Abstract
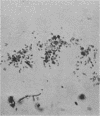
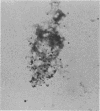
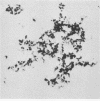
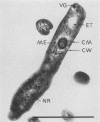
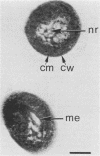
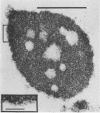
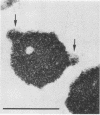
Two strains of an unclassified Mycobacterium species were isolated after 18 and 30 months of incubation of media inoculated with resected intestinal tissues from patients with Crohn's disease. These strains represented the third and fourth isolates of this organism from Crohn's disease patients. Ultrastructural examination of this strain and two previously isolated strains revealed the presence of spheroplasts which eventually transformed into the bacillary form of a previously unrecognized Mycobacterium species. These cell wall-deficient forms did not stain with conventional dyes and failed to grow on hypertonic media. Restriction polymorphism of the ribosomal DNA genes was used to determine the relationship between the cell wall-deficient and bacillary forms. Identical restriction patterns of the ribosomal DNA genes were found between the spheroplasts and Mycobacterium sp. isolates with EcoRI, BamHI, and XhoI restriction endonucleases, thus providing definitive evidence of their origin. Unidentified spheroplasts were isolated from an additional 12 patients with Crohn's disease, of which 7 of 10 seroagglutinated with antiserum prepared against the Mycobacterium sp. Spheroplasts were isolated from 16 of 26 (61%) patients with Crohn's disease but not from tissues of 13 patients with ulcerative colitis or 13 patients with other diseases of the bowel. These findings support the role of mycobacteria as etiologic agents in some cases of Crohn's disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander-Jackson E. A DIFFERENTIAL TRIPLE STAIN FOR DEMONSTRATING AND STUDYING NON-ACID-FAST FORMS OF THE TUBERCLE BACILLUS IN SPUTUM, ISSUE AND BODY FLUIDS. Science. 1944 Apr 14;99(2572):307–308. doi: 10.1126/science.99.2572.307. [DOI] [PubMed] [Google Scholar]
- Barksdale L., Convit J., Kim K. S., de Pinardi M. E. Spheroidal bodies and globi of human leprosy. Biochem Biophys Res Commun. 1973 Sep 5;54(1):290–296. doi: 10.1016/0006-291x(73)90921-2. [DOI] [PubMed] [Google Scholar]
- Belsheim M. R., Darwish R. Z., Watson W. C., Schieven B. Bacterial L-form isolation from inflammatory bowel disease patients. Gastroenterology. 1983 Aug;85(2):364–369. [PubMed] [Google Scholar]
- Burnham W. R., Lennard-Jones J. E., Stanford J. L., Bird R. G. Mycobacteria as a possible cause of inflammatory bowel disease. Lancet. 1978 Sep 30;2(8092 Pt 1):693–696. doi: 10.1016/s0140-6736(78)92699-5. [DOI] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S., Thayer W. R., Jr, Coutu J. A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J Clin Microbiol. 1984 Nov;20(5):966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Coutu J. A., Merkal R. S. In vitro antimicrobial susceptibility of a Mycobacterium sp. isolated from patients with Crohn's disease. Antimicrob Agents Chemother. 1984 Dec;26(6):930–932. doi: 10.1128/aac.26.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Merkal R. S., Coutu J. A. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1073–1079. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- DIENES L., WEINBERGER H. J. The L forms of bacteria. Bacteriol Rev. 1951 Dec;15(4):245–288. doi: 10.1128/br.15.4.245-288.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P. R., Lennard-Jones J. E., Burnham W. R., White S., Stanford J. L. Further data on skin testing with mycobacterial antigens in inflammatory bowel disease. Lancet. 1980 Aug 30;2(8192):483–484. doi: 10.1016/s0140-6736(80)91922-4. [DOI] [PubMed] [Google Scholar]
- FREEMAN B. A., RUMACK B. H. CYTOPATHOGENIC EFFECT OF BRUCELLA SPHEROPLASTS ON MONOCYTES IN TISSUE CULTURE. J Bacteriol. 1964 Nov;88:1310–1315. doi: 10.1128/jb.88.5.1310-1315.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feingold D. S. Biology and pathogenicity of microbial spheroplasts and l-forms. N Engl J Med. 1969 Nov 20;281(21):1159–1170. doi: 10.1056/NEJM196911202812106. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- Liebke H., Hatfull G. The sequence of the distal end of the E. coli ribosomal RNA rrnE operon indicates conserved features are shared by rrn operons. Nucleic Acids Res. 1985 Aug 12;13(15):5515–5525. doi: 10.1093/nar/13.15.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattman L. H. Cell wall-deficient forms of mycobacteria. Ann N Y Acad Sci. 1970 Oct 30;174(2):852–861. doi: 10.1111/j.1749-6632.1970.tb45604.x. [DOI] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Ultrastructural and chemical studies on wall-deficient forms, spheroplasts and membrane vesicles from Mycobacterium aurum. J Gen Microbiol. 1981 May;124(1):71–79. doi: 10.1099/00221287-124-1-71. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam S., Chandrasekhar S. The pathogenicity of spheroplasts of Mycobacterium tuberculosis. Am Rev Respir Dis. 1976 Sep;114(3):549–554. doi: 10.1164/arrd.1976.114.3.549. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B. Serologic identification and classification of the atypical mycobacteria by their agglutination. Am Rev Respir Dis. 1965 Dec;92(6):85–93. doi: 10.1164/arrd.1965.92.6P2.85. [DOI] [PubMed] [Google Scholar]
- Smith K. Electron microscopical observations on Mycobacterium johnei. Res Vet Sci. 1969 Jan;10(1):1–3. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thayer W. R., Jr, Coutu J. A., Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Possible role of mycobacteria in inflammatory bowel disease. II. Mycobacterial antibodies in Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1080–1085. doi: 10.1007/BF01317079. [DOI] [PubMed] [Google Scholar]
- Udou T., Ogawa M., Mizuguchi Y. An improved method for the preparation of mycobacterial spheroplasts and the mechanism involved in the reversion to bacillary form: electron microscopic and physiological study. Can J Microbiol. 1983 Jan;29(1):60–68. doi: 10.1139/m83-010. [DOI] [PubMed] [Google Scholar]
- Udou T., Ogawa M., Mizuguchi Y. Spheroplast formation of Mycobacterium smegmatis and morphological aspects of their reversion to the bacillary form. J Bacteriol. 1982 Aug;151(2):1035–1039. doi: 10.1128/jb.151.2.1035-1039.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorwell P. J., Davidson I. W., Beeken W. L., Wright R. Search by immunofluorescence for antigens of Rotavirus, Pseudomonas maltophilia, and Mycobacterium kansasii in Crohn's disease. Lancet. 1978 Sep 30;2(8092 Pt 1):697–698. doi: 10.1016/s0140-6736(78)92700-9. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]