Abstract
Adeno-associated virus (AAV) vectors are effective gene delivery vehicles mediating long-lasting transgene expression. Data from a clinical trial of AAV2-mediated hepatic transfer of the Factor IX gene (F9) into hemophilia B subjects suggests that CTL responses against AAV capsid can eliminate transduced hepatocytes and prevent long-term F9 expression. However, the capacity of hepatocytes to present AAV capsid–derived antigens has not been formally demonstrated, nor whether transduction by AAV sensitizes hepatocytes for CTL-mediated destruction. To investigate the fate of capsids after transduction, we engineered a soluble TCR for the detection of capsid-derived peptide:MHC I (pMHC) complexes. TCR multimers exhibited antigen and HLA specificity and possessed high binding affinity for cognate pMHC complexes. With this reagent, capsid pMHC complexes were detectable by confocal microscopy following AAV-mediated transduction of human hepatocytes. Although antigen presentation was modest, it was sufficient to flag transduced cells for CTL-mediated lysis in an in vitro killing assay. Destruction of hepatocytes was inhibited by soluble TCR, demonstrating a possible application for this reagent in blocking undesirable CTL responses. Together, these studies provide a mechanism for the loss of transgene expression and transient elevations in aminotransferases following AAV-mediated hepatic gene transfer in humans and a potential therapeutic intervention to abrogate these limitations imposed by the host T cell response.
Introduction
In the first phase I/II trial of hepatic gene transfer of adeno-associated virus 2 (AAV2) encoding Factor IX (AAV2-F9) in human hemophilia B subjects, transgene expression was demonstrable but short lived (1). As Factor IX activity declined 4–6 weeks after infusion, a concomitant asymptomatic, self-limited rise in hepatic aminotransferases was observed. In addition, AAV2 capsid–specific CD8+ T cells were detectable following vector administration, and their frequency expanded and contracted in temporal correlation with hepatocellular injury (2). In contrast, no T cell response against the transgene was detectable by IFN-γ ELISPOT screening of a Factor IX peptide library (1). These observations raise the possibility of capsid-directed immune destruction of transduced hepatocytes. The host CTL response to vector therefore represents a potential barrier to successful hepatic gene therapy. However, relatively little is known about the fate of a capsid once it enters the cell, including its processing, degradation, and access to antigen-presentation pathways.
Immunodominant, MHC I–restricted AAV capsid epitopes have been identified in humans (1, 2) and mice (3, 4). However, capsid-derived antigen presentation following AAV-mediated transduction has yet to be formally demonstrated on hepatocytes. Based on animal studies, this has been considered an unlikely possibility by some investigators (4, 5). Until now, no direct means for visualizing AAV capsid–derived peptide:MHC I (pMHC) complexes existed. Similarly, capsid-specific CD8+ T cells have been identified following administration of adenovirus expressing AAV2 capsid in mice (3, 6) or infusion of AAV vector in humans (2, 6). Although peptide-pulsed target cells infused in vivo are destroyed by these CTLs, the direct lysis of AAV-transduced hepatocytes has not been previously demonstrated. Finally, developing an effective strategy to circumvent host immune responses against AAV capsid will likely be important for successful gene delivery to the liver. Immunosuppression protocols have been evaluated for inhibiting undesirable host immune responses during gene therapy (7, 8). However, these agents are nonspecific and convey the risk of off-target effects such as the unwanted depletion of regulatory T cells with daclizumab, an anti–IL-2Rα antibody, resulting in loss of tolerance to the transgene product (8–10).
Soluble MHC multimers were developed over a decade ago and have been used successfully to enumerate antigen-specific T cells (11). In contrast their counterparts, soluble TCR multimers have only recently been described (12–14). These reagents can exhibit high binding affinities within the range of usual TCR:pMHC interactions (~0.1–90 μM) (15). We therefore constructed a soluble TCR to detect capsid pMHC complexes on cell surfaces. This reagent exhibited high affinity for cognate pMHC complexes and detected antigen presentation by flow cytometry. By using more sensitive confocal microscopy, TCR multimers directly visualized capsid-derived pMHC complexes following AAV-mediated transduction of human hepatocytes. Furthermore, cytotoxicity assays demonstrated that AAV-transduced human hepatocytes were specifically killed by capsid-directed CTLs. Finally, TCR multimers protected transduced hepatocytes from CTL-mediated cytolysis against cognate and cross-reactive epitopes derived from different AAV serotypes. Together, our results conclusively demonstrate that AAV sensitizes hepatocytes for CTL-mediated destruction from low levels of capsid antigen presentation. These studies provide mechanistic insight into the previously observed loss of F9 expression and elevations in aminotransferases in a human clinical trial (1). In addition, we reveal a therapeutic application for soluble TCR reagents in protecting antigen-bearing cells from undesired T cell responses.
Results
Construction of an AAV capsid–specific soluble TCR.
To construct an antigen-specific soluble TCR, an AAV2 capsid–specific CTL clone first needed to be generated. To accomplish this, HLA-B*0702+ normal human donor PBMCs were expanded in culture in the presence of AAV2 capsid peptide and growth factors. Cells were sorted by flow cytometry for antigen-specific CD8+ T cells using HLA-B*0702 pentamers bearing AAV2 capsid peptide. After sorting, single-cell clones were obtained by limiting dilution. The TCR-α and -β chain cDNAs were then cloned by SMART-RACE PCR from individual clones. A monomeric TCR fusion construct containing a BirA biotinylation tag was generated and expressed in CHO cells (Altor Bioscience). Soluble TCR monomers were then multimerized using streptavidin-conjugated fluorochromes. Non-denaturing PAGE of the resulting TCR multimers reveals that the majority of species were conjugated monomers or dimers (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/36891DS1). This is consistent with observations of other TCR multimers engineered in a similar fashion and is likely due to geometric constraints from steric hindrance (14).
Specificities of the TCR multimer.
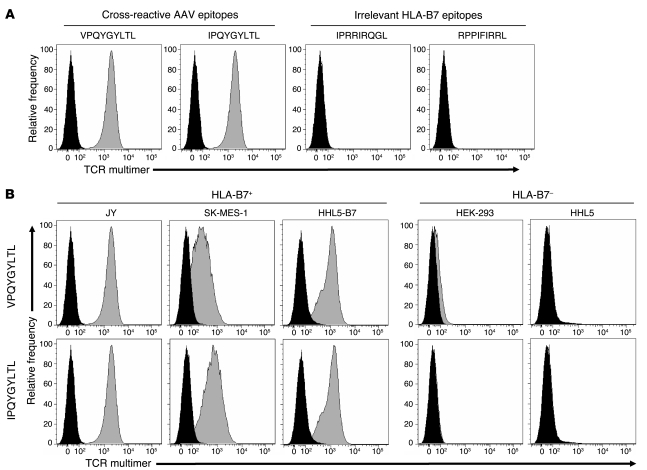
Because the TCR multimer was generated from an AAV capsid–specific CD8+ T cell clone, it was expected to manifest antigen and MHC I context specificity. To verify these critical properties, an HLA-B*0702+ cell line was pulsed with cognate AAV capsid–derived peptides or irrelevant HLA-B*0702–restricted epitopes derived from HIV-1 or EBV. The TCR multimer only detected pMHC complexes on cells pulsed with either AAV2-derived (VPQYGYLTL) or AAV1-, AAV6-, and AAV8-shared (IPQYGYLTL) capsid epitopes (Figure 1A). This concurs with previous studies demonstrating relative sequence conservation and antigenic cross-reactivity of this AAV capsid epitope across serotypes (1–3). In contrast, no TCR multimer staining was observed after pulsing cells with irrelevant peptides derived from other viruses (Figure 1A). Next, a variety of HLA-B*0702+ or HLA-mismatched cell lines were loaded with cognate AAV capsid peptides and stained with TCR multimer. Staining was observed only with cells that were an HLA match for the TCR multimer (Figure 1B). Thus, we determined that the soluble TCR retains the expected binding specificities of a bona fide T cell receptor.
Figure 1. Antigen and HLA specificities of TCR multimer staining.
(A) To demonstrate antigen specificity, HLA-B*0702+ JY cells were pulsed with 5 μg/ml of either cross-reactive capsid epitopes derived from AAV2 (VPQYGYLTL) or AAV8 (IPQYGYLTL) or irrelevant HLA-B*0702–restricted epitopes derived from HIV (IPRRIRQGL) or EBV (RPPIFIRRL). After 2 hours of incubation, cells were stained with TCR multimer for flow cytometry. Histograms depict unpulsed cells (black) and peptide-pulsed cells (gray). (B) To demonstrate HLA specificity, either HLA-matched (JY, SK-MES-1, and HHL5-B7) or HLA-mismatched (HEK-293 and HHL5) cells were pulsed with 5 μg/ml of indicated AAV capsid peptide, then stained with TCR multimer. Histograms depict unpulsed cells (black) and peptide-pulsed cells (gray).
TCR multimer binding affinity and detection sensitivity for pMHC complexes.
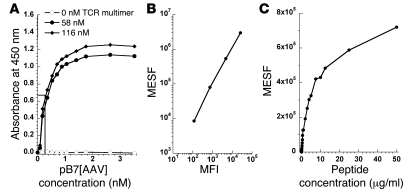
Antigen presenting cells express a finite number of MHC molecules on their surfaces, thus foreign proteins must compete with endogenously derived products for antigen presentation. It was therefore important to determine the binding affinity and detection limit of TCR multimers for pMHC complexes. The binding constant was calculated from the EC50 for TCR multimer binding to pB7[AAV] by ELISA. The concentration of pB7[AAV] required for half-maximal detection of the immobilized TCR multimer was 0.26 nM, which approximates the Kd for this interaction (Figure 2A). This indicated a relatively high affinity for pMHC complexes and suggested that the TCR multimer could bind to cells presenting low levels of antigen. To determine the detection limit for pMHC complexes by flow cytometry, the HLA-B*0702+ human hepatocyte cell line HHL5-B7 was pulsed with decreasing concentrations of AAV capsid peptide, then stained with TCR multimer. A standard curve was generated, using calibration beads containing a known number of fluorochrome molecules, that related MFI to molecules of equivalent soluble fluorochrome (MESF) (Figure 2B). Staining of peptide-pulsed cells was indistinguishable from unpulsed cells at peptide concentrations below about 25 ng/ml (23.7 nM). This corresponded to approximately 1,000 MESF (Figure 2C). However, the stoichiometry of TCR multimer binding to pMHC complexes on the cell surface was difficult to ascertain, as the conjugated reagent exists as a heterogeneous mixture of primarily monomers and dimers (Supplemental Figure 1). Thus, we conservatively estimate that under these conditions the detection of antigen by TCR multimer requires a minimum of 1,000–4,000 pMHC complexes using flow cytometry.
Figure 2. Estimation of TCR multimer binding affinity and limit of detection.
(A) TCR multimer was used to coat microtiter wells at 0 nM (open circles), 58 nM (filled circles), or 116 nM (filled diamonds). Biotinylated pB7[AAV] was then added at the indicated concentrations, followed by streptavidin-conjugated HRP. Absorbance at 450 nm was measured following addition of o-phenylenediamine substrate. Binding affinity (Kd) was estimated from the concentration of pB7[AAV] at which half-maximal detection occurred (EC50). (B) A set of APC calibration beads was used to set photomultiplier tube voltages on a FACS Canto II. Then, a calibration curve was generated using linear regression to calculate MESF from MFI (r2 ≥ 0.99). (C) After determining the MFI of peptide-pulsed HHL5-B7 hepatocytes stained with TCR multimer, the MESF was calculated and plotted against peptide concentration. The minimum number of pMHC complexes detectable by flow cytometry was estimated from the lowest MESF discernible from unpulsed cells.
Visualization of pMHC complexes following AAV-mediated transduction.
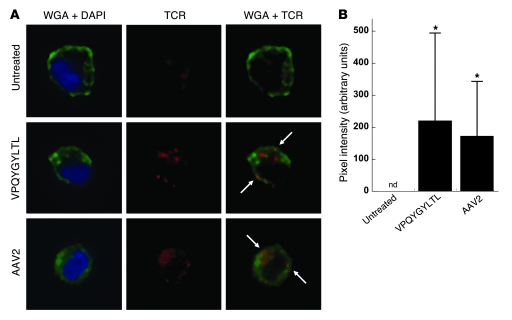
Despite the high sensitivity of TCR multimers, no pMHC complexes were detectable by flow cytometry after staining hepatocytes transduced with AAV in culture (our unpublished observations). Others have shown that flow cytometry can limit the detection of low-density pMHC complexes by soluble TCR, as compared with more sensitive confocal imaging (16). We therefore investigated whether our TCR multimer could detect pMHC complexes by confocal microscopy. HHL5-B7 human hepatocytes were incubated with medium alone, AAV2 capsid peptide, or AAV2 vector. Cells were then stained with wheat germ agglutinin (WGA) to identify the plasma membrane, DAPI to highlight the nucleus, and TCR multimer to label pMHC complexes. Untreated cells demonstrated background staining that was primarily intracellular (Figure 3A). Therefore, confocal images were analyzed for TCR staining colocalized to the plasma membrane, where specific binding of pMHC complexes was expected to occur. TCR multimer staining of cells treated with either peptide or AAV2 vector revealed modest staining that colocalized with the cell surface. Objective quantification of individual pixels for intensity of costaining on multiple cells revealed statistically significant detection of pMHC complexes above background with either treatment (Figure 3B). Although the signal from staining of the pMHC complexes formed after AAV-mediated transduction was modest, these general trends were reproduced in at least 2 independent experiments using this sensitive visualization technique.
Figure 3. Confocal microscopy of TCR multimer staining following AAV-mediated transduction.
(A) HHL5-B7 hepatocytes were cultured alone or in the presence of 10 μg/ml VPQYGYLTL capsid peptide or AAV2-F9 vector at 3 × 105 MOI. Cells were then stained with WGA (green) to visualize the plasma membrane, DAPI (blue) to identify the nucleus, and TCR multimer (red) to stain pMHC complexes. Cells were visualized using spinning disk confocal microscopy, and representative cells are shown. Arrows indicate representative areas of TCR and plasma membrane colocalization (yellow). Total original magnification, ×63. (B) Staining intensity of colocalized signal from individual cells was quantitatively measured using Volocity and graphed as average intensity per cell ± SD (n = 17–25 cells per group). After normalization as detailed in Methods, the colocalized intensity was not detectable (nd) on untreated cells. *P < 0.004 versus untreated cells.
CTL-mediated destruction of AAV-transduced hepatocytes.
Although confocal studies using TCR multimers revealed the formation of capsid-derived pMHC complexes following AAV-mediated transduction, the low density of complexes observed may not have been immunologically relevant. To investigate whether this level of antigen presentation renders transduced hepatocytes vulnerable to CTL-mediated lysis, a non-radioactive CTL cytotoxicity assay was developed. Effector cells were generated from normal human donor PBMCs that were expanded in culture with either intact AAV capsids or capsid-derived peptides. HLA-matched target hepatocytes and immunodominant capsid epitopes were chosen based upon the haplotype of the effector cells used. These studies demonstrated that effector cells were able to efficiently kill hepatocytes that were loaded with AAV2 capsid peptide (Figure 4A), transduced with AAV2 vector (Figure 4B), or treated with AAV2-empty capsids that were devoid of vector genomes (Figure 4C). Cytotoxic activity was higher with increasing antigen load over a 2.5-log range of peptide concentration (Figure 4D) and 1.5-log range of vector dose (Figure 4E). These results further demonstrate that the pMHC complexes are formed as a result of AAV entry. In addition, although the level of capsid antigen presentation is relatively low, it is nevertheless sufficient to flag transduced cells for destruction by antigen-specific CTLs.
Figure 4. Cytotoxic activity against peptide-pulsed and AAV-transduced hepatocytes.
In separate experiments, the human hepatocyte cell line HHL5 expressing HLA-A*0101 was cultured with (A) 10 μg/ml of SADNNNSEY capsid peptide, or the HLA-B*0702–expressing HHL5-B7 cell line was cultured with (B) 3 × 105 MOI AAV2-F9 vector or (C) 3 × 105 MOI AAV2-empty capsids devoid of genomes. HLA-matched human PBMCs were added at the indicated effector/target (E:T) ratios in a CTL cytotoxicity assay. The percentage of specific lysis was calculated from the release of intracellular lactate dehydrogenase. To determine the effects of antigen load on CTL cytotoxicity, HHL5-B7 target cells were incubated with the indicated concentrations of VPQYGYLTL capsid peptide (D) or MOI of AAV2-F9 (E) overnight, then effectors were added at an effector/target ratio of 10:1. Effector cells were derived as follows: (A–D) normal human donor PBMCs were independently expanded for 2 rounds with capsid peptide or AAV2-empty capsid and pooled for use as effector cells or (E) human PBMCs were expanded for 2 rounds with AAV2-empty capsid only.
Protection of transduced hepatocytes from CTL-mediated lysis by soluble TCR multimers.
The studies above support previous observations from a human clinical trial of AAV-mediated hepatic gene transfer in which transgene expression was transient and coincided with self-limited elevations in circulating markers of hepatocyte injury (1). In addition, AAV capsid–specific CTL populations were found to expand and contract in temporal correlation with serologic hepatitis (2). The experiments presented here provide direct evidence for capsid-derived antigen presentation following AAV-mediated transduction and subsequent lysis of transduced hepatocytes by CTLs. Together, these studies indicate that CTL responses directed against AAV capsid antigen are a potential barrier to successful gene therapy in the liver. One approach to overcome this immunologic limitation could be to use TCR multimers to inhibit antigen recognition by CTLs by competing for pMHC complexes (12).
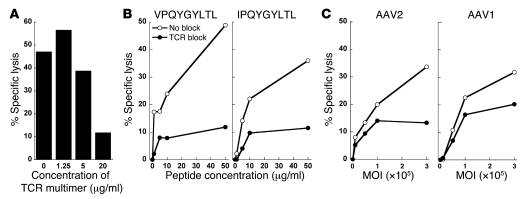
To determine whether CTL-mediated cytotoxicity could be abrogated by soluble TCR multimers, expanded PBMCs were cultured with AAV-transduced hepatocytes in the presence of increasing concentrations of TCR multimers. Indeed, TCR multimers inhibited cytolysis of sensitized target cells in a dose-dependent manner (Figure 5A). At 20 μg/ml, TCR multimers abrogated CTL-mediated destruction by more than 70%. This concentration of reagent was effective at blocking lysis of hepatocytes sensitized with AAV2-derived capsid peptide (Figure 5B) or AAV2 vectors (Figure 5C) by 68%–76% over a broad range of antigen doses. Consistent with the capsid antigen cross-reactivity of TCR multimers observed previously (Figure 1A), the reagent was also able to inhibit the lysis of hepatocytes treated with AAV1-derived capsid peptide (IPQYGYLTL) or AAV1 vector by up to 60%, although less effectively than for AAV2-directed responses. Together, these results demonstrate that CTLs require TCR recognition of capsid-derived pMHC complexes to mediate destruction of AAV-transduced human hepatocytes, and that transduced cells can be protected from CTL-mediated destruction in the presence of our soluble TCR multimer.
Figure 5. TCR multimers block CTL cytotoxicity.
(A) Normal human donor PBMCs were expanded for 3 rounds with capsid peptide or AAV2-empty capsid and pooled for use as effector cells. HLA-matched HHL5-B7 target cells were transduced with AAV2-F9 at 3 × 105 MOI. Effectors and target cells were then plated at an effector/target ratio of 10:1 in the absence or presence of indicated concentrations of TCR multimer. (B and C) In a separate experiment, HHL5-B7 hepatocytes were cultured with either AAV-derived capsid peptides (B) or AAV vectors (C) at the indicated concentrations. The following day, effector cells were added at an effector/target ratio of 10:1 in the absence (open circles) or presence (filled circles) of 20 μg/ml TCR multimer. Effectors were derived from pooled human PBMCs expanded for 1 round with capsid peptide or AAV2-empty capsid.
Discussion
An ongoing controversy in the gene therapy field is whether an effective CD8+ T cell response to vector-derived capsid sequences arises following transduction by AAV vectors. Studies of AAV-mediated gene transfer in rodents, canines, and non-human primates demonstrate long-term transgene expression and no evidence of a CD8+ T cell response to capsid (17–22). These results are in contrast to findings from a clinical trial in which hepatic infusion of AAV resulted in a transient, asymptomatic rise in liver enzymes coincident with loss of transgene expression (1). Using MHC pentamers, our group previously documented the expansion and contraction of capsid-specific CD8+ T cells in a human subject following infusion with AAV vector (2). The temporal kinetics of expansion and contraction of this antigen-specific population closely matches the asymptomatic rise and fall in liver enzymes. These observations suggested that host CTL responses directed against AAV capsid resulted in destruction of transduced hepatocytes, thereby accounting for the transient rise in hepatic enzymes and loss of transgene expression. However, there is some skepticism regarding this hypothesis, as the vector does not carry gene sequences for capsids. Consequently, it is unclear whether pre-formed capsids could gain entry into MHC class I antigen presentation pathways in sufficient quantity to drive effective capsid-directed CTL responses (23). The studies presented herein were thus undertaken in an effort to determine the molecular and cellular basis for what appeared to be destruction of transduced hepatocytes in humans infused with AAV vector.
Using our TCR multimer reagent, we have demonstrated that low levels of AAV capsid–derived antigen are indeed presented on MHC I by hepatocytes. Endogenous expression levels of MHC I on hepatocytes in vivo are not as trivial as once thought, as class I is detectable on normal hepatocytes in mice and humans and therefore is less likely to be rate limiting for antigen presentation (24–26). However, the molecular fate of AAV capsid after cellular entry, endosomal trafficking, and delivery of its genetic payload is not completely understood. In particular, it remains unclear how AAV engages class I antigen presentation pathways in vivo, as capsid is not being expressed but rather is an exogenous antigen (27). It has been demonstrated that ubiquitination of AAV capsid proteins occurs after endocytosis (28, 29) and is facilitated by EGFR-PTK signaling (30). Proteasomal inhibitors can augment transduction, suggesting that AAV capsids are degraded by proteasomes and that this process limits transduction efficiency (28, 29, 31–33). If a pre-formed capsid engages antigen processing machinery while still within endosomes, then cross-presentation pathways are likely involved (34, 35). In this regard, both liver sinusoidal endothelial cells (36) and hepatocytes (37) have been shown to be capable of presenting exogenous antigen, although the molecular details have yet to be completely elucidated. On the other hand, cross-presentation may not be required, as AAV must escape from the endosome to release its DNA. Intact pre-formed proteins in the cytosol can access MHC I pathways, as cytosolic microinjection of ovalbumin protein results in subsequent antigen presentation on MHC I (38, 39). Thus, free capsid in the cytosol may be similarly inducted into MHC I pathways without de novo protein synthesis. These possibilities are not mutually exclusive, and it is not clear whether species differences in hepatocyte antigen presentation exist as well.
We have also shown that both AAV vectors containing DNA and empty capsid particles were able to sensitize hepatocytes for cytolysis, but only the former is capable of mediating gene transfer. This underscores the importance of minimizing contamination by empty capsids during the manufacturing process (40), to increase transduction efficiency while reducing capsid antigen burden. The low numbers of pMHC complexes we observed by confocal microscopy after AAV transduction were immunologically relevant, as they rendered the transduced hepatocytes effective targets for cytolysis. Indeed, T cells have been shown to have exquisite sensitivity for antigen. Using cell lines that stably express HIV-1 to recover endogenously processed viral peptides, it has been demonstrated that target cells can be sensitized by as few as 10–100 peptides for lysis by HIV-specific CTLs (41). In an alternative approach, CTLs have been shown to have detectable increases in intracellular calcium after stimulation by 1 pMHC complex, inducible cytotoxic activity by 1–3 complexes, and maximal calcium flux with mature immunologic synapse formation by 8–10 complexes (42, 43). Thus it is not surprising that capsid-derived pMHC complexes were undetectable by flow cytometry, at the threshold for detection by confocal microscopy, and yet immunologically sufficient to trigger CTL-mediated destruction, even at 1/30th the dose of AAV vector used for visualization by confocal microscopy.
One unresolved puzzle is why the loss of transgene expression observed in a human clinical trial (1) was not predicted by rodent, canine, and nonhuman primate animal models of AAV-mediated gene transfer in which transgene expression is long lived (17–22). Perhaps the most obvious influence is that many humans have had antecedent infections with wild-type AAV2 and harbor memory CD8+ T cells not present in animal models. Also, clearly this is not the first example in which animal models have failed to recapitulate human immune responses. In a phase I clinical trial the superagonist anti-CD28 monoclonal antibody TGN1412 induced a massive cytokine storm in human recipients, resulting in multiorgan failure in 6 healthy volunteers at 1/500th the dose that appeared safe in mice and nonhuman primates (44). Subsequent investigation demonstrated that superagonist activation of CD28 leads to a sustained calcium influx in human T cells, but not cynomolgus or rhesus monkey T cells (45). Human T cells also proliferate more vigorously after TCR activation than do T cells from chimpanzees, and this difference has been attributed to loss of inhibitory Siglec expression during human evolution (46). Differences between human and murine T cells also exist, as type I IFNs can drive T cell IFN-γ production from humans but not mice due to divergent regulation of STAT1 signaling pathways (47, 48). Together, these observations indicate that human T cells are more sensitive to stimulation than their rodent or nonhuman primate counterparts and may account for the differences observed between human and animal studies with AAV-mediated gene therapy.
Finally, we have demonstrated a potential therapeutic application for soluble TCR multimers in specifically blocking T cell responses. By competing with CTLs for capsid-derived pMHC complexes, TCR multimers can interfere with immunologic synapse formation between the T cell and the antigen-presenting cell. TCR multimers have previously been shown to inhibit CTL expansion (13) and secretion of MIP-1β (12) following antigenic stimulation. We now show, for what we believe is the first time, that soluble TCR multimers are also capable of protecting cells from CTL-mediated cytolysis. This approach provides specificity to the inhibition of T cell responses, in contrast to the broad immunosuppression induced by corticosteroids or other nontargeted agents. We have demonstrated the utility of TCR multimers in preventing destruction of AAV-transduced hepatocytes in culture. This approach could potentially be generalized for other CTL-mediated diseases, such as type I diabetes mellitus or autoimmune hepatitis. However, the TCR would need to be tailored to an individual’s HLA allele and to the immunodominant epitope of interest. Further studies are necessary to delineate the optimal dosing, pharmacokinetics, and potential immunogenicity of soluble TCR therapeutics.
In conclusion, our studies provide mechanistic evidence to account for the observed loss of F9 expression and self-limited rise in aminotransferases following hepatic gene transfer by AAV in humans. These data provide the rationale for an ongoing clinical study in which AAV-mediated gene transfer to the liver is accompanied by a short course of immunomodulation to block the CD8+ T cell response to capsid (49). These results have implications for future clinical trials of AAV-mediated hepatic gene transfer and provide insights into how successful long-lived gene transfer might be achieved.
Methods
AAV vectors, peptides, and HLA pentamers.
AAV vectors were produced using previously described triple transfection methods into HEK-293 cells and subsequent CsCl density gradient purification (50). HLA-A*0101–restricted peptide from AAV2 (SADNNNSEY) and HLA-B*0702–restricted peptides derived from AAV2 (VPQYGYLTL), AAV8 (IPQYGYLTL), HIV-1 env gp120 (IPRRIRQGL), and EBV EBNA-3A (RPPIFIRRL) were commercially synthesized (Genemed Synthesis) (1–4). Custom HLA pentamers were obtained (ProImmune) and are referred to by their specific HLA molecule and loaded epitope, e.g., pB7[AAV] are pentameric complexes of HLA-B*0702 loaded with VPQYGYLTL peptide derived from the AAV2 capsid.
Cell lines.
Except as indicated, all cells were grown in MEM (ATCC) supplemented with 10% FBS, 1% l-glutamine (Gibco), and 1% penicillin-streptomycin (Gibco) at 37°C in 5% CO2. The following HLA-B*0702– cell lines were used: human embryonic kidney–derived HEK-293 cells (ATCC CRL-1573) and the human hepatocyte cell line HHL5 (51). The following HLA-B*0702+ cell lines were used: B lymphoblastoid JY cells grown in RPMI 1640 (Gibco) with 10% FBS and 1% penicillin-streptomycin, lung squamous cell carcinoma–derived SK-MES-1 (ATCC HTB-58), and HHL5-B7 cells derived from HHL5 cells that were transduced to express HLA-B*0702. Briefly, a VSV-pseudotyped lentivirus containing a CMV-based human HLA-B*0702 expression cassette was generated using a ViraPower HiPerform lentiviral expression system (Invitrogen). The HLA-B*0702 gene was cloned by RT-PCR from normal human donor splenocytes (Cooperative Human Tissue Network, NCI), then TOPO-TA–cloned into pLenti6.3/V5-TOPO to create pLenti-B*0702. 293FT cells (5 × 106 cells in a 10-cm dish) were transfected with pLenti-B*0702 in combination with ViraPower packaging mix. After 3 days the medium was harvested and centrifuged, and the supernatant containing lentivirus was aliquoted and stored at –80°C. HHL5 cells were plated into 6-well plates at 2.5 × 105 cells/well. The following day, culture medium was replaced with 2 ml transduction medium (1.8 ml fresh culture medium with 0.2 ml Lenti-B*0702 supernatant) supplemented with 3 μg/ml polybrene (Sigma-Aldrich). After overnight incubation, transduction medium was replaced with fresh culture medium containing 10 μg/ml blasticidin (Invitrogen). Five days later, cells were transferred to a 10-cm dish and allowed to expand in blasticidin-containing medium. After selection, HLA-B*0702 expression was confirmed (≥ 95%) by staining with PE-conjugated anti–HLA-B7 (US Biological) and flow cytometry.
Generation of TCR multimers.
Normal human PBMCs from an HLA-B*0702+ donor were cultured with 10 μg/ml of AAV peptide (VPQYGYLTL) as previously described (2). After 2 rounds of expansion, cells were stained with PE-conjugated pB7[AAV], allophycocyanin-conjugated (APC-conjugated) anti-CD8α antibody (BD Biosciences), and a mix of APC-Cy7–conjugated anti-CD4 and anti-CD14 antibodies (BD Biosciences). Cells were then sorted for CD8+pB7[AAV]+CD4–CD14– populations on a FACSVantage SE (BD Biosciences). Sorted cells were plated by limiting dilution into round-bottom 96-well plates containing 75,000 irradiated allogeneic PBMCs (3,300 rad) and 10,000 irradiated JY cells (8,000 rad) per well in 100 μl of RPMI 1640 supplemented with 10% human serum (Valley Biomedical), 1% Pen-Strep, 1% l-glutamine, 50 U/ml recombinant human IL-2 (Roche), and 5 μg/ml phytohemagglutinin (Sigma-Aldrich). Single-cell clones were cultured for 12 days, then restained for flow cytometry. Two clones in which 100% of the cells were CD8+pB7[AAV]+ were shipped to Altor Bioscience for generation of soluble, biotinylated TCR monomers. Briefly, TCR-α and -β chains were cloned by SMART-RACE PCR, and a fusion protein was constructed containing a BirA biotinylation tag (14). The construct was expressed in CHO cells for purification of soluble TCR monomers. Multimerization was done by conjugation with streptavidin-conjugated APC (Invitrogen) in a 1:1 molar ratio of biotin/streptavidin.
Determination of binding affinity by ELISA.
A Nunc PolySorp 96-well plate (Thermo Fisher Scientific) was coated with TCR multimer in carbonate buffer at pH 9.6. After overnight incubation at 4°C, the plate was washed 5 times with D-PBS/0.05% Tween-20, then blocked with D-PBS/0.05% Tween-20/6% BSA for 30 minutes at room temperature. After 5 washes, increasing concentrations of biotinylated pB7[AAV] were added to the wells and incubated for 2 hours at room temperature. The plate was then washed 10 times before incubating with a 1:500 dilution of streptavidin-conjugated HRP (BD Biosciences) for 2 hours at room temperature. After 10 washes, detection was performed using o-phenylenediamine dihydrochloride (Sigma-Aldrich) in a phosphate-citrate buffer with hydrogen peroxide. Absorbance was read on a SpectraMax M2e (Molecular Devices) at 450 nm.
Detection of pMHC complexes by flow cytometry.
To stain for pMHC complexes after peptide loading, 105 cells in 0.5 ml medium were plated per well on 24-well tissue culture plates (BD Biosciences). Cells were incubated with peptides for 2 hours at 37°C and washed prior to staining. Cells were stained with 0.5 μg APC-conjugated TCR multimer at 4°C for 30 minutes. Samples were washed twice with D-PBS/2% FBS/0.05% sodium azide, then fixed with 4% PFA at 4°C for 10 minutes. At least 50,000 events were acquired on a FACS Canto II (BD Biosciences) using FACSDiva version 6.1.1 (BD Biosciences). For fluorescence quantitation, a panel of APC calibration beads containing discrete numbers of fluorochrome molecules (Bangs Laboratories) was used to set PMT voltages prior to data acquisition. Flow cytometry data were analyzed using FlowJo version 8.7.1 (Tree Star). Calculation of MESF from MFI values was performed using QuickCal version 2.3 (Bangs Laboratories).
Detection of pMHC complexes by confocal microscopy.
To prepare specimens for confocal microscopy, cells were grown on 4-chamber Lab-Tek II slides (Thermo Fisher Scientific), then treated with capsid peptide (VPQYGYLTL) or AAV2-F9 vector. One day later, slides were washed twice with D-PBS, then blocked with 1% BSA in D-PBS for 20 minutes at room temperature. Slides were stained with 0.5 μg APC-conjugated TCR multimer and 6.25 ng Alexa Fluor 488–labeled WGA (Invitrogen) in 200 μl volume for 30 minutes, washed 3 times, then fixed in 3.7% formaldehyde. Specimens were washed, excess moisture was removed, and slides were cover-slipped using Prolong Gold with DAPI (Invitrogen). After curing overnight, slides were sealed with acrylic nail polish. Slides were imaged using an Olympus IX-81 DSU spinning disc confocal microscope equipped with a Hamamatsu Photonics EM-CCD camera. Exposure times for individual filter sets and gain multiplication settings were established for each experiment using unstained specimens to determine maximal settings. These settings were then adjusted to ensure no cross-channel bleed-through using positive controls (WGA and TCR multimer–stained, peptide-loaded samples). Once established, the same microscope settings were used to acquire all images. Images were analyzed with Volocity (Improvision). Cell surface and TCR multimer–binding regions were defined by creating an absolute pixel intensity threshold to include WGA or TCR staining that did not generate an area with negative controls. Once identified, this same absolute threshold was used to define regions in all samples. Regions in individual cells were measured in 17–25 randomly selected cells per experimental condition. To determine the cell surface quantities of TCR multimer, the intensity of individual pixel regions containing both WGA and TCR fluorescence above threshold were measured and averaged. Statistical differences were calculated using an unpaired 2-tailed Student’s t test.
CTL cytotoxicity assays.
To generate effector cells, cryopreserved PBMCs were purchased from a commercial supply of normal human donors (Cellular Technology). These studies were reviewed and approved by the Institutional Review Board of the Children’s Hospital of Philadelphia. Cells were expanded with either AAV2-empty capsids or VPQYGYLTL capsid peptide as described above and restimulated every 7 days for the indicated number of expansion rounds. HLA-matched target cells were prepared by plating 5,000 HHL5 cells/well in Primaria flat-bottom 96-well plates (Falcon) with serum-free DMEM (Gibco) and culturing with either 10 μg/ml HLA-matched capsid peptide or 3 × 105 MOI AAV2-F9 for 18 hours. Target cells were then washed prior to incubation with effector cells for 4 hours at the effector/target ratios indicated in the figure legends. Release of lactate dehydrogenase was detected using the CytoTox 96 non-radioactive cytotoxicity assay (Promega) and measurement of absorbance at 490 nm on a SpectraMax M2e using SoftMax Pro (Molecular Devices). The percentage of specific lysis was calculated after subtraction of the appropriate background controls.
Supplementary Material
Acknowledgments
This work was supported by institutional funding from the Children’s Hospital of Philadelphia (to G.C. Pien) and the Howard Hughes Medical Institute (to K.A. High); by NIH grants R01 AI067946S1 (to A.N. Mentlik), R01 AI067946 (to J.S. Orange), and P01 HL078810 (to K.A. High); and by NIH training grants T32 HL07439 (to D.J. Hui), T32 HL007150 (to N.C. Hasbrouck), and T32 DK07748 (to S.L. Murphy). We thank Harre Downey, Danielle Dunn, Shyrie Edmonson, and Heshu Huang for expert technical assistance and Siriam Krishnaswamy and Hing Wong for scientific input and helpful discussions.
Footnotes
Authorship note: Gary C. Pien and Etiena Basner-Tschakarjan contributed equally to this work.
Conflict of interest: Patents are held on the soluble TCR and HLA pentamers bearing AAV capsid peptides (D.J. Hui, M.V. Maus, F. Mingozzi, and K.A. High) and on certain recombinant AAV technologies (K.A. High). F. Mingozzi is a consultant for Arthrogen BV, and K.A. High is a consultant for Tacere Therapeutics Inc. In addition, the spouse of K.A. High is an employee of Wyeth and holds stock in the company. Genzyme has provided funding for an AAV-mediated hemophilia B clinical trial.
Nonstandard abbreviations used: AAV, adeno-associated virus; APC, allophycocyanin; F9, Factor IX gene; MESF, molecules of equivalent soluble fluorochrome; pMHC, peptide:MHC I; WGA, wheat germ agglutinin.
Citation for this article: J. Clin. Invest. 119:1688–1695 (2009). doi:10.1172/JCI36891
References
- 1.Manno C.S., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 2.Mingozzi F., et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 3.Sabatino D.E., et al. Identification of mouse AAV capsid-specific CD8+ T cell epitopes. Mol. Ther. 2005;12:1023–1033. doi: 10.1016/j.ymthe.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Figueredo J., Calcedo R., Lin J., Wilson J.M. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- 5.Li C., et al. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J. Virol. 2007;81:7540–7547. doi: 10.1128/JVI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H., et al. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 2007;15:792–800. doi: 10.1038/sj.mt.6300090. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H., et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mingozzi F., et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mingozzi F., et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao O., et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman J.D., et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 12.Laugel B., et al. Design of soluble recombinant T cell receptors for antigen targeting and T cell inhibition. J. Biol. Chem. 2005;280:1882–1892. doi: 10.1074/jbc.M409427200. [DOI] [PubMed] [Google Scholar]
- 13.Subbramanian R.A., et al. Engineered T-cell receptor tetramers bind MHC-peptide complexes with high affinity. Nat. Biotechnol. 2004;22:1429–1434. doi: 10.1038/nbt1024. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X., et al. Visualization of p53(264-272)/HLA-A*0201 complexes naturally presented on tumor cell surface by a multimeric soluble single-chain T cell receptor. J. Immunol. 2006;176:3223–3232. doi: 10.4049/jimmunol.176.5.3223. [DOI] [PubMed] [Google Scholar]
- 15.Davis M.M., et al. Ligand recognition by alpha beta T cell receptors. Annu. Rev. Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 16.Anikeeva N., Mareeva T., Liu W., Sykulev Y. Can oligomeric T-cell receptor be used as a tool to detect viral peptide epitopes on infected cells? Clin. Immunol. 2009;130:98–109. doi: 10.1016/j.clim.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mount J.D., et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.V99.8.2670. [DOI] [PubMed] [Google Scholar]
- 18.Snyder R.O., et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat. Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 19.Wang L., et al. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol. Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- 21.Nathwani A.C., et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- 22.Arruda V.R., et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services. 2007. Minutes of the Recombinant DNA Advisory Committee Meeting, June 19–21, 2007. Department of Health and Human Services, Public Health Service, NIH, Bethesda, Maryland, USA. http://oba.od.nih.gov/oba/rac/minutes/RAC_minutes_06-07.pdf. [Google Scholar]
- 24.Chen M., Tabaczewski P., Truscott S.M., Van Kaer L., Stroynowski I. Hepatocytes express abundant surface class I MHC and efficiently use transporter associated with antigen processing, tapasin, and low molecular weight polypeptide proteasome subunit components of antigen processing and presentation pathway. J. Immunol. 2005;175:1047–1055. doi: 10.4049/jimmunol.175.2.1047. [DOI] [PubMed] [Google Scholar]
- 25.Senaldi G., Lobo-Yeo A., Mowat A.P., Mieli-Vergani G., Vergani D. Class I and class II major histocompatibility complex antigens on hepatocytes: importance of the method of detection and expression in histologically normal and diseased livers. J. Clin. Pathol. 1991;44:107–114. doi: 10.1136/jcp.44.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu J.H., et al. Class I and class II major histocompatibility complex antigens expression on human hepatocytes and hepatoma cells: an approach with high sensitivity and specificity. Cytometry. 1997;30:317–323. doi: 10.1002/(SICI)1097-0320(19971215)30:6<317::AID-CYTO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Hauck B., et al. Undetectable transcription of cap in a clinical AAV vector: implications for preformed capsid in immune responses. Mol. Ther. 2009;17:144–152. doi: 10.1038/mt.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan D., Yue Y., Yan Z., Yang J., Engelhardt J.F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Z., et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong L., et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol. Ther. 2007;15:1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 31.Douar A.M., Poulard K., Stockholm D., Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J. Virol. 2001;75:1824–1833. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding W., et al. Second-strand genome conversion of adeno-associated virus type 2 (AAV-2) and AAV-5 is not rate limiting following apical infection of polarized human airway epithelia. J. Virol. 2003;77:7361–7366. doi: 10.1128/JVI.77.13.7361-7366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hacker U.T., et al. Adeno-associated virus serotypes 1 to 5 mediated tumor cell directed gene transfer and improvement of transduction efficiency. J. Gene Med. 2005;7:1429–1438. doi: 10.1002/jgm.782. [DOI] [PubMed] [Google Scholar]
- 34.Houde M., et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 35.Guermonprez P., et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 36.Limmer A., et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 37.Bongfen S.E., Torgler R., Romero J.F., Renia L., Corradin G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J. Immunol. 2007;178:7054–7063. doi: 10.4049/jimmunol.178.11.7054. [DOI] [PubMed] [Google Scholar]
- 38.Craiu A., Akopian T., Goldberg A., Rock K.L. Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10850–10855. doi: 10.1073/pnas.94.20.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalek M.T., Grant E.P., Rock K.L. Chemical denaturation and modification of ovalbumin alters its dependence on ubiquitin conjugation for class I antigen presentation. J. Immunol. 1996;157:617–624. [PubMed] [Google Scholar]
- 40.Qu G., et al. Separation of adeno-associated virus type 2 empty particles from genome containing vectors by anion-exchange column chromatography. J. Virol. Methods. 2007;140:183–192. doi: 10.1016/j.jviromet.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Tsomides T.J., et al. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J. Exp. Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sykulev Y., Joo M., Vturina I., Tsomides T.J., Eisen H.N. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/S1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 43.Purbhoo M.A., Irvine D.J., Huppa J.B., Davis M.M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 44.Suntharalingam G., et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 45.Waibler Z., et al. Signaling signatures and functional properties of anti-human CD28 superagonistic antibodies. PLoS ONE. 2008;3:e1708. doi: 10.1371/journal.pone.0001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen D.H., Hurtado-Ziola N., Gagneux P., Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen K.B., et al. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat. Immunol. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 48.O’Shea J.J., Visconti R. Type 1 IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nat. Immunol. 2000;1:17–19. doi: 10.1038/76872. [DOI] [PubMed] [Google Scholar]
- 49.US Department of Health and Human Services. 2007. Minutes of the Recombinant DNA Advisory Committee Meeting, December 3–5, 2007. Department of Health and Human Services, Public Health Service, NIH, Bethesda, Maryland, USA. http://oba.od.nih.gov/oba/rac/minutes/RAC_minutes_12-07.pdf. [Google Scholar]
- 50.Grimm D., et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- 51.Clayton R.F., et al. Liver cell lines for the study of hepatocyte functions and immunological response. Liver Int. 2005;25:389–402. doi: 10.1111/j.1478-3231.2005.01017.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.