Abstract
Type 1 diabetes (T1D) is an autoimmune disease that is caused by the destruction of insulin-producing β cells. Viral infections induce immune responses that can damage β cells and promote T1D or on the other hand prevent the development of the disease. However, the opposing roles of viral infections in T1D are not understood mechanistically. We report here that viruses that do not inflict damage on β cells provided protection from T1D by triggering immunoregulatory mechanisms. Infection of prediabetic NOD mice with Coxsackie virus B3 or lymphocytic choriomeningitis virus (LCMV) delayed diabetes onset and reduced disease incidence. Delayed T1D onset was due to transient upregulation of programmed cell death–1 ligand 1 (PD-L1) on lymphoid cells, which prevented the expansion of diabetogenic CD8+ T cells expressing programmed cell death–1 (PD-1). Reduced T1D incidence was caused by increased numbers of invigorated CD4+CD25+ Tregs, which produced TGF-β and maintained long-term tolerance. Full protection from T1D resulted from synergy between PD-L1 and CD4+CD25+ Tregs. Our results provide what we believe to be novel mechanistic insight into the role of viruses in T1D and should be valuable for prospective studies in humans.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease that results from immune-mediated destruction of insulin-producing β cells in the pancreatic islets of Langerhans and leads to severely deficient glucose metabolism (1). While T1D is a polygenic disorder, it is under the critical influence of epigenetic or environmental parameters, such as infections, that can either promote or diminish autoimmunity (2). Viruses are prime candidates for infectious risk factors because they can induce strong cellular immune responses and in some cases infect and damage β cells while causing local inflammation in the pancreas. These phenomena could account for presentation of β cell antigens to autoreactive T cells, resulting in their activation and attack on β cells. On the other hand, epidemiological and experimental evidence indicates that infections, and most notably those mediated by viruses, can efficiently prevent T1D (supporting the “hygiene hypothesis”). Whether a particular virus could cause or prevent T1D thus still remains unknown and is subject to much debate (3). Active investigation on this topic is currently undertaken in humans (4) as well as animal models of T1D, such as NOD mice, which develop spontaneous autoimmune diabetes that mimics many aspects of human disease. A number of different viruses can modulate the outcome of T1D in NOD mice; notably, Coxsackie virus B (CVB) and lymphocytic choriomeningitis virus (LCMV) strain Armstrong have been shown to bear both enhancing and protective capacity in NOD T1D, depending on the context. While CVB4 promotes T1D in NOD mice (5) (though possibly depending on the time point at which infection occurs; ref. 6), CVB3 significantly protects these mice from diabetes (7). And while LCMV is used as a means to induce T1D experimentally in NOD (and other) mice that transgenically express LCMV antigens in their β cells (8–10), this virus otherwise efficiently prevents T1D in these mice (11, 12). These observations suggest that when no direct β cell damage is caused during infection, pancreatotropic viruses such as CVB and LCMV can be tolerogenic rather than diabetogenic.
Prevention of T1D can be achieved in NOD mice by a number of viruses or noninfectious compounds that paradoxically induce strong inflammation and immunity activity locally in the pancreas (while sparing β cells) during the prediabetic phase (13). However, one key feature of inflammation and antiviral immunity often overlooked with respect to autoimmunity is that they have the potential to cause severe immune pathology and are therefore highly regulated by a number of feedback mechanisms that ensure that the cells participating in antiviral immunity are rapidly eliminated or kept under control following their activation. Such immunoregulatory mechanisms notably comprise (a) the expression of inhibitory molecules such as programmed cell death–1 (PD-1) on activated T cells, (b) the activation or induction of CD4+CD25+Foxp3+ Tregs, and (c) the production of suppressor cytokines such as TGF-β. Of importance is that these different factors all constitute major regulators of not only antiviral but also autoimmune responses: the PD-1 ligand 1 (PD-L1)/PD-1 interaction exerts efficient control over expansion of antiviral T cells, to the point where it is exploited by certain viruses to mediate T cell exhaustion or elimination, and ensuing viral persistence (14, 15); but this pathway also plays a critical role in the control of autoimmunity (16, 17), and in T1D it has been shown to maintain tolerance during the prediabetic phase or after therapy (18, 19). CD4+CD25+Foxp3+ Tregs are crucial for the maintenance of tolerance in the periphery (20) and can be activated or generated therapeutically to confer long-term protection from T1D (21); but these cells also appear to actively participate in the control of antiviral T cell responses, possibly with the goal of limiting immune pathology that can be caused by acute and robust antiviral mechanisms (22–26). Finally, TGF-β is a cytokine required for normal immune homeostasis (27, 28) and bearing suppressive properties that can confer efficient protection from T1D (29–33); but the suppressive effect of this cytokine also permits the control of T cells during viral infection, which is crucial for the regulation of antiviral immunity (34). In sum, similar mechanisms seem to control antiviral immunity and autoimmunity in T1D.
We thus hypothesized that immunity to viral infections might be under the control of regulatory mechanisms that, provided no extensive damage to β cells occurs, would confer protection from T1D. We used CVB3 and LCMV, which prevent rather than trigger T1D in NOD mice, and found that 2 distinct control mechanisms are indeed induced during immunity to these viruses and can operate in synergy to efficiently abort the autoimmune process leading to T1D: upregulation of PD-L1 and enhancement of CD4+CD25+Foxp3+ Tregs producing TGF-β. Our findings provide a mechanistic basis for virally mediated protection from T1D and indicate that the opposing roles of viral infections in this disease might be related to their ability to promote, on one hand, β cell damage and, on the other hand, immunoregulation.
Results
Acute infection of prediabetic mice with CVB3 or LCMV delays T1D onset and diminishes disease incidence.
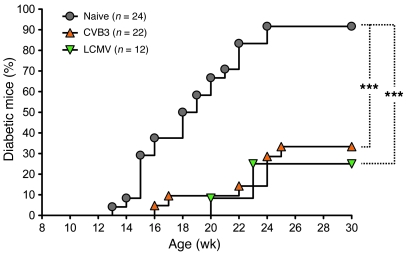
In the NOD model of spontaneous autoimmune diabetes, we used 2 different RNA viruses that, upon infection, prevent rather than induce the disease: CVB3, an enterovirus that causes a systemic, acute infection that is nonpersisting but leads to immune-mediated myocarditis; and LCMV Armstrong, an arenavirus that causes a systemic, acute infection that is asymptomatic and also nonpersisting. The majority of mice infected with CVB3 or LCMV did not develop T1D (Figure 1) (although mice challenged with CVB3 developed signs of virus-related illness). This effect of these viruses on T1D development was observed in earlier studies by us and others (7, 11, 12), but the precise underlying mechanisms were not elucidated. Interestingly, we observed that CVB3 and LCMV infections had 2 distinct effects on T1D — a delay in the onset of overt diabetes and a decrease in disease incidence, which we sought to explain mechanistically.
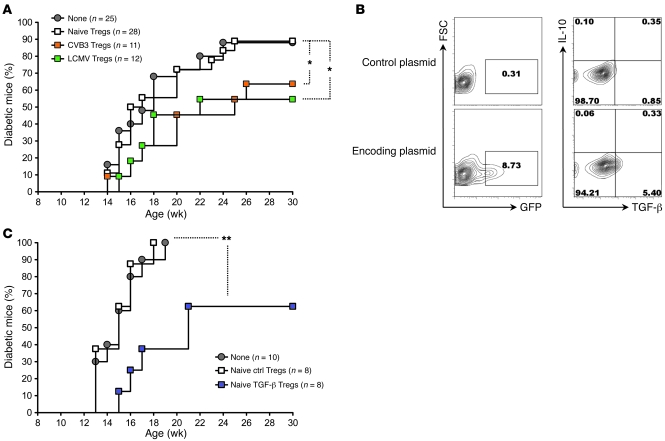
Figure 1. Acute infection of prediabetic NOD mice with CVB3 or LCMV delays T1D onset and diminishes disease incidence.
Cumulative diabetes incidence over time in NOD mice left untreated (Naive) or infected at 9 weeks of age with CVB3 (orange triangles) or LCMV (green inverted triangles). ***P < 0.001.
Viral infection of prediabetic mice causes PD-L1 upregulation on lymphoid cells and prevents the expansion of diabetogenic CD8+ T cells expressing PD-1.
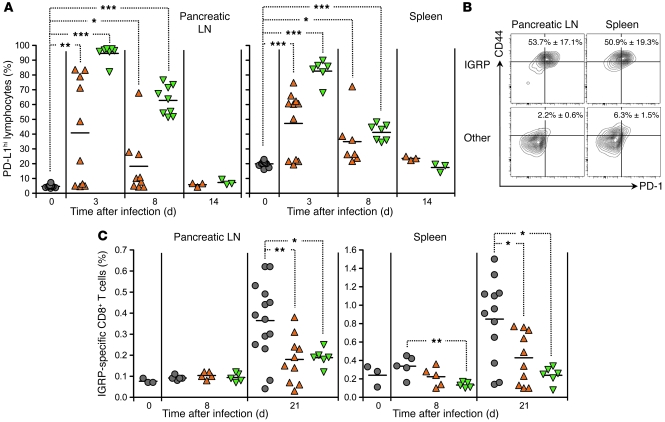
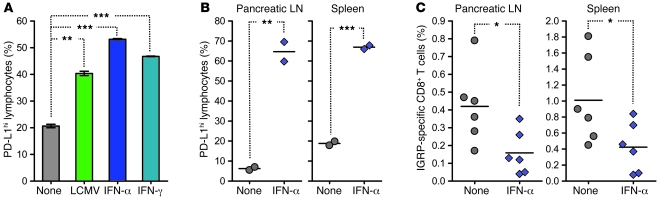
We first assessed the early changes that occurred in the lymphoid organs of NOD mice infected with CVB3 or LCMV. In particular, we assessed accessory, inhibitory molecules often upregulated during viral infection. Expression of PD-L1 was variable in CVB3-infected mice, but both CVB3 and LCMV strongly increased PD-L1 levels transiently on lymphoid cells in the pancreatic LNs and spleen of NOD mice (Figure 2A and Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI38503DS1). At the time at which infection was performed, the majority of autoreactive, diabetogenic CD8+ T cells specific for the β cell antigen islet-specific glucose-6-phosphate catalytic-related protein (IGRP) (35, 36) showed an activated phenotype and expressed the PD-L1 receptor PD-1 (Figure 2B). The PD-L1/PD-1 interaction has been shown to control not only T cell immunity in viral infection (14, 15), but also autoimmune T cell responses, notably in T1D (16, 18). Our observations thus suggested that viral infection of NOD mice rendered diabetogenic CD8+ T cells highly susceptible to PD-1–mediated impairment. Accordingly, whereas, as previously described (35), IGRP-specific CD8+ T cells expanded significantly in the pancreatic LN and spleen of untreated NOD mice, the frequency of these cells remained low in mice infected with CVB3 or LCMV (Figure 2C).
Figure 2. CVB3 or LCMV infection of prediabetic NOD mice causes PD-L1 upregulation on lymphoid cells and prevents the expansion of IGRP-specific CD8+ T cells expressing PD-1.
(A) Percentage of PD-L1hi cells over time in the pancreatic LN and spleen of individual NOD mice left untreated or infected at 9 weeks of age with CVB3 (orange triangles) or LCMV (green inverted triangles), as measured by flow cytometry. (B) PD-1 and CD44 expression by IGRP-specific or -nonspecific (Other) CD8+ T cells in the pancreatic LN and spleen of 9-week-old naive NOD mice, as measured by flow cytometry after staining with NRP-V7 tetramer. Shown are representative flow cytometry contour plots. Numbers indicate the percentage of PD-1+CD44hi cells ± SD for 3 individual mice per group. (C) Percentage of IGRP-specific CD8+ T cells over time in the pancreatic LN and spleen of individual NOD mice left untreated or infected at 9 weeks of age with CVB3 or LCMV, as measured by flow cytometry after staining with NRP-V7 tetramer. In A and C, symbols represent individual values, and horizontal lines denote mean. *P < 0.05, **P < 0.005, ***P < 0.001.
Virally induced upregulation of PD-L1 in prediabetic mice prevents the expansion of diabetogenic CD8+ T cells and is responsible for the delayed onset but not decreased incidence of T1D.
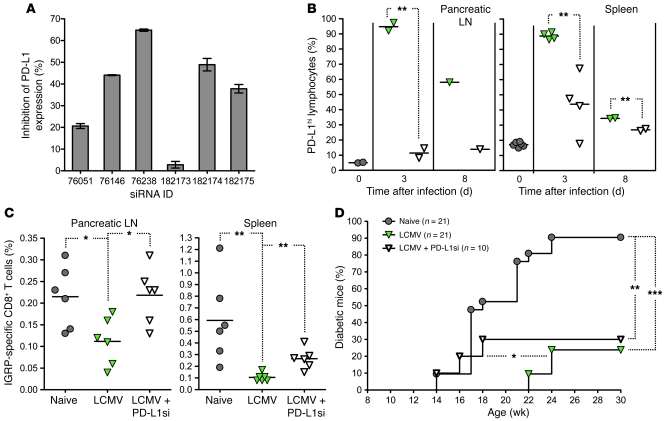
We assessed whether impaired expansion of IGRP-specific, diabetogenic CD8+ T cells was caused by the increase in PD-L1 expression triggered by viral infection. We used LCMV for this purpose, as PD-L1 was systematically upregulated in the lymphoid organs of LCMV-infected but not all CVB3-infected mice (Figure 2A). As PD-L1 blockade in NOD mice accelerated diabetes in a previous study (18), we avoided using neutralizing antibodies against PD-L1 to address this issue. Instead, we used siRNA technology with the goal of suppressing de novo expression of PD-L1 transiently in LCMV-infected mice. The efficacy of different siRNAs specific for PD-L1 was first assessed in vitro (Figure 3A and Supplemental Figure 2, A and B) to identify a candidate that could significantly inhibit upregulation of PD-L1 in the pancreatic LN and spleen of LCMV-infected NOD mice (Figure 3B). Of note, 21 days after infection, the level of expression of surface PD-L1 was reduced to normal levels in both siRNA-treated and nontreated mice (data not shown), suggesting that PD-L1 expression was indeed inhibited and not delayed by siRNA treatment. NOD mice were then challenged with LCMV and simultaneously treated with the selected PD-L1–specific siRNA or empty vehicle. We observed that LCMV infection failed to prevent the expansion of IGRP-specific CD8+ T cells in the pancreatic LN and spleen of mice treated with PD-L1 siRNA (Figure 3C). On the other hand, PD-L1 siRNA treatment did not appear to affect IGRP-specific cells in terms of PD-1 and CD44 expression (Supplemental Figure 2C). Furthermore, the delay in onset of overt diabetes mediated by LCMV infection was abolished by PD-L1 siRNA treatment, while the reduction in disease incidence was not reversed (Figure 3D). These results indicated that virally induced upregulation of PD-L1 on lymphoid cells prevented the expansion of diabetogenic CD8+ T cells and was the cause for the delayed onset but not the decreased incidence of T1D observed after viral infection.
Figure 3. LCMV-induced upregulation of PD-L1 in prediabetic NOD mice prevents the expansion of IGRP-specific CD8+ T cells and delays the onset of T1D.
(A) Different siRNAs specific for PD-L1 were assessed for efficacy by infection of NOD splenocytes for 24 hours with LCMV in vitro (MOI, 1) after transfection with different siRNAs (in duplicate). Shown is inhibition of PD-L1 upregulation, calculated as the percentage of PD-L1hi cells in populations infected with LCMV and transfected with siRNA relative to the percentage of PD-L1hi cells in the population infected with LCMV and transfected with scrambled-sequence siRNA, ± SD for duplicate samples. (B) Percentage of PD-L1hi cells over time in the pancreatic LN and spleen of individual NOD mice infected at 9 weeks of age with LCMV and simultaneously injected with cationic vehicle alone or containing 150 μg PD-L1 siRNA 76238, as measured by flow cytometry. (C) Percentage of IGRP-specific CD8+ T cells in the pancreatic LN and spleen of individual 12-week-old NOD mice injected 21 days previously with cationic vehicle alone and left untreated or simultaneously infected with LCMV (green inverted triangles) or injected 21 days previously with cationic vehicle containing PD-L1 siRNA 76238 and simultaneously infected with LCMV (white inverted triangles), as measured by flow cytometry after staining with NRP-V7 tetramer. In B and C, symbols represent individual values, and horizontal lines denote mean. (D) Cumulative diabetes incidence over time in NOD mice injected at 9 weeks of age with cationic vehicle alone and left untreated or simultaneously infected with LCMV or injected 21 days previously with cationic vehicle containing PD-L1 siRNA 76238 and simultaneously infected with LCMV. *P < 0.05, **P < 0.005, ***P < 0.001.
The frequency of CD4+CD25+ Tregs and their capacity to produce TGF-β are increased in virally immune NOD mice.
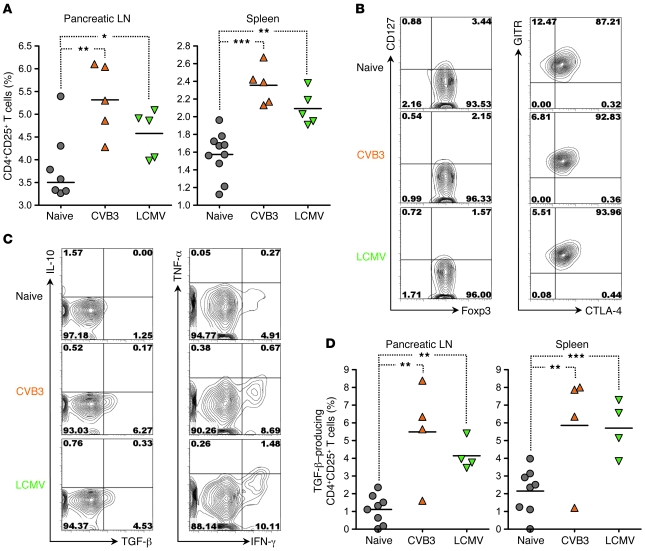
The upregulation of PD-L1 and the failure of diabetogenic CD8+ T cells to accumulate in virally infected mice caused only a delay in the onset of overt diabetes, indicating that additional factors contributed to full, long-term protection from T1D upon viral infection. It has been suggested that so-called natural CD4+CD25+ Tregs, which are crucial players in the maintenance of peripheral tolerance, progressively lose their capacity to control autoreactive T cells in the NOD model (37–39), thereby permitting T1D. We therefore assessed the number and function of these cells in virally challenged NOD mice. After viral clearance, the pancreatic LN and spleen of CVB3- and LCMV-immune NOD mice showed a significant increase in CD4+CD25+ T cells (Figure 4A). The vast majority of these cells (more than 95%) expressed Foxp3 along with low levels of CD127 (Figure 4B), indicating that they were indeed Tregs (20, 40). Furthermore, these cells expressed CTLA-4 along with high levels of glucocorticoid-induced tumor necrosis factor receptor (GITR; Figure 4B), suggesting that they might be natural Tregs (41, 42). Interestingly, while the percentage of TGF-β–producing cells was somewhat variable in CVB3-challenged mice, it was significantly higher in lymphoid organs compared with naive mice, a scenario that was also found in NOD mice following LCMV infection (Figure 4D). CD4+CD25+ T cells from both naive and virally immune mice were capable of producing IFN-γ but not IL-10 (Figure 4C). In sum, viral infection caused an increase in the frequency of CD4+CD25+ Tregs and their capacity to produce TGF-β.
Figure 4. The frequency of natural CD4+CD25+ Tregs and their capacity to produce TGF-β are increased in the lymphoid organs of CVB3- and LCMV- immune NOD mice.
(A) Percentage of CD4+CD25+ T cells in the pancreatic LN and spleen of individual 12-week-old NOD mice left untreated or infected 21 days previously with CVB3 (orange triangles) or LCMV (green inverted triangles), as measured by flow cytometry. (B) Representative flow cytometry contour plots of Foxp3, CD127, CTLA-4, and glucocorticoid-induced tumor necrosis factor receptor (GITR) expression by CD4+CD25+ T cells in the pancreatic LN of individual 12-week-old NOD mice left untreated or infected 21 days previously with CVB3 or LCMV. Quadrants were defined based on isotype control stainings showing less than 0.2% positive cells for each parameter analyzed. Numbers indicate the percentage of cells in the corresponding quadrants. Comparable results were obtained in the spleen. (C) Representative flow cytometry contour plots of TGF-β, IL-10, IFN-γ, and TNF-α expression by CD4+CD25+ T cells from the pancreatic LN of individual 12-week-old NOD mice left untreated or infected 21 days previously with CVB3 or LCMV, as measured after PMA plus ionomycin stimulation. Quadrants were defined based on isotype control stainings showing less than 0.2% positive cells for each parameter analyzed. Numbers indicate the percentage of cells in the corresponding quadrants. Comparable results were obtained in the spleen. (D) Percentage of TGF-β–producing CD4+CD25+ T cells in the pancreatic LN and spleen of individual 12-week-old NOD mice left untreated or infected 21 days previously with CVB3 or LCMV, as measured by flow cytometry after PMA plus ionomycin stimulation. In A and D, symbols represent individual values, and horizontal lines denote mean. *P < 0.05, **P < 0.005, ***P < 0.001.
CD4+CD25+ Tregs modulated by viral infection in vivo are capable of diminishing T1D incidence through TGF-β production.
We assessed whether the changes we observed in the Treg compartment of virally immune mice played a role in virally mediated prevention of T1D. We purified CD4+CD25+ T cells from naive, CVB3-immune, or LCMV-immune mice and injected them into naive, age-matched NOD recipients. The transferred CD4+CD25+ T cells were more than 95% pure and isolated 21 days after viral challenge, i.e., after viral clearance, in order to avoid injection of virus in these transfer experiments. When injected into prediabetic NOD mice, CD4+CD25+ T cells from CVB3-immune or LCMV-immune but not naive donors mediated a significant reduction in T1D incidence (Figure 5A). Based on our observation that virally exposed Tregs differed from their naive counterpart regarding TGF-β production (Figure 4, C and D), we asked whether TGF-β expression by naive Tregs would provide them with the capacity to promote tolerance in vivo. To this end, we purified CD4+CD25+ T cells from naive mice and transfected them with a TGF-β1–encoding cDNA before transferring them into naive prediabetic recipients. Transfection conditions yielded 8%–9% efficacy and allowed TGF-β expression by 4%–5% of the cells (Figure 5B), which was roughly comparable to the TGF-β production by CVB3- or LCMV-enhanced CD4+CD25+ T cells (Figure 4D). We found that naive CD4+CD25+ T cells transgenically expressing TGF-β could diminish T1D incidence in vivo (Figure 5C), suggesting that the enhanced tolerogenic function of virally modulated CD4+CD25+ T cells in T1D was exerted via their production of TGF-β.
Figure 5. CD4+CD25+ Tregs modulated during the prediabetic phase by CVB3 or LCMV infection in vivo are capable of diminishing T1D incidence in NOD mice through TGF-β production.
(A) Cumulative diabetes incidence over time in NOD mice left untreated (None) or injected at 12 weeks of age with 5 × 105 CD4+CD25+ T cells purified from 12-week-old NOD donors left untreated (Naive Tregs) or infected 21 days previously with CVB3 (CVB3 Tregs) or LCMV (LCMV Tregs). CD4+CD25– T cells from CVB3- or LCMV-immune mice had no effect on disease outcome (data not shown). (B) The efficacy of transfection in CD4+CD25+ T cells was assessed by measuring by flow cytometry GFP and TGF-β expression in naive Tregs (purified as described in A) 15 hours after transfection with an empty plasmid (Control plasmid, top) or plasmid containing a cDNA (Encoding plasmid, bottom) encoding GFP (left) or TGF-β1 (right, assessed after PMA plus ionomycin stimulation). Shown are flow cytometry contour plots representative of 2 samples per group. Numbers indicate the percentage of cells in the corresponding gate/quadrant. (C) Cumulative diabetes incidence over time in NOD mice left untreated (None) or injected at 12 weeks of age with 5 × 105 naive Tregs transfected with an empty plasmid (Naive ctrl Tregs) or plasmid containing a cDNA encoding human TGF-β1 (Naive TGF-β Tregs). *P < 0.05, **P < 0.005.
Treatment of prediabetic mice with IFN-α induces PD-L1 upregulation on lymphoid cells and prevents the expansion of diabetogenic CD8+ T cells in vivo.
We investigated the mechanism by which viral infection could have induced PD-L1 upregulation in vivo. Previous work by others had shown that PD-L1 expression by microvascular endothelial cells can be acquired as a direct consequence of exposure to type I and II interferon (43). As these cytokines are produced in vast amounts during viral infections, we asked whether they might be capable of directly inducing PD-L1 upregulation on NOD lymphoid cells. We found that PD-L1 expression was increased on these cells in response to IFN-α or IFN-γ in vitro (Figure 6A). Similarly, administration of IFN-α to prediabetic NOD mice rapidly induced upregulation of PD-L1 on lymphoid cells in the pancreatic LN and spleen in vivo (Figure 6B). In order to determine whether the effects of this cytokine-induced PD-L1 expression on T1D were comparable to those of the virally triggered increase, we measured the frequency of diabetogenic CD8+ T cells in lymphoid organs. We observed that expansion of IGRP-specific CD8+ T cells in the pancreatic LN and spleen was significantly hampered by IFN-α treatment, similar to our observation using CVB3 or LCMV (Figure 2C). These results suggested that during viral infection, upregulation of PD-L1 on lymphoid cells and subsequent control over expanding diabetogenic CD8+ T cells was caused, at least in part, by interferon production.
Figure 6. Treatment of prediabetic NOD mice with IFN-α induces PD-L1 upregulation on lymphoid cells and prevents the expansion of IGRP-specific CD8+ T cells in vivo.
(A) PD-L1 expression on NOD splenocytes incubated for 24 hours with LCMV (MOI, 1), IFN-α (104 U/ml), or IFN-γ (10 μg/ml), as measured by flow cytometry. Numbers indicate the percentage of PD-L1hi cells ± SD for 2 individual mice per group. (B) Percentage of PD-L1hi cells in the pancreatic LN and spleen of individual 9-week-old NOD mice left untreated (None) or injected 24 hours previously with 105 U IFN-α (blue diamonds), as measured by flow cytometry. (C) Percentage of IGRP-specific CD8+ T cells in the pancreatic LN and spleen of individual 12-week-old NOD mice left untreated or injected 21 days prior with 105 U IFN-α, as measured by flow cytometry. In B and C, symbols represent individual values, and horizontal lines denote mean. *P < 0.05, **P < 0.005, ***P < 0.001.
PD-L1 upregulation in prediabetic mice synergistically increases the capacity of virally enhanced CD4+CD25+ Tregs to protect from T1D.
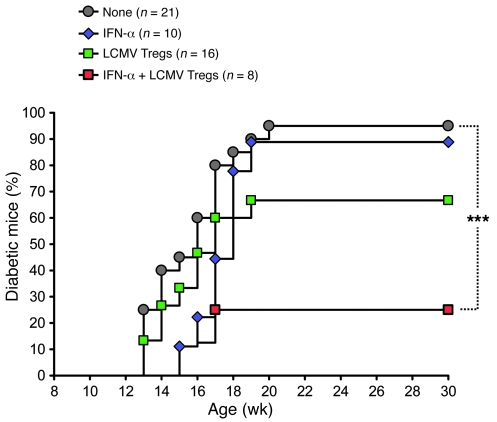
Our results so far indicated that viral infection was capable of modulating 2 distinct regulatory components of the immune system, both acting to protect from T1D. First, PD-L1 upregulation prevented the expansion of diabetogenic, PD-1–expressing CD8+ T cells and delayed the onset of overt diabetes. Second, CD4+CD25+ Tregs were enhanced in number and TGF-β–producing function and diminished diabetes incidence. Our finding that these 2 regulatory components had distinct, partially protective effects in T1D (Figure 3D and Figure 5A) suggested that upon viral infection, full protection might be conferred by their combination. We thus addressed whether virally enhanced CD4+CD25+ T cells might confer enhanced protection from T1D when diabetogenic CD8+ T cells had been previously curbed by upregulation of PD-L1 in vivo. To this end, we treated NOD mice with IFN-α and LCMV-enhanced CD4+CD25+ T cells in combination. IFN-α was administered at 9 weeks of age to confer upregulation of PD-L1 and hinder the accumulation of IGRP-specific diabetogenic CD8+ T cells (Figure 6, B and C), and CD4+CD25+ T cells were purified from LCMV-immune mice and injected at 12 weeks of age to diminish T1D incidence (Figure 5A). We found that treatment with IFN-α alone delayed the onset of T1D (consistent with a PD-L1–mediated effect), while injection of LCMV-exposed CD4+CD25+ Tregs alone diminished disease incidence (Figure 7). Strikingly, coinjection of IFN-α and LCMV-enhanced CD4+CD25+ Tregs was highly effective in protecting from T1D (Figure 7), to a degree comparable to LCMV infection (Figure 1). This indicated that virally enhanced CD4+CD25+ Tregs synergized with PD-L1 upregulation and ensuing impairment of autoreactive T cells to efficiently protect from T1D.
Figure 7. Early PD-L1 upregulation in prediabetic NOD mice synergistically increases the capacity of LCMV-enhanced CD4+CD25+ Tregs to protect from T1D.
Cumulative diabetes incidence over time in NOD mice left untreated or injected at 9 weeks of age with 105 U IFN-α or at 11 weeks of age with 5 × 105 LCMV Tregs (purified as described in Figure 5A), or receiving both treatments (red squares). ***P < 0.001.
Discussion
In the present study, we showed that regulatory mechanisms similarly induced by infection with 2 distinct viruses during the prediabetic phase can act in synergy to protect from T1D (Supplemental Figure 3). While LCMV and CVB differ in terms of genome structure (strand polarity), surface structure (enveloped), and particular tissue tropism, we found that both viruses caused PD-L1 upregulation and enhanced CD4+CD25+ Treg number and tolerogenic function, which acted in concert to efficiently curb diabetogenic CD8+ T cells. Interestingly, this is comparable to anti-CD3 treatment of T1D, which causes the direct demise of autoreactive effectors along with their indirect control via CD4+CD25+ Tregs and TGF-β (32). We previously proposed that such synergistic effects can be exploited therapeutically by combining anti-CD3 with induction of insulin-specific Tregs to maintain long-term tolerance (44).
Overall, the precise association between viruses and T1D is not understood. While CVB3 can protect from T1D, CVB4 has diabetogenic properties (5, 6). Similarly, while LCMV can prevent T1D in the NOD model, this virus is also used experimentally to trigger autoimmune diabetes in RIP-LCMV mice (8–10, 45). To explain the difference between induction and prevention of T1D by viruses, one has to consider the factors required for the initiation of the autoimmune process upon infection. Triggering of T1D by CVB4 is the consequence of direct tropism of the virus for β cells, which causes their damage and releases antigen in an inflammatory milieu where APCs are activated and expand autoreactive T cells (5, 46). In the RIP-LCMV model, diabetes results from direct antigenic recognition of β cells by committed antiviral T cells interacting with activated APCs (9, 10, 47). Thus, in order to expand autoreactive T cells and cause diabetes, viral infections have to either directly lyse β cells or inflict damage on these cells via antigenic recognition of β cell proteins within the proinflammatory milieu. On the other hand, our present results indicate that proinflammatory signals can invoke important regulatory mechanisms that protect from autoimmunity by promoting PD-L1 upregulation, which controls the expansion, rather than causing the activation, of diabetogenic CD8+ T cells. Furthermore, antiviral immunity triggered the expansion of CD4+CD25+ Tregs with enhanced tolerogenic function in T1D in vivo. In sum, it thus appears that bystander inflammation and antiviral immunity systemically or in the vicinity of the pancreatic islets are not detrimental but rather beneficial in T1D when occurring in the absence of direct β cell damage. Accordingly, CVB and LCMV cause T1D in NOD mice in contexts where they mediate β cell injury (in CVB4 and RIP-LCMV mice, respectively) but prevent T1D when infection does not directly harm β cells (in CVB3 and wild-type mice, respectively). This suggests that induction versus prevention of T1D by viruses depends on the extent to which antiviral inflammation and immunity alter the balance between β cell injury and immunoregulation.
The PD-L1/PD-1 interaction is a crucial regulator of immunity in numerous systems, including T1D. Blockade of this pathway in NOD mice in vivo precipitates T1D (18), indicating that the slow prediabetic process leading to overt disease is the result of attempted control over autoreactive T cells via their expression of PD-1. In our study, PD-L1 was rapidly upregulated in the pancreatic LN and spleen of virally infected NOD mice, a phenomenon that could be induced by IFN-α in the absence of infection. This might constitute a feedback mechanism ensuring that upregulation of PD-L1 to control antiviral immunity is proportional to the extent of immune pathology the inflammatory response might cause. The majority of diabetogenic, IGRP-specific CD8+ T cells present in the lymphoid organs of NOD mice before infection showed an activated phenotype and expressed PD-1. This might be reflective of advanced autoimmunity in NOD mice at this time point shortly preceding overt diabetes. Consequently, upregulation of PD-L1, most likely to control acute antiviral T cell responses during viral infection, led to the nonspecific demise of activated, PD-1–expressing diabetogenic T cells along with antiviral effectors. But this was not sufficient to fully prevent T1D in the absence of an additional mechanism, namely enhancement of natural Tregs, further containing the remaining autoreactive effectors and/or preventing their replenishment from naive precursors.
While natural Tregs are crucial in maintaining or restoring peripheral tolerance to self antigens, they also actively participate in the control of antiviral immunity. These cells usually have only a minimal impact on viral elimination but play an important part in limiting collateral tissue damage usually caused by strong antiviral T cell responses (22, 48). Importantly, however, we found that enhancement of Tregs did not coincide with priming of antiviral CD8+ T cells (data not shown) but rather succeeded it. This might constitute a safety mechanism ensuring that viral clearance was not hindered. We propose that enhancement of natural Tregs during viral infection does not occur virus-specifically, in the same way that expansion of antiviral T cells is controlled nonspecifically via PD-L1. As natural Tregs in antiviral immunity might serve to prevent immune pathology caused by inflammation, these cells might acquire upon infection the capacity to respond, not to viral antigen, but to common inflammatory signals such as modulated self antigen presentation by APCs. In this way, natural Tregs would become “inflammation-specific” and thereby responsive to the inflammatory processes that can expand autoreactive effectors and cause T1D. Acute inflammation thus seems centrally involved in triggering the regulatory mechanisms that protect from T1D upon viral infection.
Previous work has suggested that CD4+CD25+ Tregs from NOD mice gradually lose their suppressive properties over time (37–39). Our results indicate that viral infection during the prediabetic phase could not only prevent the progressive loss of function of these cells but also increase their frequency in lymphoid organs. We also found that Tregs might be rescued or enhanced during CVB3 and LCMV infection by acquiring the ability to produce TGF-β. Interestingly, a recent report indicates that in the context of TGF-β production, CVB4 switches from a causative to a protective infection in T1D as a consequence of Treg enhancement (49). Thus, a long-term increase in TGF-β production caused by viral infections during early life might not only abort ongoing autoimmune processes but also be able to subsequently counter the diabetogenic effect of viruses such as CVB4. Of note, we recently reported that TGF-β plays a dual role in T1D via its ability to inhibit the activation of naive CD8+ T cells but promote their survival and function after their encounter with antigen (50). As we observed here that the diabetogenic CD8+ T cells that were curbed by interaction with PD-L1 harbored an activated phenotype, we propose that CD4+CD25+ Tregs prevented T1D by controlling, via TGF-β, activation of new autoreactive cells. Synergy between virally enhanced Tregs and PD-L1 in T1D might thus be conferred by the selective impairment of activated autoreactive effectors, enabling an optimal effect of TGF-β on their naive precursors.
In conclusion, we found that virally induced inflammatory events, some of which may directly contribute to autoimmunity when β cells are damaged in the pancreas, trigger regulatory mechanisms that can not only control antiviral immunity but also synergize to halt autoimmunity. In this way, the same factors that might be causative or detrimental in T1D by expanding autoreactive effectors are also beneficial by enhancing immunoregulation. Our study provides what we believe to be a novel mechanistic insight into the current controversy about the role of viruses and inflammation in T1D (3) and should be important for prospective studies in humans such as The Environmental Determinants of Diabetes in the Young (TEDDY) (4).
Methods
Mice and virus.
Female NOD/ShiLtJ mice, which develop spontaneous T1D, were purchased from The Jackson Laboratory. Protection from diabetes was mediated by intraperitoneal infection of mice with a single dose of 103 PFU CVB3 Nancy or 105 PFU LCMV Armstrong 53b at 9 weeks of age. Blood glucose was monitored using the OneTouch Ultra system (LifeScan Inc.), and mice exhibiting values greater than 300 mg/dl were considered diabetic. Animal work in all studies was approved by the La Jolla Institute for Allergy & Immunology Animal Care Committee.
Flow cytometry.
NRP-V7 tetramers (35) were obtained from Rusung Tan of the University of British Columbia, Vancouver, British Columbia, Canada. After staining with fluorescently labeled mAbs (BD Biosciences, eBioscience, BioLegend, and Caltag [Invitrogen]), cells were processed on an LSRII or FACScalibur (BD Biosciences) and results analyzed using FlowJo version 7.2 (Tree Star). For surface staining, cell suspensions were incubated at 4°C for 20 minutes with different combinations of mAbs diluted in PBS (Invitrogen) containing 0.1% BSA (Calbiochem) and 2 mM EDTA (Invitrogen) (FACS buffer). Nonspecific binding was blocked using unlabeled anti-FcγR (BD Biosciences). Intracellular Foxp3 expression was assessed using a Foxp3 detection kit (eBioscience). For intracellular staining of cytokines, CD4+CD25+ T cells were stimulated at 37°C in RPMI 1640 (Invitrogen) supplemented with 10% FCS (HyClone, Thermo Scientific) (for TGF-β staining, stimulation was performed in low-FCS media), 2 mM l-glutamine (Sigma-Aldrich), and 50 μM 2-mercaptoethanol (Sigma-Aldrich) containing 5 μg/ml Brefeldin A (Sigma-Aldrich) for 3 hours with PMA and ionomycin (10 ng/ml and 0.5 μg/ml, respectively). After surface staining, cells were fixed in 2% paraformaldehyde (Sigma-Aldrich), permeabilized in FACS buffer containing 0.05% saponin (Sigma-Aldrich), and stained at 4°C for 20 minutes with different combinations of mAbs diluted in the same buffer. For TGF-β staining in the spleen, values obtained using isotype control mAb were subtracted from values using anti–TGF-β mAb for each individual sample.
CD4+CD25+ T cell purification.
Female NOD mice were sacrificed 21 days after CVB3 or LCMV infection, at which point virus was cleared from lymphoid tissue (data not shown). Cell suspensions prepared from pooled spleens and mesenteric, inguinal, and pancreatic LN of 10–25 mice per group were incubated at 4°C for 30 minutes with the following mAb cocktail in PBS containing 2% FCS and 2 mM EDTA: purified rat anti–mouse CD8, B220, I-A/I-E, CD11c, CD11b, and FcγR (BD Biosciences). CD4+ T cells were then negatively selected by magnetic separation using sheep anti-rat Dynabeads (DYNAL, Invitrogen). CD4+ T cells were then labeled with biotinylated anti-CD25 mAb (BD Biosciences) at 4°C for 20 minutes, and CD4+CD25+ (Tregs) and CD4+CD25– cells were purified by magnetic separation using anti-streptavidin MACS microbeads (Miltenyi Biotec). Cell purity was measured by flow cytometry and was determined to be greater than 95% in each sample.
Treatments.
With the exception of siRNA treatment, which was given both intraperitoneally and intravenously, all injections were performed intraperitoneally in 200 μl. Tregs and IFN-α (PBL Interferon Source) were injected in PBS, and PD-L1 siRNA was injected in jetPEI (Polyplus-transfection) at an N/P ratio of 8.
Transfection.
Cells were transfected with a plasmid DNA (circular form) containing a ubiquitous viral promoter driving expression of TGF-β1, GFP, or no protein (InvivoGen, Clontech) or with PD-L1 siRNA (Ambion, Applied Biosystems) using a transfection kit (Mouse T Cell Nucleofector; Amaxa) according to the manufacturers’ instructions.
Statistics.
Statistical significance was determined using a log-rank test (for T1D assessment) or an unpaired, 2-tailed t test. In all experiments, differences were considered significant when P was less than 0.05.
Supplementary Material
Acknowledgments
We thank Damien Bresson for help with molecular biology techniques, Malina McClure for mouse colony maintenance, Amira Belhani for technical help, Urs Christen for discussions, and Priscilla Colby for administrative support. This work was supported by NIH grant P01 AI58105-03 from the National Institute of Allergy and Infectious Diseases to M.G. von Herrath; and grants from the Juvenile Diabetes Research Foundation and the Fondation pour la Recherche Médicale to C.M. Filippi. We thank the Brehm Coalition for generous support.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: CVB, Coxsackie virus B; IGRP, islet-specific glucose-6-phosphate catalytic-related protein; LCMV, lymphocytic choriomeningitis virus; PD-1, programmed cell death–1; PD-L1, PD-1 ligand 1; T1D, type 1 diabetes.
Citation for this article: J. Clin. Invest. 119:1515–1523 (2009). doi:10.1172/JCI38503
See the related Commentary beginning on page 1458.
References
- 1.Eisenbarth G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 3.Filippi C.M., von Herrath M.G. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57:2863–2871. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.No authors listed. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr. Diabetes. 2007;8:286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz M.S., et al. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 6.Serreze D.V., Ottendorfer E.W., Ellis T.M., Gauntt C.J., Atkinson M.A. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes. 2000;49:708–711. doi: 10.2337/diabetes.49.5.708. [DOI] [PubMed] [Google Scholar]
- 7.Tracy S., et al. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 2002;76:12097–12111. doi: 10.1128/JVI.76.23.12097-12111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinic M.M., et al. Minimal impact of a de novo-expressed beta-cell autoantigen on spontaneous diabetes development in NOD mice. Diabetes. 2007;56:1059–1068. doi: 10.2337/db05-0062. [DOI] [PubMed] [Google Scholar]
- 9.Oldstone M.B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-U. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi P.S., et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-T. [DOI] [PubMed] [Google Scholar]
- 11.Christen U., et al. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J. Clin. Invest. 2004;113:74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldstone M.B. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science. 1988;239:500–502. doi: 10.1126/science.3277269. [DOI] [PubMed] [Google Scholar]
- 13.Shoda L.K., et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Barber D.L., et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 15.Day C.L., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 16.Keir M.E., et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latchman Y.E., et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansari M.J., et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fife B.T., et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 21.Filippi C., Bresson D., Herrath M. Antigen-specific induction of regulatory T cells for type 1 diabetes therapy. Int. Rev. Immunol. 2005;24:341–360. doi: 10.1080/08830180500371116. [DOI] [PubMed] [Google Scholar]
- 22.Suvas S., Kumaraguru U., Pack C.D., Lee S., Rouse B.T. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aandahl E.M., Michaelsson J., Moretto W.J., Hecht F.M., Nixon D.F. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boettler T., et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J. Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson S.J., Messer R.J., Carmody A.B., Hasenkrug K.J. In vitro suppression of CD8+ T cell function by friend virus-induced regulatory T cells. J. Immunol. 2006;176:3342–3349. doi: 10.4049/jimmunol.176.6.3342. [DOI] [PubMed] [Google Scholar]
- 26.Truong P., McGavern D.B. A novel virus carrier state to evaluate immunotherapeutic regimens: regulatory T cells modulate the pathogenicity of antiviral memory cells. J. Immunol. 2008;181:1161–1169. doi: 10.4049/jimmunol.181.2.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelik L., Flavell R.A. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/S1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 28.Green E.A., Gorelik L., McGregor C.M., Tran E.H., Flavell R.A. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccirillo C.A., Chang Y., Prud’homme G.J. TGF-beta1 somatic gene therapy prevents autoimmune disease in nonobese diabetic mice. J. Immunol. 1998;161:3950–3956. [PubMed] [Google Scholar]
- 30.King C., et al. TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity. 1998;8:601–613. doi: 10.1016/S1074-7613(00)80565-8. [DOI] [PubMed] [Google Scholar]
- 31.Grewal I.S., et al. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J. Autoimmun. 2002;19:9–22. doi: 10.1006/jaut.2002.0599. [DOI] [PubMed] [Google Scholar]
- 32.Belghith M., et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 33.Peng Y., Laouar Y., Li M.O., Green E.A., Flavell R.A. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M.O., Wan Y.Y., Sanjabi S., Robertson A.K., Flavell R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 35.Trudeau J.D., et al. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J. Clin. Invest. 2003;111:217–223. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieberman S.M., et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salomon B., et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/S1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 38.Tritt M., Sgouroudis E., d’Hennezel E., Albanese A., Piccirillo C.A. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 39.You S., et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 40.Liu W., et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi T., et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McHugh R.S., et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/S1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 43.Eppihimer M.J., et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002;9:133–145. doi: 10.1080/713774061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bresson D., et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J. Clin. Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Herrath M.G., Dockter J., Oldstone M.B. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 46.Horwitz M.S., Ilic A., Fine C., Rodriguez E., Sarvetnick N. Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J. Clin. Invest. 2002;109:79–87. doi: 10.1172/JCI11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Herrath M., Holz A. Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM. J. Autoimmun. 1997;10:231–238. doi: 10.1006/jaut.1997.0131. [DOI] [PubMed] [Google Scholar]
- 48.Suvas S., Azkur A.K., Kim B.S., Kumaraguru U., Rouse B.T. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 49.Richer M.J., Straka N., Fang D., Shanina I., Horwitz M.S. Regulatory T-cells protect from type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-beta. Diabetes. 2008;57:1302–1311. doi: 10.2337/db07-1460. [DOI] [PubMed] [Google Scholar]
- 50.Filippi C.M., et al. Transforming growth factor-beta suppresses the activation of CD8+ T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes. 2008;57:2684–2692. doi: 10.2337/db08-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.