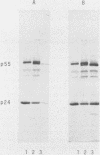
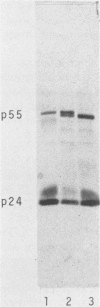
Abstract
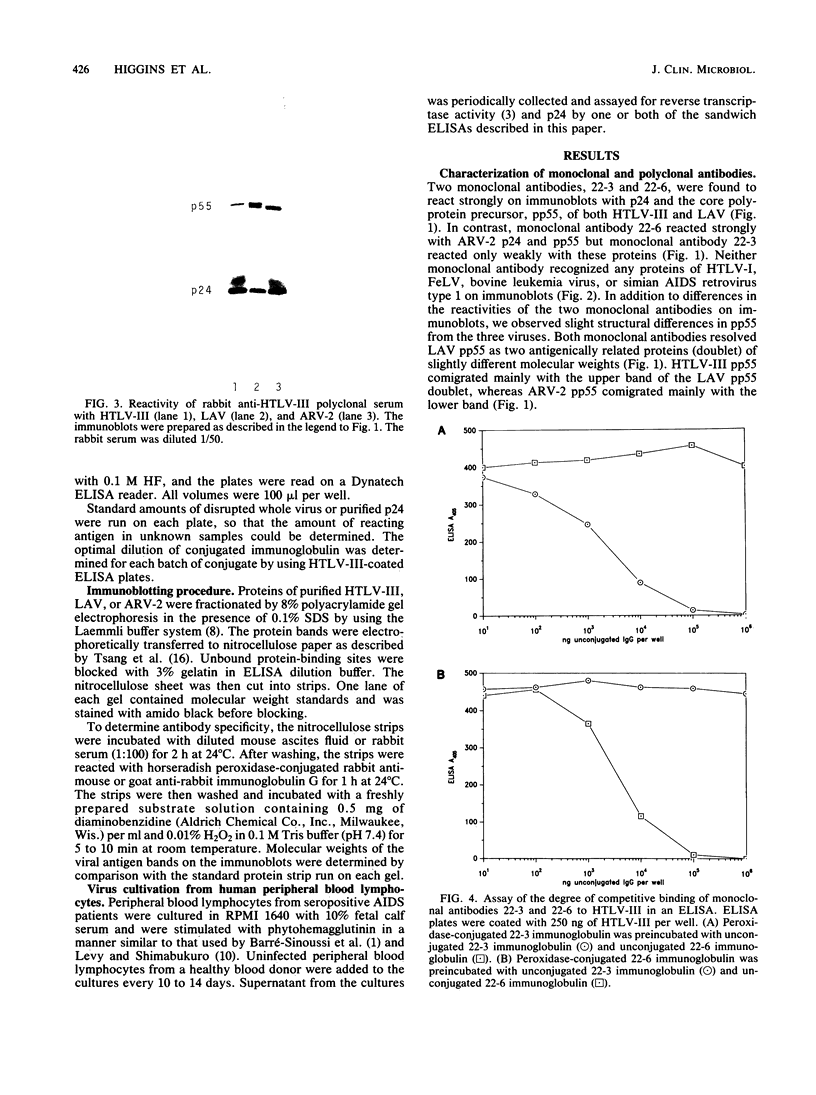
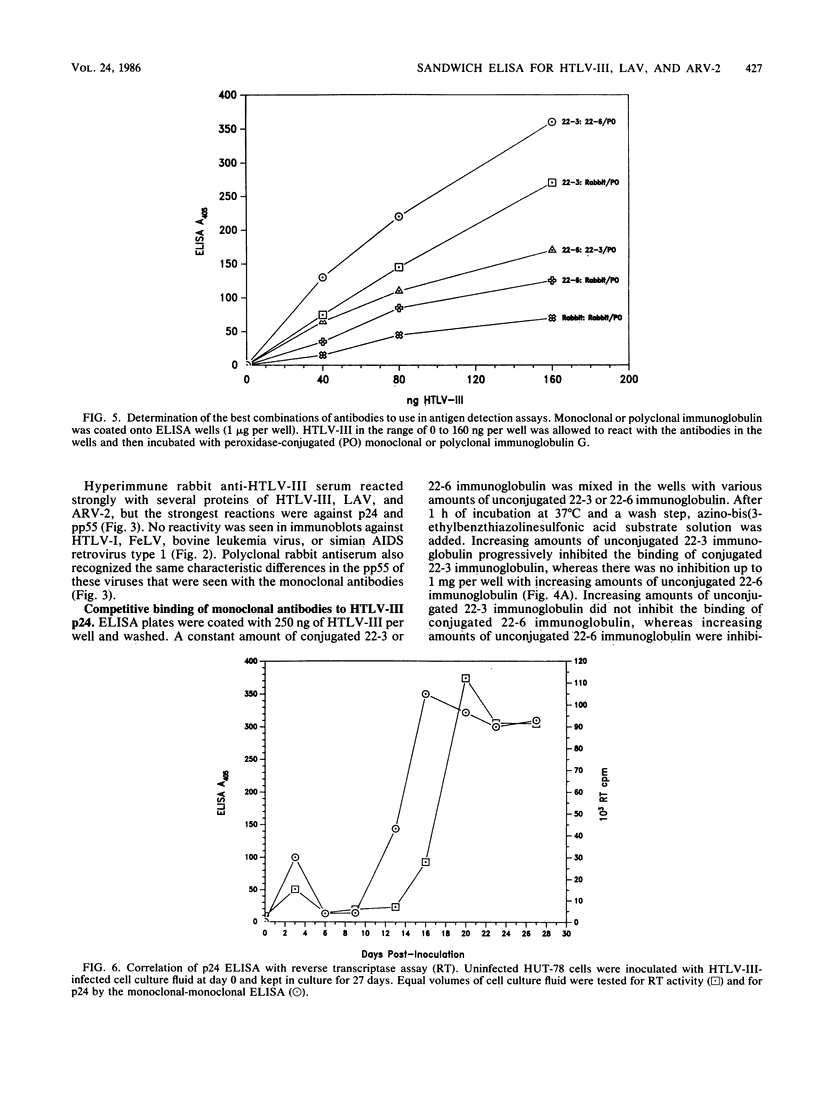
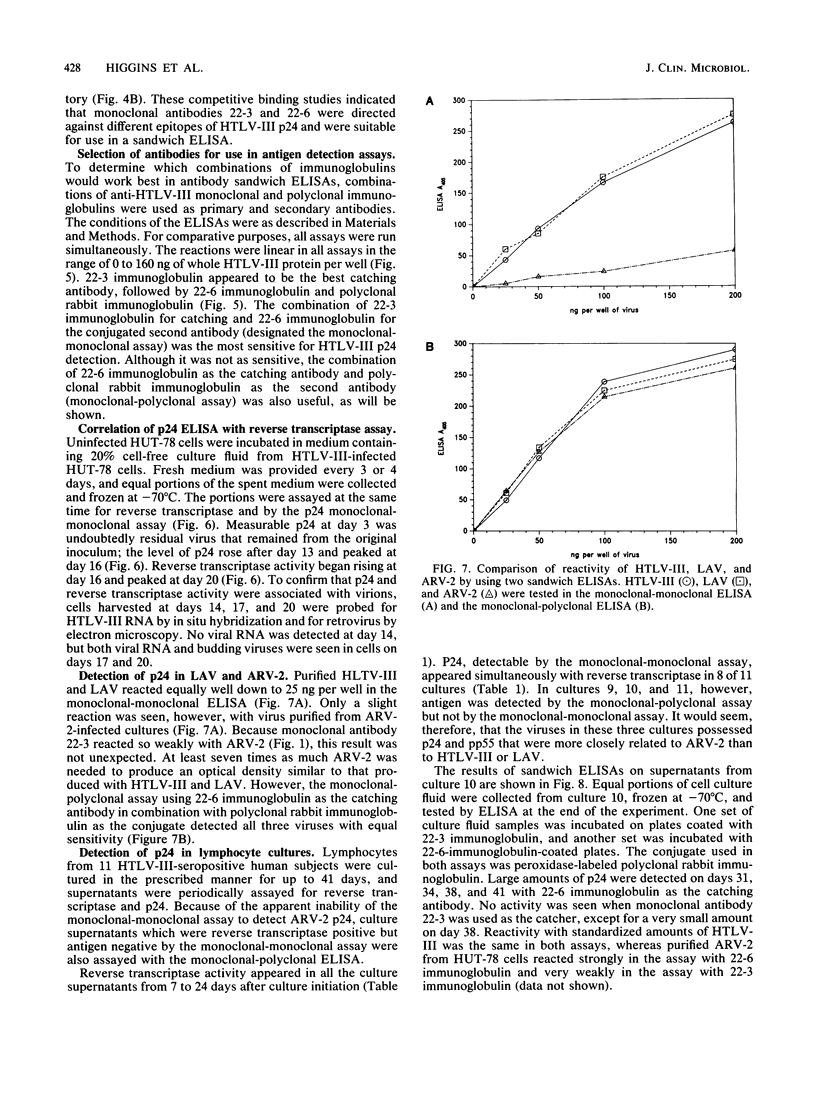
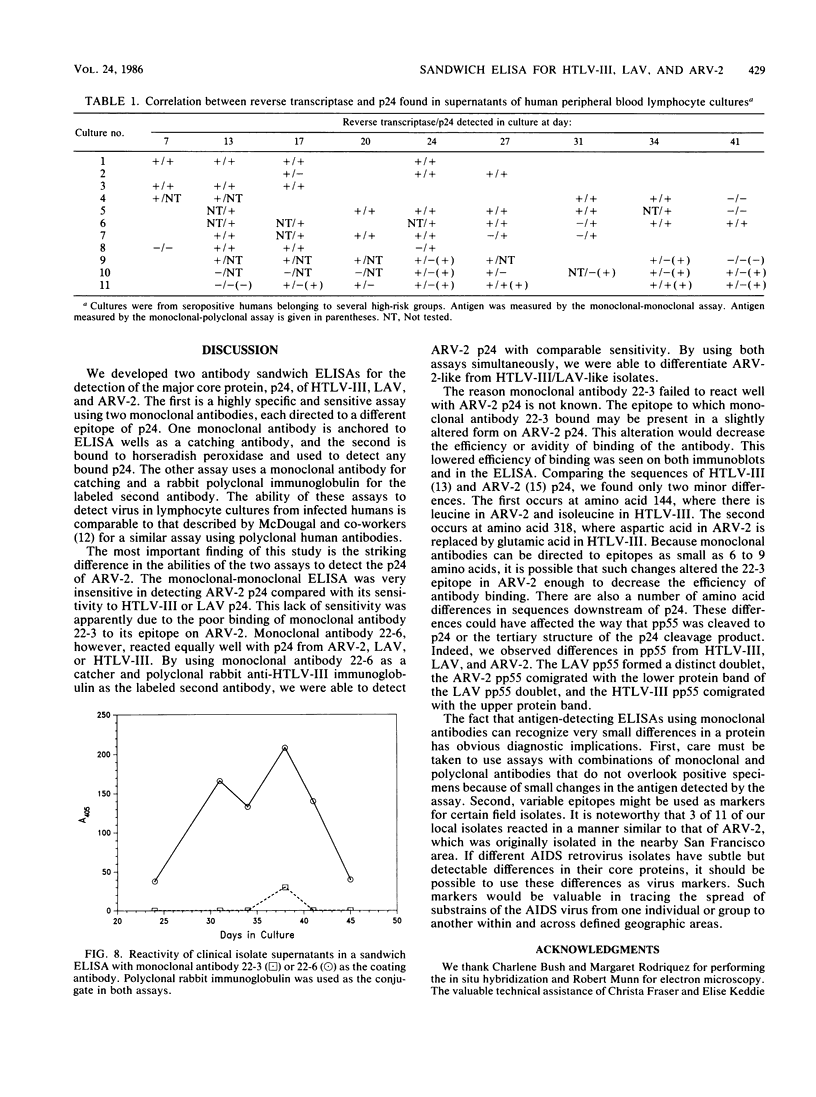
Monoclonal antibodies can be used in sandwich enzyme-linked immunosorbent assays to measure viral antigens. Such an assay was developed to detect the core protein, p24, of human T-cell lymphotropic virus type III and lymphadenopathy-associated virus, etiologic agents of the acquired immunodeficiency syndrome (AIDS). Another AIDS-associated virus, AIDS-associated retrovirus type 2 (ARV-2) could not be detected in this assay because of the low affinity of one of the monoclonal antibodies to ARV-2 p24. Detection of ARV-2 was accomplished with a monoclonal antibody-rabbit polyclonal antibody sandwich enzyme-linked immunosorbent assay. These two assays were used to efficiently detect AIDS-related viruses in lymphocyte cell cultures and to distinguish strains of the viruses.
Full text
PDF
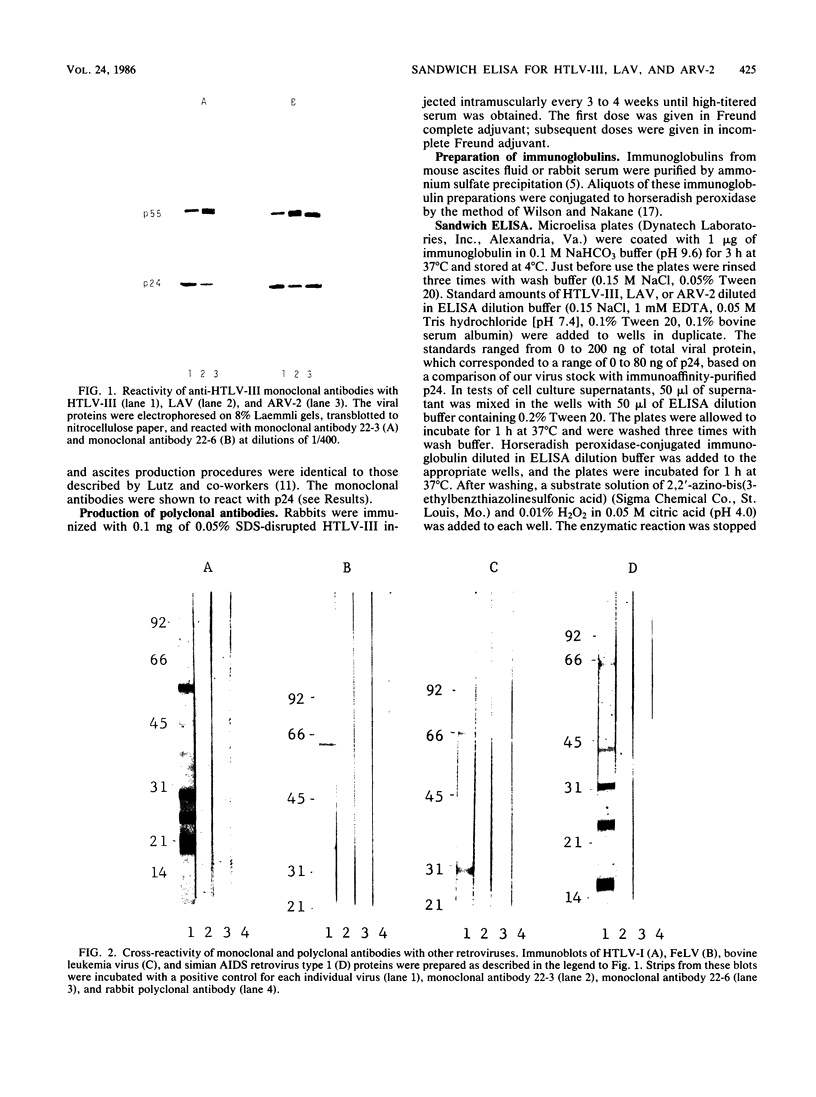

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Colcher D., Schlom J. Purification and characterization of the RNA-directed DNA polymerase of a primate type-D retrovirus: Mason-Pfizer virus. Biochim Biophys Acta. 1980 May 30;607(3):445–456. doi: 10.1016/0005-2787(80)90155-0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985 Oct;152(4):734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- Lutz H., Pedersen N. C., Durbin R., Theilen G. H. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J Immunol Methods. 1983 Jan 28;56(2):209–220. doi: 10.1016/0022-1759(83)90413-1. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Cort S. P., Kennedy M. S., Cabridilla C. D., Feorino P. M., Francis D. P., Hicks D., Kalyanaraman V. S., Martin L. S. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV). J Immunol Methods. 1985 Jan 21;76(1):171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]