Abstract
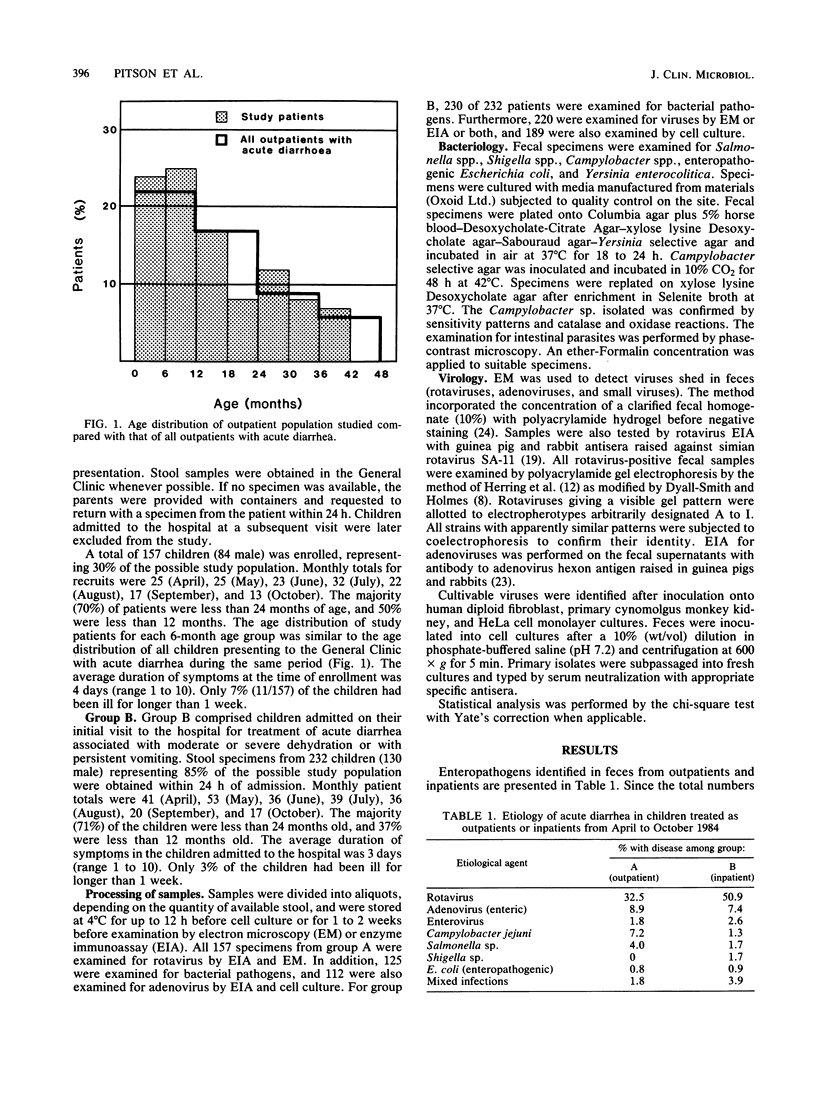
The etiology of acute diarrhea in children less than 42 months of age attending one pediatric hospital in Melbourne, Australia, was studied during a 7-month period encompassing the winter of 1984. Pathogens identified in 157 children treated as outpatients with mild disease were compared with those in 232 children hospitalized with severe disease. The pathogens (and frequencies among outpatients and inpatients, respectively) detected were rotaviruses (32.5 and 50.9%), enteric adenoviruses (8.9 and 7.4%), Campylobacter jejuni (7.2 and 1.3%), and Salmonella sp. (4.0 and 1.7%). Electropherotypes of rotavirus strains from outpatients and inpatients were compared. Two strains predominated during the 7 months of this study and were observed with equal frequency from outpatients and inpatients. Rotaviruses of the same electropherotype caused a wide spectrum of disease, with symptoms ranging from mild to severe, life-threatening diarrhea. The similarity of etiological agents identified from children with mild and severe forms of acute diarrhea suggests that the etiology of community enteric illness can be reasonably inferred from the etiology of inpatient disease in children in the same geographic area. During the winter epidemic period, the severity of symptoms associated with rotavirus infection in young children is likely to be determined by the inherent susceptibility of the host rather than by genetic differences in the strains of infecting rotaviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott M. R., Cloonan M. J., Montgomery J., Smith D. D. Pathogens detected in the faeces of children with diarrhoea in a Sydney hospital. Med J Aust. 1984 Jul 7;141(1):26–28. doi: 10.5694/j.1326-5377.1984.tb132663.x. [DOI] [PubMed] [Google Scholar]
- Bartlett A. V., Moore M., Gary G. W., Starko K. M., Erben J. J., Meredith B. A. Diarrheal illness among infants and toddlers in day care centers. I. Epidemiology and pathogens. J Pediatr. 1985 Oct;107(4):495–502. doi: 10.1016/s0022-3476(85)80004-4. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Black R. E., Merson M. H., Rahman A. S., Yunus M., Alim A. R., Huq I., Yolken R. H., Curlin G. T. A two-year study of bacterial, viral, and parasitic agents associated with diarrhea in rural Bangladesh. J Infect Dis. 1980 Nov;142(5):660–664. doi: 10.1093/infdis/142.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Stallings E. P., Lewis C., Miles A. J., Gardner M. K., Parrott R. H. Adenoviruses and pediatric gastroenteritis. J Infect Dis. 1985 Mar;151(3):437–443. doi: 10.1093/infdis/151.3.437. [DOI] [PubMed] [Google Scholar]
- Davidson G. P., Bishop R. F., Townley R. R., Holmes I. H. Importance of a new virus in acute sporadic enteritis in children. Lancet. 1975 Feb 1;1(7901):242–246. doi: 10.1016/s0140-6736(75)91140-x. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Sequence homology between human and animal rotavirus serotype-specific glycoproteins. Nucleic Acids Res. 1984 May 11;12(9):3973–3982. doi: 10.1093/nar/12.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Muñz O., Serafin F., Romero P. Shift in the prevalent human rotavirus detected by ribonucleic acid segment differences. Infect Immun. 1980 Feb;27(2):351–354. doi: 10.1128/iai.27.2.351-354.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. R. Infectious diarrhoea in children. Aust Paediatr J. 1979 Mar;15(Spec No):25–29. doi: 10.1111/j.1440-1754.1979.tb01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirubakaran C., Davidson G. P., Darby H., Hansman D., McKay G., Moore B., Lee P. Campylobacter as a cause of acute enteritis in children in South Australia. I. A 12-month study with controls. Med J Aust. 1981 Oct 3;2(7):333–335. doi: 10.5694/j.1326-5377.1981.tb100991.x. [DOI] [PubMed] [Google Scholar]
- Konno T., Sato T., Suzuki H., Kitaoka S., Katsushima N., Sakamoto M., Yazaki N., Ishida N. Changing RNA patterns in rotaviruses of human origin: demonstration of a single dominant pattern at the start of an epidemic and various patterns thereafter. J Infect Dis. 1984 May;149(5):683–687. doi: 10.1093/infdis/149.5.683. [DOI] [PubMed] [Google Scholar]
- Koopman J. S., Turkish V. J., Monto A. S., Gouvea V., Srivastava S., Isaacson R. E. Patterns and etiology of diarrhea in three clinical settings. Am J Epidemiol. 1984 Jan;119(1):114–123. doi: 10.1093/oxfordjournals.aje.a113712. [DOI] [PubMed] [Google Scholar]
- Monto A. S., Koopman J. S., Longini I. M., Isaacson R. E. The Tecumseh study. XII. Enteric agents in the community, 1976-1981. J Infect Dis. 1983 Aug;148(2):284–291. doi: 10.1093/infdis/148.2.284. [DOI] [PubMed] [Google Scholar]
- Monto A. S., Koopman J. S. The Tecumseh Study. XI. Occurrence of acute enteric illness in the community. Am J Epidemiol. 1980 Sep;112(3):323–333. doi: 10.1093/oxfordjournals.aje.a112998. [DOI] [PubMed] [Google Scholar]
- Murphy A. Aetiology of viral gastroenteritis: a review. Med J Aust. 1981 Aug 22;2(4):177–182. [PubMed] [Google Scholar]
- Roberton D. M., Harrison M., Hosking C. S., Adams L. C., Bishop R. F. Rapid diagnosis of rotavirus infection: comparison of electron microscopy and enzyme linked immunosorbent assay (Elisa). Aust Paediatr J. 1979 Dec;15(4):229–232. doi: 10.1111/j.1440-1754.1979.tb01235.x. [DOI] [PubMed] [Google Scholar]
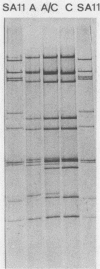
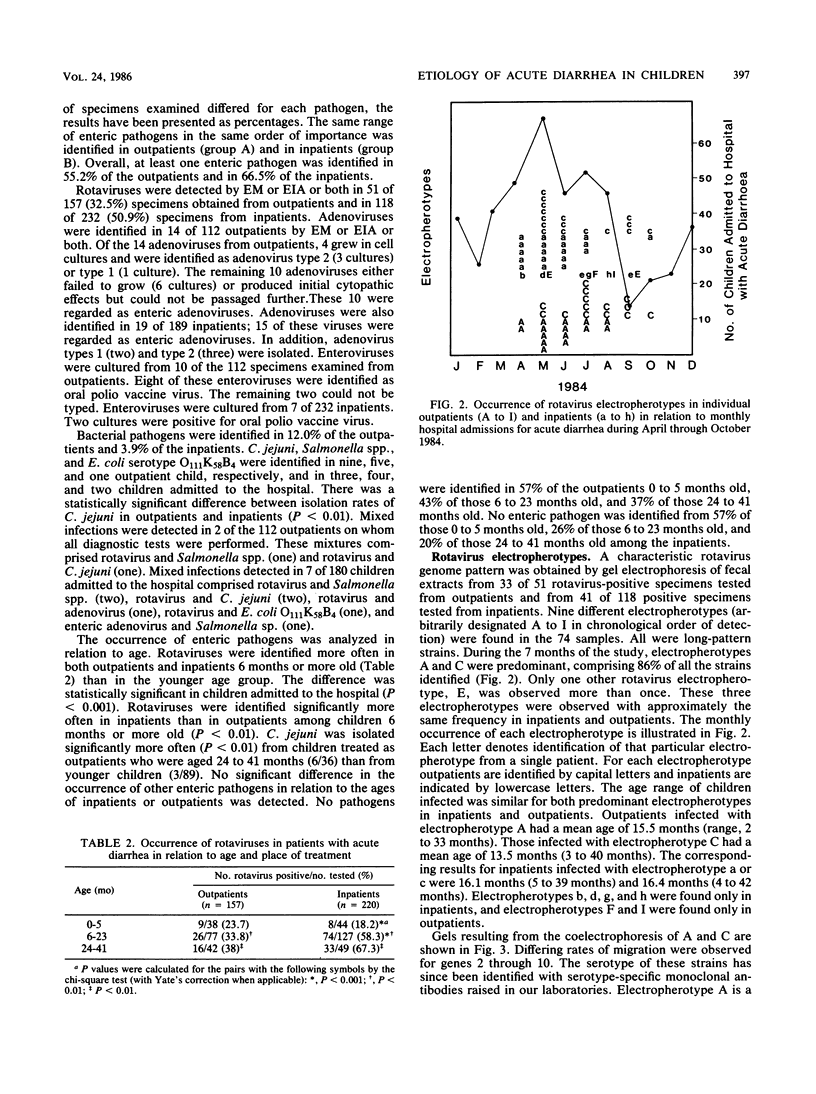
- Rodger S. M., Bishop R. F., Birch C., McLean B., Holmes I. H. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. J Clin Microbiol. 1981 Feb;13(2):272–278. doi: 10.1128/jcm.13.2.272-278.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W. J., Kim H. W., Brandt C. D., Bise B., Kapikian A. Z., Chanock R. M., Curlin G., Parrott R. H. Rotavirus gastroenteritis in the Washington, DC, area: incidence of cases resulting in admission to the hospital. Am J Dis Child. 1980 Aug;134(8):777–779. doi: 10.1001/archpedi.1980.02130200047015. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby H. J., Rodgers F. G. Detection of virus particles by electron microscopy with polyacrylamide hydrogel. J Clin Pathol. 1980 May;33(5):484–487. doi: 10.1136/jcp.33.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mol P., Brasseur D., Hemelhof W., Kalala T., Butzler J. P., Vis H. L. Enteropathogenic agents in children with diarrhoea in rural Zaire. Lancet. 1983 Mar 5;1(8323):516–518. doi: 10.1016/s0140-6736(83)92202-x. [DOI] [PubMed] [Google Scholar]