composition further pointing to the importance of continued studies of APOAV in humans and model systems such as the mouse.
Overall, APOA5 represents a potential therapeutic target for modulation of plasma triglyceride levels and obesity. Of particular interest to us is whether chronic reduction of circulating triglyceride levels protects against the development of adiposity and insulin resistance. We used mice overexpressing human APOA5 (7) to test the hypothesis that chronically reduced plasma triglyceride levels predisposes to reduced adiposity and improved insulin sensitivity following a high fat diet challenge. We report for the first time, responses to high fat and sucrose feeding for APOA5 transgenic mice, and show that reduced triglyceride levels do not protect these mice from obesity and changes in glucose homeostasis associated with body weight gain.
MATERIALS AND METHODS
Mice
APOA5 transgenic mice (A5tg) on the FVB/NJ background (The Jackson Laboratories, Bar Harbor, ME) were obtained as a generous gift from Drs. Len A. Pennacchio and Edward M. Rubin and (Joint Genome Institute, Lawrence Berkeley National Laboratory, Berkeley, CA) and their development using a human BAC and singular expression in the liver has been described (7). Isogenic FVB/NJ mice were purchased from The Jackson Laboratory as described (7). All animals were maintained in a specific pathogen free animal facility at the University of Washington in a temperature-controlled room (25°C) with a fixed 12-hour light/dark cycle. Mice had free access to food and water.
Experimental design
Male and female mice were initially fed pelleted rodent chow (Wayne Rodent BLOX 8604, Teklad, Madison, WI) and randomly assigned to one of two diet groups: rodent chow or high fat/high sucrose (HFHS) diet. Rodent chow contained 4% fat (w/w), 24% protein and 4.5% crude fiber. The HFHS diet (Bioserve No. F1850, Frenchtown, NJ) contained 35.5% fat (primarily lard), 20 % protein, and 36.6% carbohydrate (primarily sucrose) as described previously (22–24). At 8–10 weeks of age, diet groups were initiated and diets were fed for 18 weeks. During weeks 14–16, mice were subjected to glucose tolerance and insulin sensitivity tests. At the end of the study, mice were fasted for 4 hours in the morning, bled from the retro-orbital sinus into tubes containing 1 mM EDTA, killed by cervical dislocation and tissues collected for analyses. Plasma and tissues were stored at −80°C until analyses. All procedures were done in accordance with current NIH guidelines and approved by the Animal Care and Use Committee of the University of Washington.
Analytical procedures
Blood glucose levels were measured with a portable glucose measuring device (Accu-Chek Advantage®). Plasma insulin levels were determined using a commercial Elisa kit (Cat. #EZRMI-13K; Linco Research, Inc., St. Charles, MO) using rat insulin as a standard. Plasma total cholesterol levels were determined using a colorimetric kit (Diagnostic Chemicals Limited, Oxford, CT) with cholesterol standards (Cat.#c0532, Sigma Chemical Co., Inc., St. Louis, MO). Plasma triglyceride levels were determined colorimetrically following the removal of free glycerol (Trig/GB Kit #450032, Roche Diagnostics, Indianapolis, IN). Plasma lipoproteins were separated by fast-performance liquid chromatography gel filtration using a Superose 6 column (GE Healthcare, Piscataway, NJ). A 150 μl aliquot of plasma from pools of 4 mice for each sex and sex group was analyzed at a flow rate of 0.2 ml/min using phosphate-buffered saline (PBS). Next, 100 μl aliquots from each 0.5 ml fraction were used for total lipid determinations.
Glucose tolerance test
Mice were fasted overnight (18 hours) and glucose is injected intraperitoneally at a dose of 1g glucose/kg body wt. Blood glucose was monitored before glucose injection and at 15, 30, 60, and 120 minutes after injection (25).
Insulin sensitivity assay
Mice were fasted overnight and injected intraperitoneally with 0.1 U/ml Humulin R insulin (Eli Lilly and Co., Indianapolis, IN) in sterile PBS at a dose of 0.5 units insulin/kg body weight. Blood glucose was monitored before and at 5, 15, 30, 60 and 120 minutes after insulin injection as described (25).
Lipoprotein lipase
Adipose tissue samples from abdominal and inguinal depots were weighed and made 20% (w/v) in ice-cold buffer (10 mM Tris-HCL, pH 8.0, containing 0.1% Triton X-100, 10% glycerol, 10 U/ml heparin). Tissues were homogenized using a polytron (output setting 40) for 1 minute on ice, then centrifuged for 10 min. at 20,000 × g. The infranatent region above the cell debris and below the floating fat layer was removed and stored at −80°C for further analyses. Neutral lipase activity was determined using radiolabeled triolein as outlined previously (26). Briefly, phosphatidylcholine and triolein were sonicated together, then mixed with buffer containing albumin. Rat serum was added as a source of apoC-II to maximize lipoprotein lipase activity. Infranatent samples were assayed in duplicate and were found to have equivalent activity levels compared to samples not previously frozen and were linear with added protein.
Statistics
Values are reported as means ± SEM. Statistical differences were assessed using the SPSS program (SPSS Inc., Chicago, Il.). Groups were compared using ANOVA analyses and Tukey’s posthoc tests were applied to determine differences between means. In some cases the Student’s t-test was used to compare independent means. P<0.05 was accepted as statistically significant.
RESULTS
Plasma lipid and lipoprotein profiles for wild-type and APOA5 transgenic mice
In rodents, insulin resistance is frequently induced by feeding high fat diets. Further, fructose feeding also leads to a stimulation of VLDL secretion, and obesity and insulin resistance (22, 27–29). Thus, it was important to establish that for the human APOA5 transgenic (A5tg) mice, plasma triglyceride levels were maintained when mice were challenged with dietary fat and sucrose.
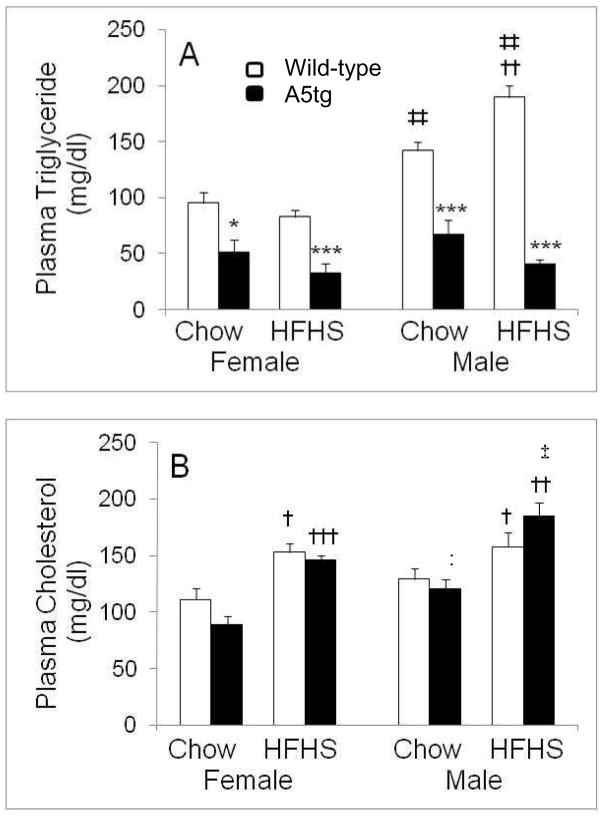
As seen previously (7, 9, 30), A5tg mice fed rodent chow had nearly 2-fold lower plasma triglyceride levels than wild-type mice and importantly for our study, levels did not change for the A5tg mice upon feeding the HFHS diet (Figure 1A). In contrast to these results, plasma total cholesterol (TC) levels for A5tg mice were comparable to those of wild-type mice, and values increased significantly upon feeding the HFHS diet (Figure 1B). Overall, A5tg mice were resistant to diet induced changes in plasma triglyceride but not TC levels.
Figure 1.
Plasma total triglyceride (A) and cholesterol (B) levels for mice fed rodent chow or the high fat/high sucrose (HFHS) diet for 18 weeks. Wild-type (open bars) and APOA5 transgenic (A5tg) (filled bars) mice were fasted for 4 hours in the morning prior to bleeding via the retro-orbital sinus and plasma lipids were quantified as described in Methods and Materials using colorimetric kits purchased from commercial vendors. Plasma triglyceride levels for A5tg mice were significantly lower than wild-type values for both sexes and diets. Male wild-types but not females showed increased triglyceride levels with the HFHS diet. Data are presented as Mean ± SE for 6–10 mice per group; between genotypes as *p<0.05, ***p<0.0001; between diets as †p<0.05, ††p<0.005, †††p<0.0001; between sexes as ‡p<0.03, ‡‡p<0.002.
There were several significant interactions between diet and sex (p<0.05) or genotype (p<0.0003). Among wild-type mice, males but not female mice showed a significant increase in plasma triglyceride levels with HFHS diet feeding (Figure 1A). Because A5tg showed no such change for plasma triglyceride levels, there was also a significant genotype by diet interaction for plasma triglyceride levels. For both A5tg and wild-type mice, plasma TC levels (Figure 1B) increased significantly with feeding of the HFHS diet. However, male A5tg mice had higher TC values than females under both rodent chow and HFHS diet conditions. Overall, both sex and genotype interacted with diet changes which resulted in differential responses in plasma lipid levels.
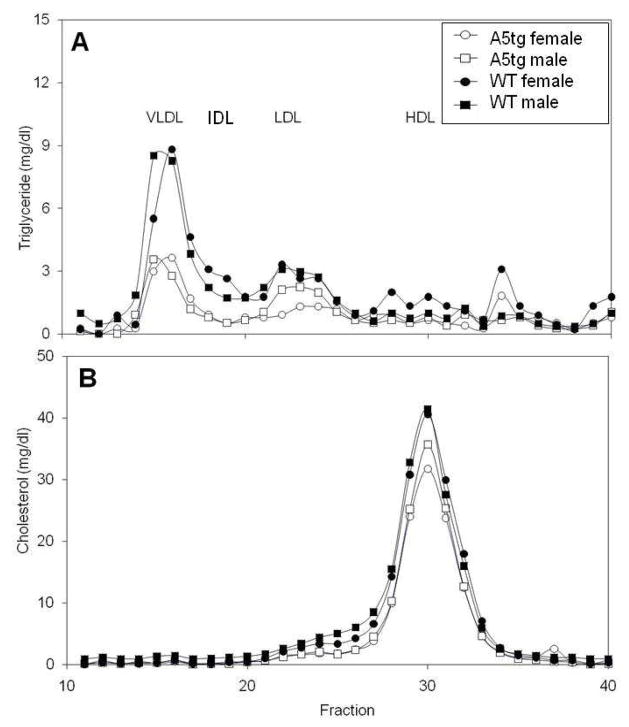
To investigate the nature of the lipoprotein particles seen for A5tg mice, we compared the lipoprotein profiles for A5tg and wild-type mice fed the HFHS diet. Equal plasma aliquots were pooled from 4 mice in each sex and sex group to create the profiles shown in Figure 2. Cholesterol was mainly carried by particles within the HDL fraction and levels were modestly reduced for A5tg mice (Figure 2B). Triglyceride was dispersed among VLDL, IDL and LDL fractions for wild-type mice (Figure 2A). For A5tg mice, triglyceride particles consisted primarily of VLDL and LDL and the VLDL fraction was markedly reduced compared to wild-types. Overall, lipoprotein profiles showed several quantitative differences in lipoprotein fractions between A5tg and wild-type animals.
Figure 2.
Lipoprotein profiles for wild-type (WT) and APOA5 transgenic (A5tg) mice fed the HFHS diet for 18 weeks. Equal plasma aliquots were pooled from 4 mice in each sex and sex group and lipoproteins separated using fast-performance liquid chromatography as described in Methods and Materials. (A) Triglyceride and (B) cholesterol contents are presented for male (square) and female (circle) mice of strains A5tg (open symbols) and FVB (filled symbols) mice. VLVL, IDL, LDL and HDL elution profiles are indicated.
Body weight, fat pad size and tissue lipase levels
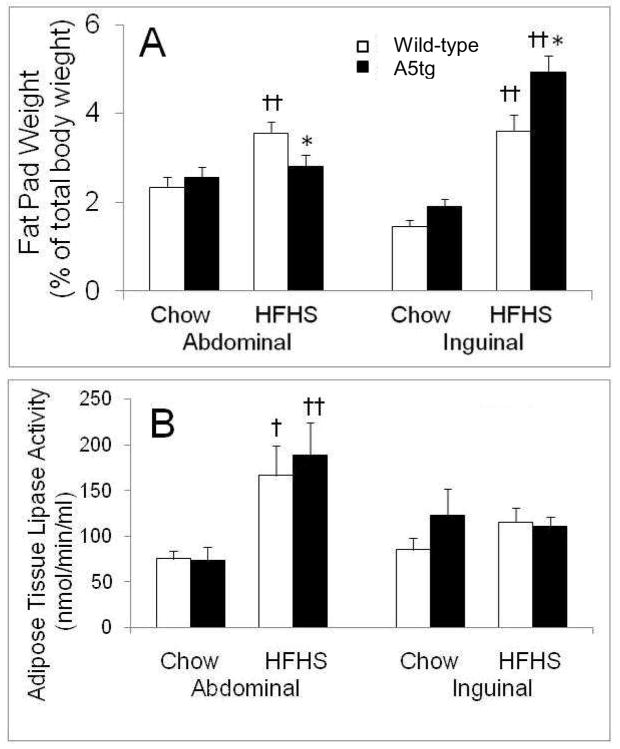
Initial body weights were comparable between genotypes, ranging between 19 and 23 g for males and females. Final weights for mice fed rodent chow were not significantly different between the A5tg and wild-type genotypes (data not shown). For mice fed the HFHS diet, no differences were seen among females (29 ± 0.9 g and 30 ± 0.6 g for wild-type and A5tg, respectively). For the males fed HFHS, A5tg mice were 3 g heavier than wild-types although differences were not significantly different (35 ± 1 g versus 38 ± 1 g for wild-type and A5tg, respectively, p=0.06, n=11–12). The greater body weight gain for A5tg males was reflected in part by significant increases in relative inguinal (~20%; p<0.05) (Figure 3A) and BAT (p<0.05, not shown) fat pad weights. A5tg mice also showed a small but significant decrease in relative abdominal fat pad weight (p<0.05). No differences in renal fat pad weights were seen between genotypes (data not shown).
Figure 3.
Fat depot weights as a percent of body weight (A) and lipase activity values (B) for male mice fed diets for 18 weeks. Wild-type (open bars) and A5tg (filled bars) mice were killed after 4 hours of fasting and individual fat depots were removed and weighed. Adipose tissue fat pads are given as a percent of total body weight. Adipose tissue lipase activity measurements were performed as described in Methods and Materials. Briefly, tissues were homogenized and neutral lipase activity was determined using radiolabeled triolein and using rat serum as a source of apoC-II as described in the text. Activity units are given as nmol free fatty acid hydrolyzed/min/ml of lysate (nmol/min/ml). Data are presented as Mean ± SE for 6–13 mice per group. Significance between genotypes is given as *p<0.05 and between diet as †p<0.025, ††p<0.002.
Adipose tissues are the major storage sites for triglyceride, primarily due to the rapid hydrolysis of circulating TG by lipoprotein lipase. Since APOA5 stimulates the activity of lipoprotein lipase (9), we tested whether changes in adipose tissue lipase activity contributed to the selective changes in size of fat pad as seen for male A5tg mice. Lipase activity was not different between genotypes but increased 2-fold with HFHS diet feeding in abdominal fat tissue (Figure 3b). Thus, lipase activity did not mediate differences in adipose depot sizes between genotypes.
Assessment of glucose homeostasis
A role for APOA5 in glucose homeostasis has been indicated by others since polymorphisms in the human APOA5 locus are associated with altered glucose tolerance in humans (31). In addition, mice deficient in APOA5 are hypertriglyceridemic and develop insulin resistance when fed high fat diets (16). Thus, at the beginning of our study, we hypothesized that APOA5 transgenic mice fed a HFHS diet would show improved glucose homeostasis as compared to wild-type mice.
Glucose homeostasis was examined in several ways. We first compared plasma glucose and insulin levels taken after 4 hours of fasting and examined the ratio of glucose to insulin (G/I), which would be reduced for insulin resistant mice. Table 1 shows that overall, plasma glucose and insulin levels increased and the G/I decreased for mice fed the HFHS diet as compared to rodent chow. An exception to these changes was female A5tg mice that showed comparable insulin and G/I values for both diets. The most important observation is that male A5tg mice showed the highest levels of circulating glucose and insulin, and the G/I value reflected a requirement for higher insulin to maintain their glucose levels. These data suggest that no benefit to glucose homeostasis is seen with overexpression of APOA5 and this was actually detrimental in male mice.
Table 1.
Plasma glucose and insulin levels for wild-type (WT) and transgenic (A5tg) mice fed rodent chow or the high fat/high sucrose (HFHS) diets for 18 weeks.
| Genotype | Glucose (mg/ml) | Insulin (pg/ml) | Ratio (Glucose/Insulin) | |
|---|---|---|---|---|
| Female | ||||
| Chow | WT | 133 ± 10 | 0.64 ± 0.24 | 388 ± 95 |
| Chow | A5tg | 100 ± 12* | 0.57 ± 0.10 | 186 ± 22* |
| HFHS | WT | 151 ± 6 | 1.51 ± 0.33†† | 144 ± 30† |
| HFHS | A5tg | 133 ± 7*†† | 0.82 ± 0.18 | 194 ± 28 |
| Male | ||||
| Chow | WT | 138 ± 3 | 0.51 ± 0.05 | 280 ± 19 |
| Chow | A5tg | 155 ± 7* | 0.56 ± 0.03 | 282 ± 23 |
| HFHS | WT | 158 ± 12 | 1.31 ± 0.33†† | 164 ± 30† |
| HFHS | A5tg | 213 ± 10**†† | 3.89 ± 1.06*†† | 64 ± 13*†† |
Data are presented as Mean ± SE with significance between genotypes as *p<0.05, **p<0.005 and between diets as †p<0.03, ††p<0.005.
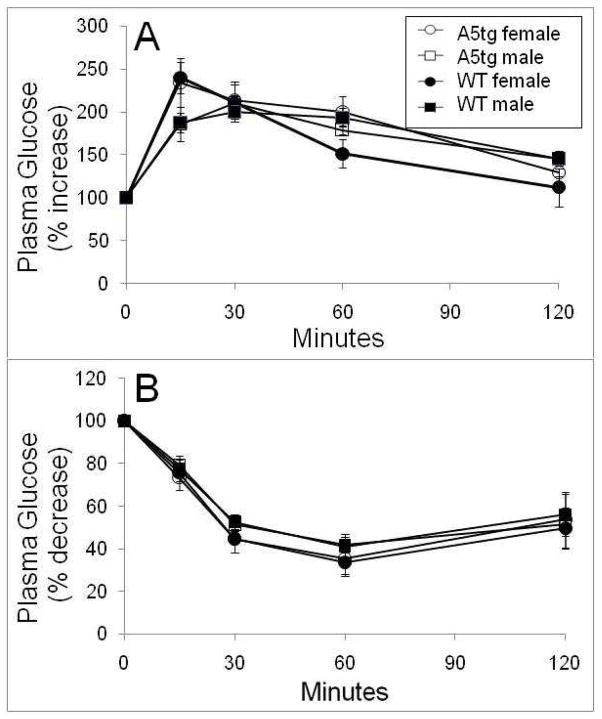
To examine glucose homeostasis in more detail, we measured plasma glucose levels over time in response to injections of glucose (glucose tolerance) or insulin (insulin sensitivity). As shown in Figure 4, except for a significant difference at 60 min between females, responses for these measures of glucose homeostasis were the same between A5tg and wild-type mice.
Figure 4.
Glucose tolerance (A) and insulin sensitivity (B) curves for mice fed the HFHS diet for 16 or 14 weeks, respectively. For glucose tolerance, mice were fasted overnight, gAavaged with a 25% glucose solution (in sterile PBS) at a dose of 2 g glucose/kg body weight and blood glucose monitored over 120 minutes. For insulin sensitivity, mice were fasted overnight, injected intraperitoneally with 0.1 U/ml Humulin R insulin in sterile PBS at a dose of 0.5 units insulin/kg body weight. Wild-type (WT) (open symbols) and APOA5 transgenic (A5tg) (filled symbols) mice show comparable responses between genotypes for both male (square) and female (circle) mice. Values are presented as percent changes with respect to starting plasma glucose levels.
Overall, our data do not support a beneficial effect on body weight or glucose homeostasis following overexpression of APOA5 in mice.
DISCUSSION
Metabolic syndrome is a risk factor for type 2 diabetes and cardiovascular diseases (32). It is characterized by a collection of factors including hypertriglyceridemia, reduced HDL levels, obesity and insulin resistance. The onset is progressive and mechanisms for aberrant changes in lipoprotein profiles include increased rates of production of triglyceride rich lipoproteins from the intestine and liver, decreased hydrolysis of circulating triglycerides, alterations in specific concentrations of circulating apolipoproteins and changes in gene expression in tissues responsible for the maintenance of lipid homeostasis (33–35). We used the hypotriglyceridemic APOA5 transgenic mouse strain to test the hypothesis that chronic reduction in circulating triglycerides would protect animals from diet-induced obesity and insulin resistance. Our data did not support this idea, as there was no improvement in body weight or glucose homeostasis accompanying sustained reductions in plasma triglyceride levels. Sexual dimorphism was evident as male A5tg mice showed worsened metabolic traits as compared to the wild-type mice.
When fed the HFHS diet, male A5tg showed a modest increase in body weight as compared with wild-types attributed to increases in inguinal and BAT fat pad weights. Further, an assessment of G/I suggested that male A5tg mice were more glucose intolerant than wild-types. Of note is that all A5tg mice retained markedly reduced plasma TG levels with feeding of the HFHS diet suggesting that plasma TG levels were not predictive of metabolic clinical outcomes among these mice. The results involving the males were surprising and demonstrate that an interaction exists between sex-based factors and expression of the APOA5 transgene.
In humans, interactions between sex and APOA5 polymorphisms have been difficult to discern. One report found an association between the APOA5 promoter allele 1131C and postprandial triglyceride levels which was more significant for males than females among a cohort of healthy participants in the United Kingdom (36). Among males and females selected from a Czech population, another APOA5 polymorphism (Val153>Met) associated with plasma HDL cholesterol levels in females but not males (37). In concert with apolipoprotein E alleles, the APOA5-1131C genotype was related to plasma lipid levels in females but not males (38). In contrast to these reports, Corella et al. (21) found no sex by genotype differences in outcome measures of BMI among individuals participating in the Framingham Heart Study with APOA5 alleles (−1131T>C and 56C>G). Additional epidemiological studies are still needed to investigate whether APOA5 alleles, especially those affecting APOA5 plasma levels, combine with sex to influence outcomes of obesity and risk factors for heart disease.
We also found interactions between APOA5 genotype (wild-type versus transgenic) and diet which influenced body weight. These data are in concert with human studies by Corella et al. (21) which showed association between the APOA5 promoter allele-1131T>C and body mass index among individuals of the Framingham Heart Study. Further, Lai et al. (39) showed interactions between type of dietary fat and the APOA5-1131T>C polymorphism such that higher n−6 (but not n−3) polyunsaturated fat diets increased triglyceride levels and modified lipoprotein sizes resulting in a more atherogenic lipid profile. Overall, multiple contributions of diet, fasting time, and population heterogeneity interact with APOA5 alleles to alter outcomes of obesity, lipid levels and heart disease risk factors.
Humans and mice differ in many regards, and in mice, sexual dimorphism in body fat and type 2 diabetes mellitus is known to occur more easily in males which show greater disease severity in clinical traits than females (40–42). For wild-type FVB mice fed the HFHS diet, plasma triglyceride levels were higher for males than females, but body weights and levels of plasma glucose, insulin and cholesterol were comparable between males and females. However, for A5tg mice, sexual dimorphic effects were magnified as male A5tg mice showed more body weight gain and significant increased in relative inguinal fat pad weight and levels of glucose and insulin. There are other examples for which a genetic modification in genes involved in lipid metabolism or energy balance result in significant interactions with ex as described (43–45). Thus, it is important to carefully access the action of a new genetic modifications using both sexes and when possible, multiple genetic backgrounds (41).
Mechanisms by which sexual dimorphism interacts with the APOA5 transgene are likely to be complex. Sexual dimorphism for leptin responsiveness has been reported for FVB mice (40). Leptin is a hormone primarily secreted by adipocytes and known to contribute to body weight regulation through signals to CNS which control food intake (46,47). Reduced leptin levels are expected for mice with reduced insulin resistance (48). We quantified plasma leptin levels at the end of the study and found essentially no differences between male leptin levels (23 ± 4.0 ng/ml versus 29 ± 2.3 ng/ml for wild-type and A5tg males, respectively) which suggest a lack of contribution through this axis. Alternatively, it is known that the liver, a key tissue mediating lipid and glucose metabolism, has sex-specific gene expression patterns for a variety of transcripts involved in determining fat mass and biotransformation enzymes (49–51). In our mice, plasma TC levels were higher for males than females (p<0.03). Since the liver is responsible in large part for lipoprotein production and clearance, and is the site of APOA5 synthesis in wild-types and these transgenic mice (7), sex specific factors influencing lipid metabolism in the liver may have contributed to final clinical outcomes.
In summary, since its identification in 2001 as a new apolipoprotein (7, 8), APOA5 has generated much interest due to its role in modulating plasma triglyceride levels. There are strong associations between elevated plasma TG levels, obesity and insulin resistance and so, we tested whether mice made hypotriglyceridemic by overexpression of human APOA5 would be protected against diet-induced obesity and insulin resistance. There was no improvement in body weight, or glucose homeostasis accompanying sustained reductions in plasma triglyceride levels. Sexual dimorphism was evident as male A5tg mice showed worsened metabolic traits as compared to the wild-type mice. Overall, there was significant interaction between excessive APOA5 expression and sex and diet factors which resulted in lack of protection from obesity and glucose tolerance at least in male mice. These results suggest that caution should be used in considering APOA5 for therapeutic intervention strategies. However, it is difficult to extrapolate these findings directly to humans because major differences in lipid biology exist between these species.
Acknowledgments
This work was supported by grants from NIH (RO1 DK063159 [RCL]) and a VA Merit Review Grant (HW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. American Journal Cardiology. 1998;81:7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 2.Brunzell JD, Ayyobi AF. Dyslipidemia in the metabolic syndrome and type 2 diabetes mellitus. American Journal Medicine. 2003;115(Suppl 8A):24S–28S. doi: 10.1016/j.amjmed.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto WY. The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. American Journal Medicine. 2000;108(Suppl 6a):9S–14S. doi: 10.1016/s0002-9343(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 4.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Journal of the American Medical Association. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 5.Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109:III15–19. doi: 10.1161/01.CIR.0000131513.33892.5b. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 7.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 8.van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoproteins associated with an early phase of liver regeneration. The Journal of Biological Chemistry. 2001;276:44512–44521. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 9.Fruchart-Najib J, Bauge E, Niculescu LS, Pham T, Thomas B, Rommens C, Majd A, Brewer B, Pannacchio LA, Fruchart JC. Mechanism of triglyceride lowering in mice expressing human apolipoproteins A5. Biochem Biophys Res Commmun. 2004;319:397–404. doi: 10.1016/j.bbrc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Austin MA, Talmud PJ, Farin FM, Nicherson DA, Edwards KL, Leonetti D, McNeely MJ, Viernes HM, Humphries SE, Fujimoto WY. Association of apolipoproteins A5 variants with LDL particle size and triglyceride in Japanese Americans. Biochem Biophys Acta. 2004;1688:1–9. doi: 10.1016/j.bbadis.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Horinek A, Vrablik M, Ceska R, Adamkova V, Poledne R, Hubacek JA. T1131 → C polymorphism within the apolipoprotein A5 gene in hypertriglyceridemic individuals. Atherosclerosis. 2003;167:369–370. doi: 10.1016/s0021-9150(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 12.Hubacek JA, Skodova Z, Adamkova V, Lanska V, Poledne R. The influence of APOA5 polymorphisms (T-1131>C and S19>W) on plasma triglyceride levels and risk of myocardial infarction. Clinical Genetics. 2004;65:126–130. doi: 10.1111/j.0009-9163.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 13.Lai CQ, Tai ES, Tan CE, Cutter J, Chew SK, Zhu YP, Adiconis X, Ordovas JM. The APOA5 locus is a strong determinant of plasma triglyceride concentrations across ethnic groups in Singapore. Journal of Lipid Research. 2003;44:2365–2373. doi: 10.1194/jlr.M300251-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Mar R, Pajukanta P, Allayee H, Groenendijk M, Dallinga-Thie G, Krauss RM, Sinsheimer JS, Cantor RM, de Bruin TW, Lusis AJ. Association of the APOLIPOPROTEIN A1/C3/A4? A5;gene cluster with triglyceride levels and LDL particle size in familial combined hyperlipidemia. Circulation Research. 2004;94:993–999. doi: 10.1161/01.RES.0000124922.61830.F0. [DOI] [PubMed] [Google Scholar]
- 15.Olivier M, Wang X, Cole R, Gau B, Kim J, Ruben EM, Pennacchio LA. Haplotype analysis of the apolipoprotein gene cluster on human chromosome 11. Genomics. 2004;83:912–923. doi: 10.1016/j.ygeno.2003.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosskopf I, Baroukh N, Lee SJ, Kamari Y, Harats D, Rubin EM, Pennacchio LA, Cooper AD. Apolipoprotein A-V deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arteriosclerosis, Thrombosis and Vascular Biology. 2005;25:2573–2579. doi: 10.1161/01.ATV.0000186189.26141.12. [DOI] [PubMed] [Google Scholar]
- 17.Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein A5 accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycans-bound lipoprotein lipase. The Journal of Biological Chemistry. 2005;280:21553–21560. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- 18.Schaap FG, Rensen PC, Voshol PJ, Vrins C, van der Vliet HN, Chamuleau RA, Havekes LM, Groen AK, van Dijk KW. ApoA5 reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. The Journal of Biological Chemistry. 2004;279:27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 19.Priore Oliva C, Pisciotta L, Li Volti G, Sambataro MP, Cantafora A, Bellocchio A, Catapano A, Tarugi P, Bertolini S, Calandra S. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:411–417. doi: 10.1161/01.ATV.0000153087.36428.dd. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg RB, Cook VR, Beckstead JA, Martin DD, Gallagher JW, Shelness GS, Ryan RO. Structure and interracial properties of human apolipoproteins AV. The Journal of Biological Chemistry. 2003;278:34438–34444. doi: 10.1074/jbc.M303784200. [DOI] [PubMed] [Google Scholar]
- 21.Corella D, Chao-Qiang L, Demissie S, Cupples LA, Manning AK, Tucker KL, Ordovas JM. APOA5 gene variation modulates the effects of dietary fat intake on body mass index and obesity risk in the Framingham Heart Study. Jounal of Molecular Medicine. 2007;85:119–128. doi: 10.1007/s00109-006-0147-0. [DOI] [PubMed] [Google Scholar]
- 22.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes n mice. American Journal of Physiology, Endocrinology Metabolism. 2002;282:E207–214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 23.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 24.Surwit RS, Seldin MF, Kuhn CM, Cochrane C, Feinglos MN. Control of expression of insulin resistance and hyperglycemia by different genetic factors in diabetic C57BL/6J mice. Diabetes. 1991;40:82–87. doi: 10.2337/diab.40.1.82. [DOI] [PubMed] [Google Scholar]
- 25.Schreyer SA, Cummings DE, McKnight GS, LeBoeuf RC. Mutation of the RIIbeta subunit of protein kinase A prevents diet-induced insulin resistance and dyslipidemia in mice. Diabetes. 2001;50:2555–2562. doi: 10.2337/diabetes.50.11.2555. [DOI] [PubMed] [Google Scholar]
- 26.Veronique Briquet-Laugier OB-Z, Doolittle MH. Determining lipoprotein lipse and hepatic lipase activity using radiolabeled substrates. Totawa, NJ: Humana Press inc.; 1998. [DOI] [PubMed] [Google Scholar]
- 27.Noguchi T, Tanaka T. Insulin resistance in obesity and its molecular control. Obesity Research. 1995;3 (Suppl 2):195S–198S. doi: 10.1002/j.1550-8528.1995.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 28.Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53:454–457. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Zammit VA, Waterman IJ, Topping D, McKay G. Insulin stimulation of hepatic triacylglycerol secretion and the etiology of insulin resistance. Journal of Nutrition. 2001;131:2074–2077. doi: 10.1093/jn/131.8.2074. [DOI] [PubMed] [Google Scholar]
- 30.Baroukh N, Bauge E, Akiyama J, Chang J, Afzal V, Fruchart JC, Rubin EM, Fruchart-Najib J, Pannacchio LA. Analysis of apolipoproteins A5, C3, and plasma triglyceride concentrations in genetically engineered mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:1297–1302. doi: 10.1161/01.ATV.0000130463.68272.1d. [DOI] [PubMed] [Google Scholar]
- 31.Martin S, Nicaud V, Humphries SE, Talmud PJ. Contribution of APOA5 gene variants to plasma triglyceride determination and to the response to both fat and glucose tolerance challenges. Biochim biophys Acta. 2003;1637:217–225. doi: 10.1016/s0925-4439(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg HN. Insulin resistance and cardiovascular disease. The Journal of Clinical Investigation. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunzell JD, Hazzard WR, Porte D, Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. The Journal of Clinical Investigation. 1973;52:1578–1585. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan DC, Barrett PH, Watts GF. Recent studies of lipoprotein kinetics in the metabolic syndrome and related disorders. Current Opinion in Lipidology. 2006;17:28–36. doi: 10.1097/01.mol.0000199815.46720.ca. [DOI] [PubMed] [Google Scholar]
- 35.Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoproteins B-48-containing lipoproteins in humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:1357–1363. doi: 10.1161/01.ATV.0000222015.76038.14. [DOI] [PubMed] [Google Scholar]
- 36.Olano-Martin E, Abraham EC, Gill-Garrison R, Valdes AM, Grimaldi K, Tang F, Jackson KG, Williams CM, Minihane AM. Influence of apoA-V gene variants on postprandial triglyceride metabolism: impact of gender. Journal of Lipid Research. 2008;49:945–953. doi: 10.1194/jlr.M700112-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Hubacek JA, Skodová Z, Adámková V, Lánská V, Poledne R. Sex-specific effect of APOAV variant (Val153>Met) on plasma levels of high-density lipoprotein cholesterol. Metabolism. 2005;54:1632–1635. doi: 10.1016/j.metabol.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Hubacek JA, Lánská V, Skodová Z, Adámková V, Poledne R. Sex-specific interaction between APOE and APOA5 variants and determination of plasma lipid levels. European Journal of Human Genetics. 2008;16:135–138. doi: 10.1038/sj.ejhg.5201941. [DOI] [PubMed] [Google Scholar]
- 39.Lai C-Q, Corella D, Demissie S, Cupples LA, Adiconis X, Zhu Y, Parnell LD, Tucker KL, Ordovas JM. Dietary intake of n−6 fatty acids modulates effect of apolipoprotein A5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size. The Framingham Heart Study. Circulation. 2006;113:2062–2070. doi: 10.1161/CIRCULATIONAHA.105.577296. [DOI] [PubMed] [Google Scholar]
- 40.Harris RB, Bowen HM, Mitchell TD. Leptin resistance in mice is determined by gender and duration of exposure to high-fat diet. Physiological BehA5ior. 2003;78:543–555. doi: 10.1016/s0031-9384(03)00035-0. [DOI] [PubMed] [Google Scholar]
- 41.Leiter EH. The genetics of diabetes susceptibility in mice. Faseb Journal. 1989;3:2231–2241. doi: 10.1096/fasebj.3.11.2673897. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp Anim. 2007;56:263–272. doi: 10.1538/expanim.56.263. [DOI] [PubMed] [Google Scholar]
- 43.Costet P, Lagendre C, More J, Edgar A, Galtier P, Pneau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. The Journal of Biological Chemistry. 1998;273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- 44.Tiraby C, TA5ernier G, Capel F, Mairal A, Crampes F, Rami J, Pujol C, boutin JA, Langin D. Resistance to high-fat-diet-induced obesity and sexual dimorphism in the metabolic responses of transgenic mice with moderate uncoupling protein 3 overexpression in glycolytic skeletal muscles. Diabetologia. 2007;50:2190–2199. doi: 10.1007/s00125-007-0765-2. [DOI] [PubMed] [Google Scholar]
- 45.Zammaretti F, Panzica G, Eva C. Sex-dependent regulation of hypothalamic neuropeptide Y-Y1 receptor gene expression in moderate/high fat, high-energy diet-fed mice. Journal of Physiology. 2007;583:445–454. doi: 10.1113/jphysiol.2007.133470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. The Journal of Clinical Investigation. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metabolism. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Willi SM, Kennedy A, Wallace P, Ganaway E, Rogers NL, Garvey WT. Troglitazone antagonizes metabolic effects of glucocorticoids in humans: effects on glucose tolerance, insulin sensitivity, suppression of free fatty acids, and leptin. Diabetes. 2002;51:2895–2902. doi: 10.2337/diabetes.51.10.2895. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez JP, Chapman LM, Kretschmer XC, Baldwin WS. Gender-specific induction of cytochrome P450s in nonylphenol-treated FVB/NJ mice. Toxicology Applied Pharmacology. 2006;216:186–196. doi: 10.1016/j.taap.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park SH, Liu X, Hennighausen L, DA5ey HW, Waxman DJ. Distinctive role of STAT5a and STAT5b in sexual dimorphism of hepatic P450 gene expression. Impact of STAT5a gene disruption. The Journal of Biological Chemistry. 1999;274:7421–7430. doi: 10.1074/jbc.274.11.7421. [DOI] [PubMed] [Google Scholar]
- 51.Wang S, Yehya N, Schadt EE, Wang H, Drake TA, Lusis AJ. Genetic and genomic analysis of a fat mass trait with complex inheritance reveals marked sex specificity. PLoS Genetics. 2006;2:e15. doi: 10.1371/journal.pgen.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]