Abstract
Melanoma is a particularly aggressive tumor-type that exhibits a high level of resistance to apoptosis. The serine/threonine kinase, B-RAF, is mutated in 50–70% of melanomas and protects melanoma cells from anoikis, a form of apoptosis induced by lack of adhesion or adhesion to an inappropriate matrix. Mutant B-RAF down-regulates two BH3-only pro-apoptotic proteins, BimEL and Bad. BH3-only proteins act, at least in part, by sequestering pro-survival B-cell lymphoma (Bcl)-2 family proteins and preventing them from inhibiting the mitochondrial apoptotic pathway. Several Bcl-2 proteins are up-regulated in melanoma; however the mechanisms of up-regulation and their role in melanoma resistance to anoikis remain unclear. Utilizing RNA interference, we show that depletion of myeloid cell leukemia-1 (Mcl-1) renders mutant B-RAF melanoma cells sensitive to anoikis. By contrast, minor effects were observed following depletion of either Bcl-2 or Bcl-XL. Mcl-1 expression is enhanced in melanoma cell lines compared to melanocytes and up-regulated by the B-RAF-MEK-ERK1/2 pathway through control of Mcl-1 protein turnover. Similar to B-RAF knockdown cells, adhesion to fibronectin protected Mcl-1 knockdown cells from apoptosis. Finally, expression of Bad, which does not sequester Mcl-1, further augmented apoptosis in non-adherent Mcl-1 knockdown cells. Together, these data support the notion that BH3 mimetic compounds that target Mcl-1 may be effective for the treatment of melanoma in combinatorial strategies with agents that disrupt fibronectin-integrin signaling.
Introduction
Anoikis is a form of apoptosis induced by loss of adhesion or adhesion to an inappropriate extracellular matrix (1). The susceptibility of cells to anoikis controls their numbers during development and normal homeostasis. By contrast, malignant cells display resistance to anoikis, a trait that permits their survival at sites distant from the primary tumor. Resistance to various forms of apoptosis is a critical factor contributing to the aggressive nature of melanoma cells. Once this form of skin cancer has metastasized, the clinical prognosis and five year survival rates of patients are poor since current treatments are few and often ineffective.
Anoikis is controlled by activation of the mitochondrial apoptotic pathway involving sub-families of Bcl-2 proteins that differ in their activities (2). Pro-apoptotic Bcl-2 proteins, Bcl-2 antagonist/killer 1 (Bak) and Bcl-2 associated X protein (Bax), mediate release of apoptogenic factors from the mitochondrial membrane and activation of the caspase pathway. Bax/Bak activation is modulated by pro-apoptotic BH3-only proteins including Bcl-2-associated death promoter (Bad), Bcl-2 interacting mediator of cell death (Bim), NOXA, and p53 up-regulated modulator of apoptosis (PUMA). BH3-only proteins sense cellular damage but whether they directly activate Bax/Bak or rather act indirectly by sequestering pro-survival Bcl-2 family proteins from inactivating Bax/Bak is currently under debate (3–5). Pro-survival Bcl-2 proteins such as Bcl-2, Bcl-XL and Mcl-1, antagonize this pathway through interactions with BH3 domains of BH3-only proteins and Bak/Bax (6). The balance between the expression/activation of the various Bcl-2 family proteins ultimately determines the cellular response.
B-RAF, a serine-threonine kinase, is mutated in 50–70% of human melanomas to a form that activates the MEK-ERK1/2 signaling cascade (7). We have previously shown that mutant B-RAF and MEK signaling are required for melanoma cell resistance to anoikis (8, 9). Oncogene-mediated resistance to anoikis has also been demonstrated in other tumor cell types for example by over-expression of EGFR in breast cancer cells (10). In melanoma, B-RAF-mediated protection from anoikis is mediated, at least in part, by the down-regulation of two BH3-only proteins, BimEL and Bad (9).
Targeting pro-survival members of the Bcl-2 family holds therapeutic potential for many cancer types. BH3 mimetic compounds that bind to a variety of pro-survival proteins have already been described (11, 12). These small molecules insert into the groove formed by the BH1, BH2 and BH3 domains on the surface of Bcl-2/Bcl-XL and block their inhibitory potential. However, some of these BH3 mimetic compounds target only a subset of Bcl-2 family proteins; thus it is important to determine which members contribute to resistance to apoptosis in response to different stimuli. Immunohistochemistry studies in melanoma indicate up-regulation of Bcl-XL and Mcl-1 correlates with melanoma progression (13), but the role of Bcl-2 family proteins in resistance to melanoma anoikis remains unknown. Here, we demonstrate that Mcl-1 expression mediates resistance to anoikis in mutant B-RAF human melanoma cells. By contrast, Bcl-2 and Bcl-XL exhibited minor activity in protecting melanoma cells from anoikis. Mcl-1 expression was elevated in human melanoma cell lines and its protein stability was regulated by mutant B-RAF/MEK signaling.
Results
Mcl-1 expression is required for resistance of melanoma cells to anoikis
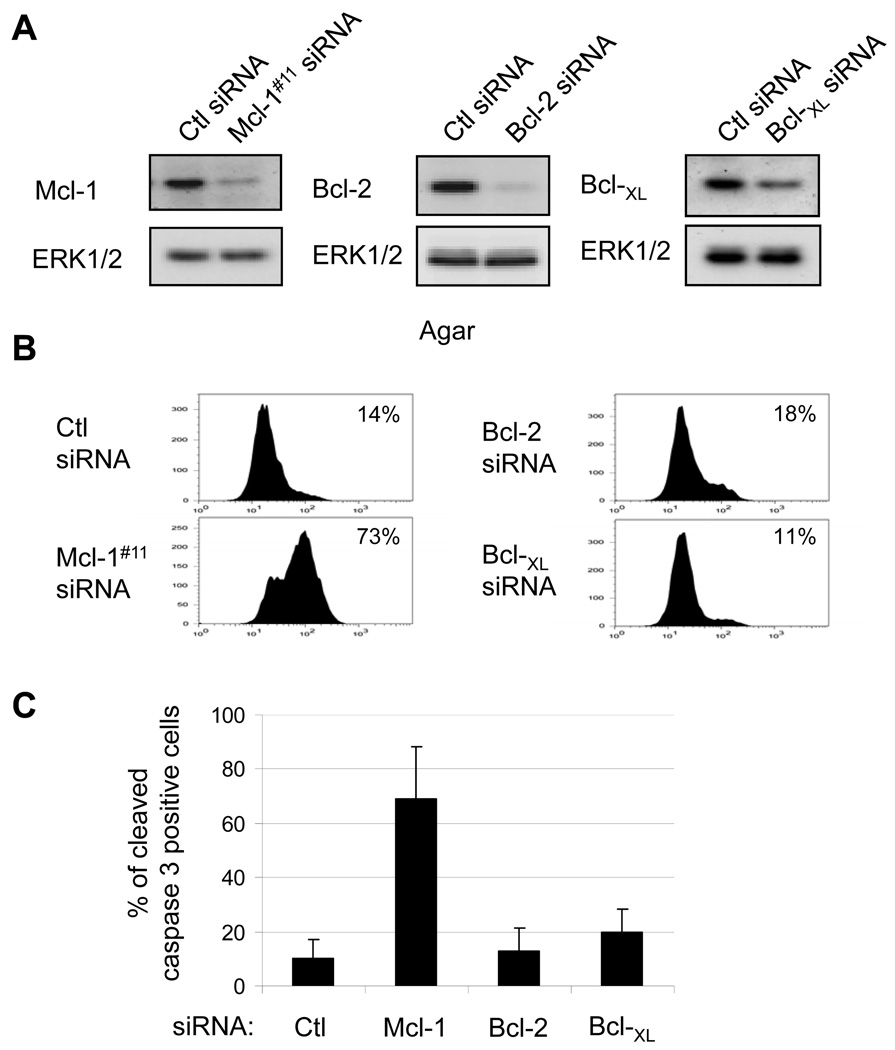
We have previously shown that mutant B-RAF promotes resistance to anoikis in melanoma cells via down-regulation of BimEL and Bad (8, 9). BH3-only proteins act, at least in part, by sequestering pro-survival Bcl-2 proteins and preventing them from inhibiting the essential pro-apoptotic proteins, Bak and Bax (14–16). We investigated the role of pro-survival Bcl-2 proteins in resistance to anoikis in mutant B-RAF melanoma cells. We employed a knockdown approach to individually deplete Mcl-1, Bcl-2, and Bcl-XL from WM793 cells that harbor mutant B-RAF (17, 18). Efficient knockdowns were confirmed by Western blotting both at 72 hrs post-knockdown (Fig. 1A) and 144 hrs equivalent to the time of cleaved caspase 3 analysis (Supplemental Fig. 1). Bcl-XL knockdowns were less efficient than for Mcl-1 and Bcl-2. Knockdown cells were cultured in serum-free conditions on agar-coated plates for 48 hours before assaying for cleaved caspase 3, a marker of activation of the intrinsic apoptosis pathway. In these conditions, Mcl-1 knockdown cells but not control, Bcl-2 or Bcl-XL knockdown cells displayed a large population of cleaved caspase 3-positive cells (Fig. 1B and quantitated in 1C).
FIGURE 1.
Mcl-1 knockdown promotes cleavage of caspase 3 in non-adherent melanoma cells. WM793 cells were transfected with control, Mcl-1, Bcl-2 or Bcl-XL siRNA, as indicated. (A) Seventy-two hours post-transfection, cell lysates were analyzed by Western blotting for Mcl-1, Bcl-2 or Bcl-XL. Total ERK1/2 was used as a loading control. (B) 72 hours following transfection, WM793 cells were serum starved for 24 hours and then replated onto agar-coated dishes in serum-free medium. After 48 hours, cells were analyzed for cleaved caspase 3 using flow cytometry. The fluorescence intensity is measured on the x axis and cell counts on the y-axis. The percentages of cells staining positive in each condition are indicated. (C) Quantitation of the data from (B) is presented as the average percentage of cells staining positive for cleaved caspase 3 from three independent experiments.
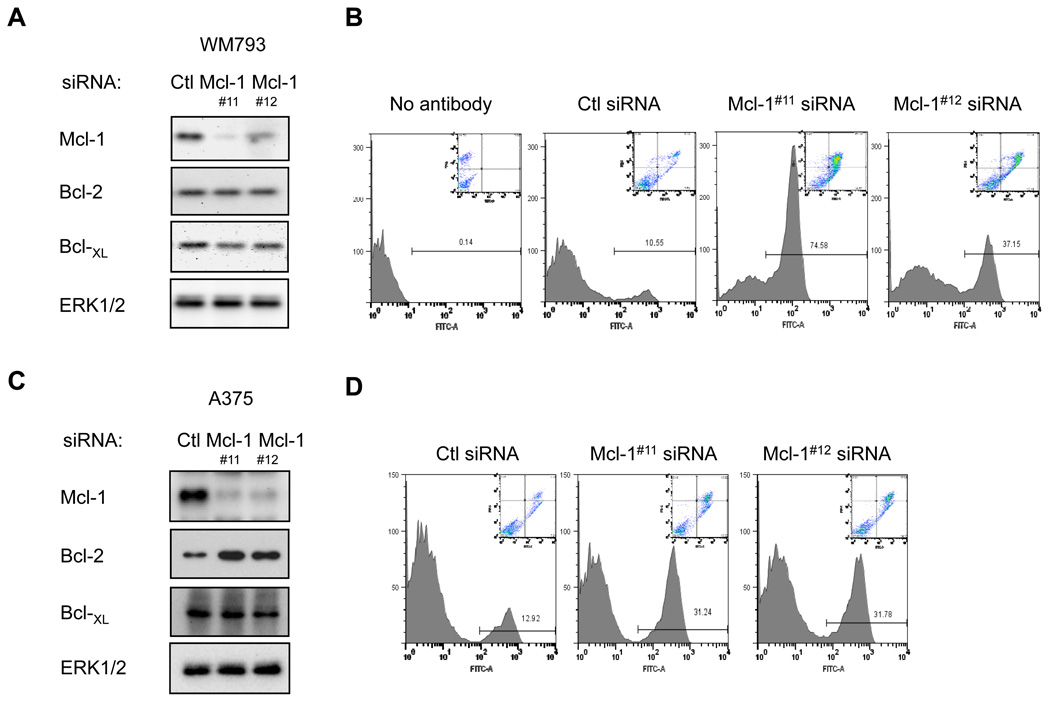
Since cleavage of caspase 3 is an early event leading to apoptosis, we next analyzed annexin V staining and propidium iodide uptake, as measures of later apoptotic events. Furthermore, to reduce concerns about off-target actions, we utilized a second, independent siRNA sequence targeting Mcl-1. Both siRNAs reduced Mcl-1 expression without altering levels of Bcl-2 and Bcl-XL, although Mcl-1 siRNA #11 was more efficient than duplex #12 (Fig. 2A). Importantly, both siRNAs enhanced annexin V staining and incorporation of propidium iodide in suspended WM793 cells (Fig. 2B). Since our serum-free medium conditions may confound the analysis, we performed similar experiments in medium containing 2% serum and insulin. Mcl-1 knockdown with either of two siRNAs were susceptible to anoikis in serum and growth factor containing medium albeit at a reduced extent compared to serum-free conditions (Supplemental Fig. 2). To extend these findings to more than one melanoma line, we performed similar experiments in A375 cells, which also harbor mutant B-RAF. Selective knockdown of Mcl-1 led to increased annexin-V staining and propidium iodide incorporation in suspended A375 cells (Fig. 2C &D). We also performed Mcl-1 knockdowns in Sbcl2 cells that are wild-type for B-RAF but harbor an N-RAS mutation (18, 19). Sbcl2 cells display high sensitivity to anoikis that was further enhanced following Mcl-1 knockdown (Supplemental Fig. 3). These data show that depletion of Mcl-1 renders melanoma cells susceptible to anoikis.
FIGURE 2.
Mcl-1 knockdown sensitizes WM793 and A375 cells to anoikis. (A) WM793 cells were transfected with control, Mcl-1#11, or Mcl-1#12 siRNA. Cell lysates were analyzed by Western blotting for Mcl-1, Bcl-2, Bcl-XL and ERK1/2. (B) Following transfection and serum starvation, WM793 cells were replated onto agar-coated dishes for 48 hours in serum-free medium. Harvested cells were analyzed for annexin-V staining and propidium iodide uptake. The main trace shows annexin V-FITC staining versus relative cell number. Inset depicts annexin V-FITC staining versus PI incorporation. (C) and (D) As for A and B, except that A375 cells were utilized. Shown are representative traces from one of three independent experiments.
Regulation of Mcl-1 in melanoma cells
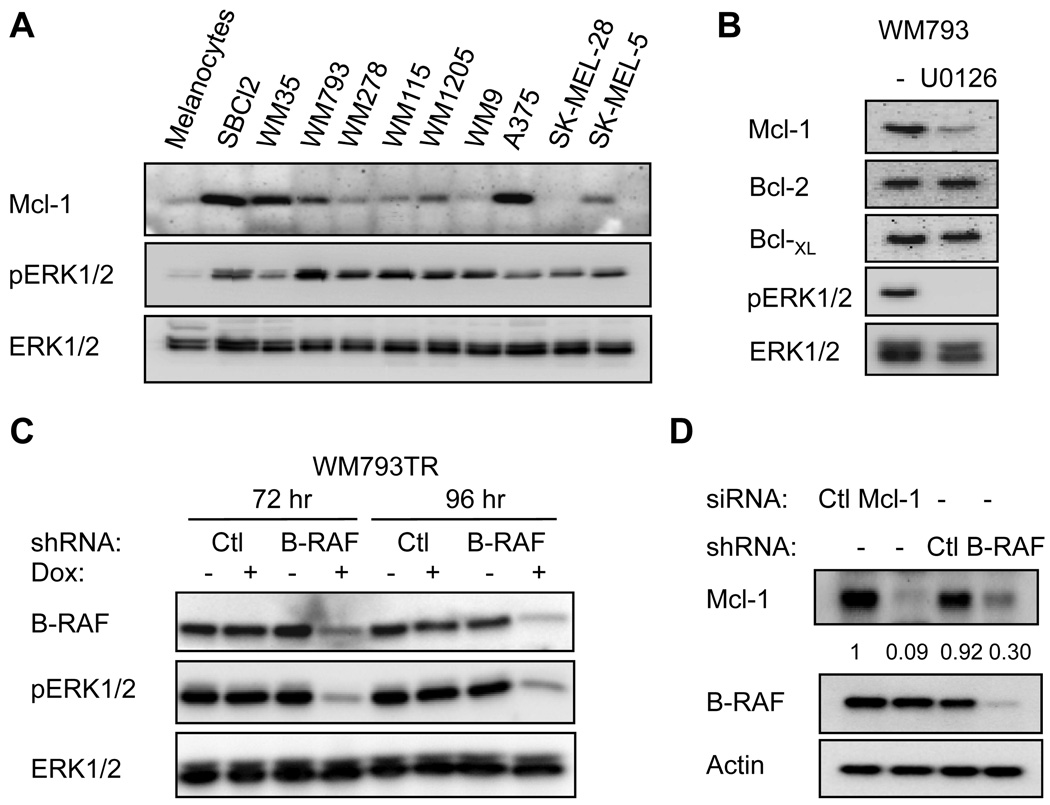
Increased staining for Mcl-1 has been observed in primary and metastatic melanomas (13). We analyzed Mcl-1 levels in a panel of melanoma cells compared to human melanocytes. All the melanoma lines harbor mutant B-RAF with the exception of Sbcl2, which is mutant for N-Ras (18, 19). Mcl-1 protein levels were enhanced in the majority of these cells compared to melanocytes (Fig. 3A). The MEK-ERK1/2 pathway is elevated in melanoma cell lines (Fig. 3A, (17, 18)) and Mcl-1 levels decreased following inhibition of MEK with U0126 (Fig. 3B and Supplemental Fig. 3C). No alterations in the levels of Bcl-2 and Bcl-XL were detected in these conditions. Additionally, we generated WM793 cell lines expressing inducible control or B-RAF shRNAs. Treatment of B-RAF shRNA cells with doxycycline led to an efficient decrease in the levels of B-RAF and concomitant decrease in phospho-ERK1/2 (Fig. 3C). No effect was observed in Ctl shRNA WM793 cells. In support of MEK inhibitor experiments, inducible knockdown of B-RAF in WM793 cells led to a decrease in Mcl-1 expression (Fig. 3D).
FIGURE 3.
Mcl-1 is highly expressed in melanoma cells. (A) Protein cell lysates from human melanocytes (NHEM) and melanoma cells (Sbcl2, WM35, WM793, WM278, WM115, WM1205, WM9, A375, SK-MEL-28 and SK-MEL-5) were analyzed for Mcl-1, phospho-ERK1/2 and total ERK1/2 levels. (B) WM793 cells were treated with U0126 for 5 hours and lysates were analyzed by Western blotting. (C) WM793TR-Ctl shRNA and WM793TR-B-RAF shRNA cell lines were untreated or treated with 100 ng/ml doxycycline for 72 and 96 hours. Cell lysates were analyzed by Western blotting for B-RAF, phospho-ERK1/2, and total ERK1/2. (D) Ctl and Mcl-1 knockdown WM793 cells, and WM793TR-Ctl shRNA and WM793TR-B-RAF shRNA treated with doxycycline for 96 hours were analyzed by Western blotting for levels of Mcl-1, B-RAF and actin. The levels of Mcl-1 normalized to actin are indicated for each sample.
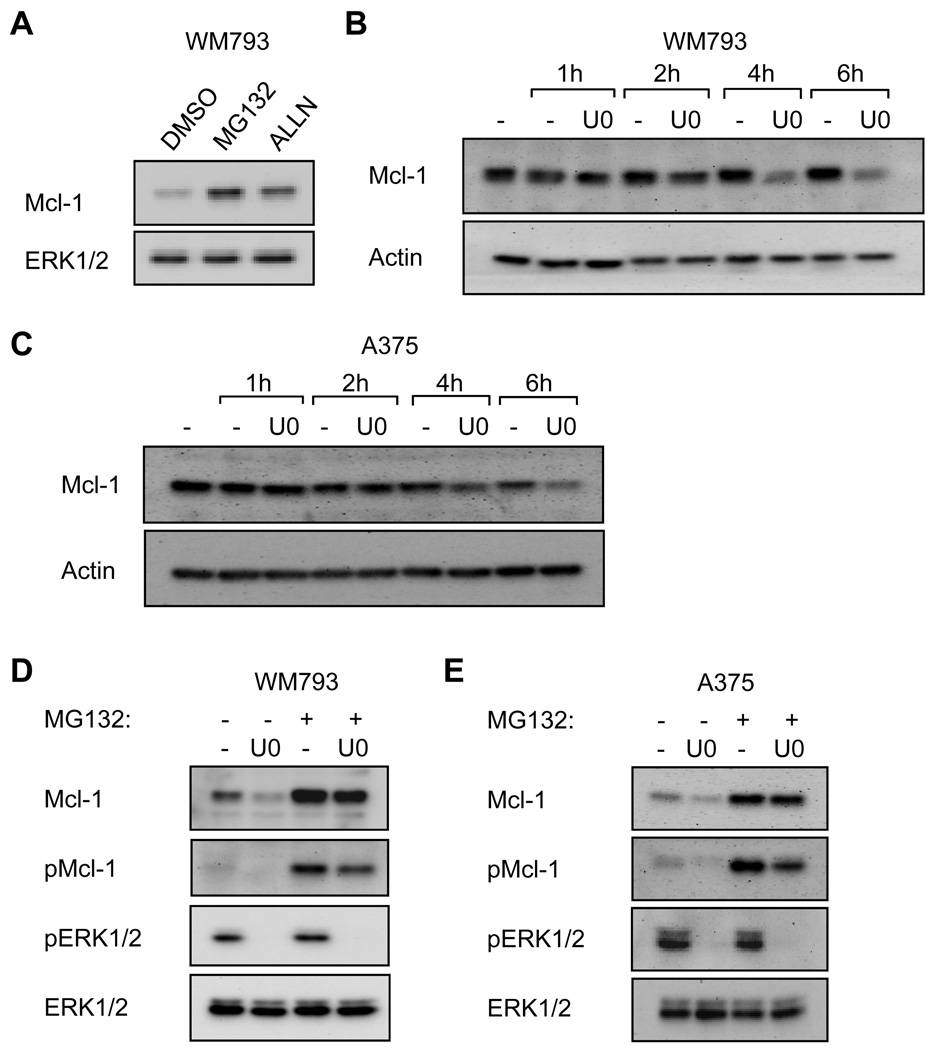
Mcl-1 levels can be regulated by the proteasome (20, 21). Treatment of WM793 cells with proteasomal inhibitors increased expression of Mcl-1 (Fig. 4A). To determine whether turnover of Mcl-1 was regulated by MEK-ERK1/2, we next analyzed protein turnover in cycloheximide chase experiments in the absence/presence of MEK inhibitor. Mcl-1 levels were efficiently decreased in U0126-treated WM793 cells compared to DMSO controls over a 6 hour time-course (Fig. 4B). Similarly, turnover of Mcl-1 was increased in U0126-treated A375 cells compared to control treatment although basal turnover of Mcl-1 was more rapid in these cells compared to WM793 (Fig. 4C). Mcl-1 levels were up-regulated in U0126-treated cells following proteasomal inhibition (Fig. 4D and 4E). Phosphorylation of Mcl-1 within PEST (proline, glutamate, serine, and threonine) sequences regulates Mcl-1 protein turnover (22–24). Levels of phospho-Mcl-1 were also up-regulated following MG132 treatment alone. Consistent decreases in the levels of phospho-Mcl-1 were observed following MG132/U0126 treatment compared to MG132 alone (22–24). The incomplete inhibition of phosphorylation was expected because multiple phosphorylation sites are recognized by this antibody. Together, these data show that Mcl-1 protein turnover is regulated by the MEK-ERK1/2 pathway in mutant B-RAF melanoma cells, likely through phosphorylation within the PEST domain.
FIGURE 4.
Mcl-1 protein turnover is regulated by B-RAF-MEK signaling. (A) WM793 cells were treated with 10 µM MG132 or 50 µM ALLN for 5 hours. Cell lysates were analyzed by Western blotting. (B) WM793 cells were treated with 10 µg/ml cycloheximide for 1 hour after which one culture dish was taken as the time 0. Cells were then treated with either DMSO or 5 µM U0126 for the indicated time. Cell lysates were analyzed by Western blotting for Mcl-1 and actin (loading control). (C) As for B, except A375 cells were used. (D) WM793 cells were treated for 6 hours with 10 µM MG132 and/or 5 µM U0126, as indicated. Cell lysates were analyzed by Western blotting for levels of total and phospho-Mcl-1, and total and phospho-ERK1/2. (E) As for D, except that A375 cells were utilized. Shown are representative blots from one of three independent experiments.
Survival of Mcl-1 knockdown cells is adhesion-dependent
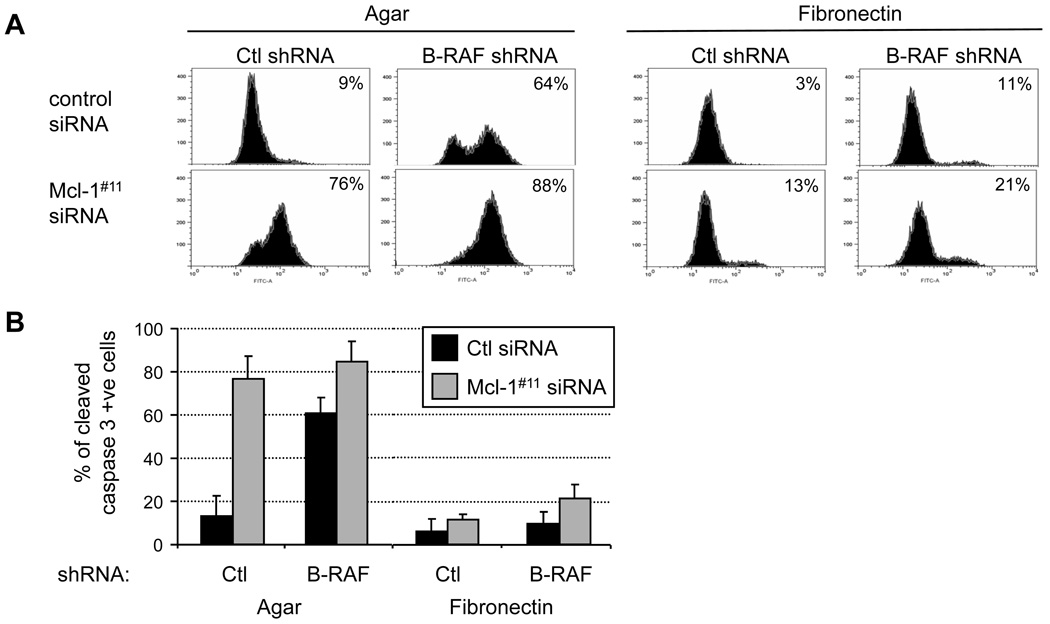
We have previously shown that adhesion to fibronectin protects mutant B-RAF depleted melanoma cells from apoptosis (8). Next, we determined whether fibronectin protected Mcl-1 knockdown cells from apoptosis and the effects of combined B-RAF/Mcl-1 knockdown. We utilized inducible B-RAF shRNA WM793 cells in which B-RAF was efficiently knocked down and ERK1/2 activation impaired over the time period of experiments (Fig. 3C). Consistent with results from siRNA knockdown, shRNA depletion of B-RAF rendered WM793 cells sensitive to anoikis, and adhesion to fibronectin was protective (Fig. 5A and quantitated in 5B). Similar to B-RAF depletion, apoptosis in Mcl-1 knockdown cells was protected by adhesion to fibronectin. Indeed, adhesion to fibronectin protected cells in which both B-RAF and Mcl-1 were depleted. We consistently observed higher levels of cleaved caspase 3 levels following Mcl-1 knockdown compared to B-RAF knockdown. This is likely due to more Mcl-1 being present in B-RAF knockdown cells compared to Mcl-1 knockdowns (Fig. 3D). In summary, adhesion to fibronectin confers protection to Mcl-1 depleted melanoma cells.
FIGURE 5.
Adhesion to fibronectin protects Mcl-1 knockdown cells from apoptosis. (A) Doxycycline-treated WM793TR-Ctl shRNA and WM793TR-B-RAF shRNA cells were transfected with either Ctl or Mcl-1 siRNAs. Following transfection and serum starvation, WM793 cells were replated onto agar or fibronectin-coated dishes for 48 hours in serum-free medium. Harvested cells were analyzed for cleaved caspase 3 staining by flow cytometry. (B) Quantitation of cleaved caspase 3 staining from three independent experiments.
Expression of Bad enhances susceptibility of Mcl-1 knockdown cells to anoikis
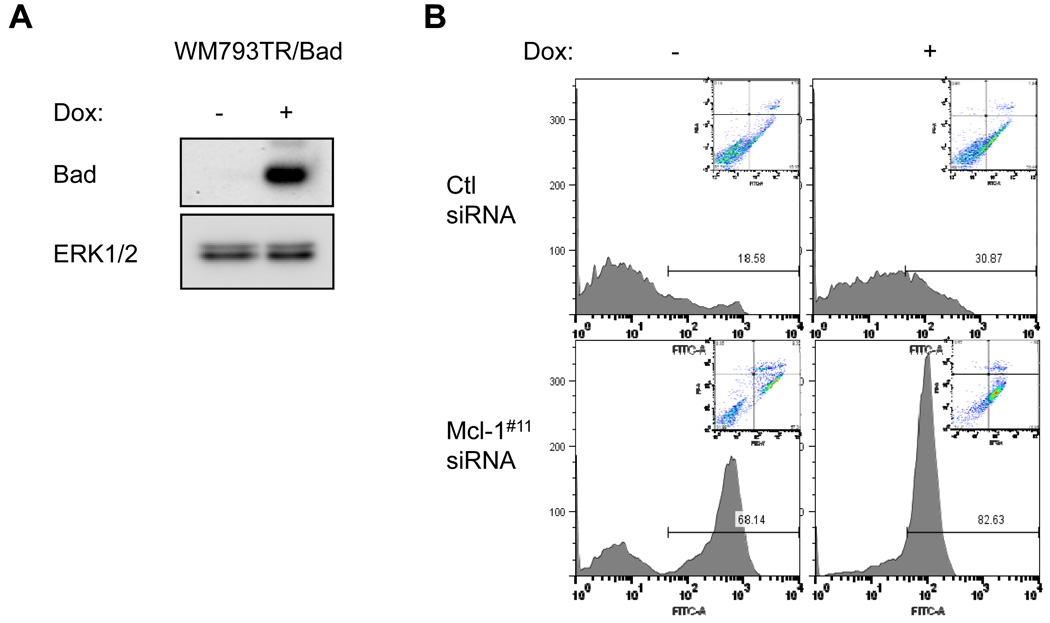
B-RAF knockdown in combination with Mcl-1 knockdown slightly enhanced susceptibility to anoikis (Fig. 5). We have previously shown that the BH3-only protein, Bad is up-regulated following B-RAF knockdown and is required, in part, for sensitivity to anoikis in B-RAF knockdowns (8, 9). Work from others has demonstrated that Bad does not target Mcl-1 (14–16); thus, we tested whether Bad expression enhanced apoptosis in Mcl-1 knockdown cells. We generated an inducible system to express wild-type Bad following addition of doxycycline to cells (Fig. 6A). Expression of Bad alone increased slightly the sensitivity of WM793 to anoikis, consistent with our previous study (8, 9). Additionally, Bad expression further enhanced the susceptibility of Mcl-1 knockdown cells to anoikis (Fig. 6B). These data suggest that mechanisms in addition to inactivation of Mcl-1 contribute to melanoma cell susceptibility to anoikis.
FIGURE 6.
Bad expression augments sensitivity to anoikis in Mcl-1 knockdown cells. (A) WM793TR-Bad cells were treated for 72 hrs with 100 ng/ml doxycycline. Lysates were analyzed by Western blotting for Bad and ERK1/2, as a loading control. (B) WM793TR-Bad cells were transfected with Ctl or Mcl-1 siRNA and used in anoikis assays. Harvested cells were analyzed for annexin-V staining and propidium iodide uptake. Shown are representative results from one of three independent experiments.
Discussion
Melanoma is renowned for its resistance to apoptosis. In this study, we examined the role of pro-survival Bcl-2 family members. These studies are the first to demonstrate a role for Mcl-1 in melanoma cell resistance to anoikis. Our data add to growing evidence implicating Mcl-1 in melanoma. Antisense oligonucleotide/siRNA strategies to down-regulate Mcl-1 increases melanoma cell sensitivity to apoptosis induced by dacarbazine treatment in vivo (25), exposure to ionizing radiation in vitro (26), the proteasome inhibitor, Bortezomib (27), and endoplasmic reticulum stress (28). Additionally, a small molecule BH3 mimetic, obatoclax, which targets Mcl-1, renders melanoma cells sensitive to the Bcl-2/ Bcl-XL/ Bcl-WL selective antagonist, ABT-737, and to Bortezomib (29, 30). Thus, Mcl-1 may underlie resistance to several forms of pro-apoptotic signals.
Our studies focus on anoikis, a form of apoptosis induced by loss of/inappropriate adhesion. We have previously shown that B-RAF knockdown was associated with the up-regulation of two pro-apoptotic proteins, BimEL and Bad (9). BimEL is known to bind Mcl-1 (5, 11, 31) and thus the effects of up-regulated BimEL following B-RAF knockdown are likely to prevent Mcl-1 actions on Bax/Bak. Bad, however, does not bind Mcl-1 and likely further enhances sensitivity to anoikis in Mcl-1 knockdown cells. This result indicates the multi-factorial mechanism underlying B-RAF-dependent resistance to anoikis. Similar to our findings with B-RAF depletion, adhesion to fibronectin was protective for Mcl-1 knockdown cells. The integrins αvβ3 and α4β1 are up-regulated in invasive melanomas (32) and both are capable of binding fibronectin in addition to other ligands (33). Fibronectin deposits are found in the dermis of human skin (34) and also at pre-metastatic niche sites (35). Thus, combinatorial targeting of fibronectin integrins and Mcl-1 (or B-RAF) may represent an effective strategy to target melanoma cells.
Additionally, our study shows that Mcl-1 expression is up-regulated in multiple human melanoma lines compared with normal human epidermal melanocytes. However, there is not an exact correlation between Mcl-1 levels and resistance to anoikis indicating that mechanisms in addition to up-regulation of Mcl-1 exist. Nevertheless, these data support immunohistochemistry studies showing that Mcl-1 expression is increased in primary and metastatic melanomas compared to benign melanocytic lesions (13). Mcl-1 is regulated by the proteasome through the action of E3 ubiquitin ligases including Mule and beta-TRCP1/2 (20, 21, 36). It is possible that high expression of Mule and/or beta-TRCP1/2 levels in cell lines such as WM9 and SK-MEL-28 mediates the low Mcl-1 levels. GSK3β-mediated serine 159 phosphorylation of Mcl-1 leads to increased ubiquitylation and proteasomal degradation (24). By contrast, phosphorylation of threonine 163 in response to ERK1/2 activation inhibits the proteasomal turnover of Mcl-1 (23). Thus, it is likely that decreased phosphorylation of Mcl-1 following B-RAF knockdown promotes Mcl-1 turnover. Although our findings show proteasomal regulation of Mcl-1 levels, we cannot rule out that B-RAF regulates Mcl-1 expression in part through control of mRNA levels.
In summary, our findings show that Mcl-1 is required for protection from anoikis in melanoma and Mcl-1 protein turnover is regulated by the B-RAF-MEK pathway. These studies underscore the importance of targeting Mcl-1, likely in combination with integrin antagonists, as a possible therapeutic strategy for melanoma.
Materials and Methods
Cell culture
All melanoma cells, except A375, were routinely sub-cultured in MCDB 153 containing 20% Leibovitz L-15 medium, 2% FBS and 5 µg/ml insulin. A375 cells were cultured in DMEM containing 10% FBS. Isolation of neonatal human melanocytes has been described elsewhere (17).
Short-interfering RNA (siRNA) knockdowns
WM793 cells were transfected with siRNA at a final concentration of 25 nM using Oligofectamine (Invitrogen Carlsbad, CA), as previously described. A375 cells were electroporated using program K-017 on a Nucleofector (Amaxa Biosystems, Gaithersburg, MD). The siRNA sequences (Dharmacon, Lafayette, CO) used were Ctl: UAGCGACUAAACACAUCAAUU; Mcl-1 #11: GCAUCGAACCAUUAGCAGAUU; Mcl-1 #12: GCUAAACACUUGAAGACCAUU; Bcl-2: GGGAGAUAGUGAUGAAGUAUU; Bcl-XL: GGAGAUGCAGGUAUUGGUGUU.
Western blotting
Western blotting was completed, as previously described (8, 9). The following primary antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA: Mcl-1 (sc20679), B-RAF (sc5284), and ERK 1 (sc94). Bad (#9292), Bcl-XL (#2762), phospho-ERK (Thr202/Tyr204, #9106) and phospho-Mcl-1 (Ser159/Thr163, #4579) were obtained from Cell Signaling Technology, Beverley, MA. Bcl-2 (551107) antibody was purchased from BD Biosciences, San Jose, CA. Anti-actin (A2066) was purchased from Sigma-Aldrich, St. Louis, MO.
Apoptosis assays
Cells were serum-starved for 24 hours before replating onto either 10 µg/ml fibronectin or 1% bactoagar for 48 hours in serum-free MCDB 153 medium containing 0.5% BSA. Cells were processed for either cleaved caspase 3 flow cytometry, as we have previously described (8, 9), or annexin V/propidium iodide staining. For the latter, cells were harvested off agar, washed in PBS/0.1% BSA and resuspended in 100 µL binding buffer (10 mM Hepes/NaOH (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2) at a concentration of 1 × 106 cells/mL. Cells were then incubated for 15 min at ambient temperature with Annexin V-FITC (BD Biosciences) and propidium iodide (final concentration of 100 µM; Molecular Probes Inc., Eugene, OR). After incubation, cells were diluted 1:4 in binding buffer and analyzed by flow cytometry.
Recombinant lentiviral transduction
Inducible short-hairpin RNA (shRNA) knockdown of B-RAF was achieved using the BLOCK-iT™ lentiviral expression system (Invitrogen) according to the manufacturer’s instructions. The generation of WM793TR cells has been described (9). The sequences for the B-RAF shRNA oligos were: 5’ CACCACAGAGACCTCAAGAGTAATTCAAGAGATTACTCTTGAGGTCTCTG and 5' AAAACAGAGACCTCAAGAGTAATCTCTTGAATTACTCTTGAGGTCTCTGT (underlined is the hairpin). Oligonucleotide sequences for the control shRNA were: 5’-CACCGTAGCGACTAAACACATCAATTCAAGAGATTGATGTGTTTAGTCGCTA and 5’-AAAATAGCGACTAAACACATCAATCTCTTGAATTGATGTGTTTAGTCGCTAC. Oligos were annealed and ligated into the pENTR/H1/TO vector and analyzed for errors by DNA sequencing. Correct constructs were then recombined into the pLenti4/BLOCK-iT™-DEST vector. Bad cDNA in pENTR/3C vector (gift from Dr. Matthew VanBrocklin (Van Andel Research Institute) was recombined with pLenti4/TO/V5-DEST using the LR Clonase II kit. Stop codons from cDNAs were maintained to omit the inclusion of a V5 epitope tag contained within the vectors. Lentiviral particles were generated, as previously described, and used to transduce WM793TR cells. Transduced cells were selected with zeocin over 2 weeks. Inducible expression of the transgene was obtained by treatment of cultures with doxycycline at a final concentration of 100 ng/ml.
Protein turnover assays
For cycloheximide chase assays, cells were pre-treated with 10 µg/ml cycloheximide (Sigma) for 1 hour. Zero time point samples were lysed. U0126 (5 µM) or DMSO (vehicle control) was added and cells incubated for 1, 2, 4 and 6 hours. In other experiments, the proteasome inhibitors, MG132 (Calbiochem, La Jolla, CA) and ALLN were used at final concentrations of 10 µM and 50 µM, respectively.
Acknowledgements
We thank Dr. Meenhard Herlyn (Wistar Institute, Philadelphia) for WM melanoma cells, Dr. Matthew VanBrocklin (Van Andel Research Institute) for Bad vector, and the flow cytometry facility at Albany Medical College for use of the FACSCanto and FlowJo software.
This work was supported by NIH R01 grant, GM067893, to A.E.A.
Abbreviations
- Bak
Bcl-2 antagonist/killer 1
- Bax
Bcl-2 associated X protein
- Bcl
B cell lymphoma
- Mcl-1
myeloid cell leukemia-1
- shRNA
short-hairpin RNA
- siRNA
short interfering RNA.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 2.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letai A, Bassik MC, Walensky LD, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 4.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 6.Lindsten T, Ross AJ, King A, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 8.Boisvert-Adamo K, Aplin AE. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene. 2006;25:4848–4856. doi: 10.1038/sj.onc.1209493. [DOI] [PubMed] [Google Scholar]
- 9.Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–3312. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- 10.Reginato MJ, Mills KR, Paulus JK, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;6:6. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 11.Letai A. Pharmacological manipulation of Bcl-2 family members to control cell death. J Clin Invest. 2005;115:2648–2655. doi: 10.1172/JCI26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 13.Tang L, Tron VA, Reed JC, et al. Expression of apoptosis regulators in cutaneous malignant melanoma. Clin Cancer Res. 1998;4:1865–1871. [PubMed] [Google Scholar]
- 14.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Swanson BJ, Wang M, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci USA. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conner SR, Scott G, Aplin AE. Adhesion-dependent activation of the ERK1/2 cascade is by-passed in melanoma cells. J Biol Chem. 2003;278:34548–34554. doi: 10.1074/jbc.M305797200. [DOI] [PubMed] [Google Scholar]
- 18.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- 19.Ikediobi ON, Davies H, Bignell G, et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther. 2006;5:2606–2612. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Ding Q, He X, Hsu J-M, et al. Degradation of Mcl-1 by {beta}-TrCP Mediates Glycogen Synthase Kinase 3-Induced Tumor Suppression and Chemosensitization. Mol Cell Biol. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domina AM, Smith JH, Craig RW. Myeloid Cell Leukemia 1 Is Phosphorylated through Two Distinct Pathways, One Associated with Extracellular Signal-regulated Kinase Activation and the Other with G2/M Accumulation or Protein Phosphatase 1/2A Inhibition. J Biol Chem. 2000;275:21688–21694. doi: 10.1074/jbc.M000915200. [DOI] [PubMed] [Google Scholar]
- 23.Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- 24.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Thallinger C, Wolschek MF, Wacheck V, et al. Mcl-1 antisense therapy chemosensitizes human melanoma in a SCID mouse xenotransplantation model. J Invest Dermatol. 2003;120:1081–1086. doi: 10.1046/j.1523-1747.2003.12252.x. [DOI] [PubMed] [Google Scholar]
- 26.Skvara H, Thallinger C, Wacheck V, et al. Mcl-1 blocks radiation-induced apoptosis and inhibits clonogenic cell death. Anticancer Res. 2005;25:2697–2703. [PubMed] [Google Scholar]
- 27.Qin JZ, Xin H, Sitailo LA, Denning MF, Nickoloff BJ. Enhanced Killing of Melanoma Cells by Simultaneously Targeting Mcl-1 and NOXA. Cancer Res. 2006;66:9636–9645. doi: 10.1158/0008-5472.CAN-06-0747. [DOI] [PubMed] [Google Scholar]
- 28.Jiang CC, Lucas K, Avery-Kiejda KA, et al. Up-regulation of Mcl-1 Is Critical for Survival of Human Melanoma Cells upon Endoplasmic Reticulum Stress. Cancer Res. 2008;68:6708–6717. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15–070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin J-Z, Xin H, Sitailo LA, Denning MF, Nickoloff BJ. Enhanced Killing of Melanoma Cells by Simultaneously Targeting Mcl-1 and NOXA. Cancer Res. 2006;66:9636–9645. doi: 10.1158/0008-5472.CAN-06-0747. [DOI] [PubMed] [Google Scholar]
- 31.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albelda SM, Mette SA, Elder DE, et al. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 33.Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, Ig-CAMs and selectins. Pharm Rev. 1998;50:197–262. [PubMed] [Google Scholar]
- 34.Gaggioli C, Robert G, Bertolotto C, et al. Tumor-Derived Fibronectin Is Involved in Melanoma Cell Invasion and Regulated by V600E B-Raf Signaling Pathway. J Invest Dermatol. 2007;127:400–410. doi: 10.1038/sj.jid.5700524. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warr MR, Acoca S, Liu Z, et al. BH3-ligand regulates access of MCL-1 to its E3 ligase. FEBS Letts. 2005;579:5603–5608. doi: 10.1016/j.febslet.2005.09.028. [DOI] [PubMed] [Google Scholar]