Abstract
Of the many signals in the developing nervous system, GABA (γ-aminobutyric acid) has been shown to be one of the earliest neurotransmitters present. Unlike in the adult, where this transmitter acts synaptically to inhibit neurons, during development, GABA can depolarize progenitor cells and their progeny due to their high intracellular chloride concentration. This early form of GABA signalling may provide the main excitatory drive for the immature cortical network and play a central role in regulating cortical development. Many features of GABA signalling are conserved in different species and are recapitulated during neurogenesis in the adult brain, demonstrating the importance of this versatile molecule in driving cortical formation. Here, we present recent evidence supporting the multiple functions of GABA during embryonic development and adult neurogenesis, from regulating progenitor proliferation to influencing the migration and maturation of newborn neurons.
The construction of a three-dimensional cerebral cortex from a single sheet of neuroepithelium requires multiple tightly regulated developmental processes working in concert. In order to form this complex structure, neurons must be generated in the correct numbers, migrate to the proper position in the cortex, and form intricate connections with neighbours and targets. Of the many cell-intrinsic and extrinsic signals involved in neocortical development, neurotransmitters have been shown to be key players in building the cortical network, and GABA in particular proves to be an important regulator in this process.
GABA is the main inhibitory neurotransmitter in the adult brain and acts primarily by binding to GABAA or GABAB receptors. The GABAA receptor gates a Cl− channel, and in the mature brain, GABA activation causes Cl− influx and membrane hyperpolarization due to the low intracellular Cl− concentration established by the K+–Cl− cotransporter KCC2 (Rivera et al. 1999; Li et al. 2002; Wang et al. 2002). However, during development, GABA exerts a different effect by depolarizing cortical progenitors (radial glia) and immature neurons. This is due to the Cl− gradient established by the Na+–K+–2Cl− cotransporter NKCC1, expressed from mid-embryonic stages until the first week of postnatal life in rodents (Li et al. 2002; Wang et al. 2002). NKCC1 imports Cl− into immature cells, thereby causing Cl− efflux and membrane depolarization upon GABAA receptor activation (Plotkin et al. 1997; Wang et al. 2002). Interestingly, radial glia and migrating neurons express GABAA receptors early in development, thus providing them with the mechanism to respond to GABA (LoTurco et al. 1995; Owens et al. 1996, 1999). Why is a GABA signalling system established well before functional synapses are formed? GABA-mediated signalling has been implicated in most of the developmental steps from cell proliferation to synaptic integration. As it is the first neurotransmitter active in the immature brain and provides the main excitatory drive, GABA is poised to serve as an ideal signal to coordinate corticogenesis. Here, we will discuss the developmental roles of GABA in modulating progenitor proliferation, neuronal migration, and circuit formation in the cortex. We will focus on recent studies and address controversial as well as emerging functions of GABA in network construction.
GABA and stem cell proliferation
During development, radial glia, which are the cortical neural stem cells, express functional GABAA receptors (LoTurco et al. 1995; Owens et al. 1996). This finding has led to the hypothesis that GABA supports a trophic function besides its normal role in synaptic transmission. A study from our lab demonstrated that GABA and glutamate decreased net DNA synthesis and the number of progenitor cells that incorporate BrdU in acute cortical slices (LoTurco et al. 1995). Interestingly, incubating the slices with the GABAA receptor antagonist bicuculline increased DNA synthesis, suggesting that there is tonic release of endogenous GABA to regulate the rate of neurogenesis. GABA's effect on cortical progenitor proliferation is likely to be due to its ability to depolarize the cell and activate voltage-gated Ca2+ channels (VGCCs) that in turn regulate DNA synthesis (LoTurco et al. 1995; Owens & Kriegstein, 2002; Represa & Ben-Ari, 2005). Another study compared the proliferative effects of GABA between the two proliferative zones in the embryonic cortex, the ventricular (VZ) and subventricular (SVZ) zones (Haydar et al. 2000). Using organotypic slices of mouse embryonic cortex and BrdU labelling, the authors found that GABA appeared to have different effects: promoting VZ cell division while inhibiting SVZ cell divisions (Haydar et al. 2000). With molecular markers now available to selectively label neurogenic and gliogenic precursors, it would be interesting to explore GABA's effects on the proliferation of these two cell types and the mechanism for potential differences.
Likewise, studies have shown that GABA can inhibit cell cycle progression in other systems, such as the neural precursors in neurospheres and organotypic brain slices (Nguyen et al. 2003) and in the adult neurogenic SVZ (Liu et al. 2005). In the adult SVZ, immature neurons synthesize and release GABA (Stewart et al. 2002; Bolteus & Bordey, 2004; Liu et al. 2005), which in turn activates GABAA receptors in stem cells and immature neurons. Tonic GABAA activation depolarizes stem cells, causes transient intracellular Ca2+ increases, and reduces the number of proliferative stem cells (Liu et al. 2005). This provides an intriguing mechanism in which the generation of young GABAergic neurons would increase ambient GABA levels and serve as a negative feedback loop to reduce their generation by slowing down stem cell proliferation (Platel et al. 2008). Apparently, the theme of GABA inhibiting stem cell proliferation is developmentally conserved as this occurs even before the formation of the nervous system. Recent evidence suggests that in mice, embryonic stem cells as well as neural crest stem cells express glutamic acid decarboxylase (GAD) and functional GABAA receptors, and just like in the developing cortex, GABAA activation decreased cell proliferation (Andang et al. 2008). However, here GABA hyperpolarizes the stem cells and activates the S/G2 DNA-damage checkpoint pathway to inhibit cell cycle progression. This novel mode of neurotransmitter involvement in S-phase checkpoint control may operate in other areas of the nervous system, and disruptions in this pathway may contribute to various congenital CNS malformations (Andang & Lendahl, 2009). It seems that even though GABA can activate different signalling pathways depending on cell-intrinsic factors or extrinsic environmental cues, it generally functions to decrease progenitor or stem cell proliferation.
GABA and migration
Chemotropic actions of GABA were first identified using chemotaxis chambers, where dissociated cortical cells were allowed to undergo gradient-dependent migration, and it was found that extremely low concentrations of GABA induced cell migration by activating GABAA receptors (Behar et al. 1996). However, these studies remain controversial as GABA produces half-maximal responses in the developing neocortex in the micromolar range and GABAA receptors are not sensitive in the femtomolar range (Owens et al. 1999). Another study showed that blocking GABAA receptors in hippocampal slice cultures reduced the migration of neuroblasts (Manent et al. 2005). Following these experiments, Behar and colleagues incubated organotypic neocortical explants with specific GABA receptor antagonists to show that activation of different GABA receptor subtypes regulated various parts of neural migration to the cortical plate. Activation of GABAA/C receptor promoted migration of neuroblasts from the VZ/SVZ to the intermediate zone (IZ), GABAB receptor the entry of migrating neuroblasts from the IZ to the cortical plate, and finally GABAA receptor to provide a stop signal to end migration (Behar et al. 2000).
More recently, in vivo and in vitro studies demonstrated that GABAA receptor desensitization by agonist activation or blockade with antagonists caused heterotopias in the most superficial cortical layers, likely to be due to an overmigration from the loss of a stop signal (Heck et al. 2007). Heck and colleagues demonstrated that GABAA receptor activation leads to transient Ca2+ channel oscillations that may be important for promoting neuronal migration. These studies suggest that the effect of GABA on migration is highly concentration- and location-dependent. GABAA receptors confer a high degree of variability due to their subunit composition. Interestingly, different GABAA receptor subunits are expressed in a time- and location-specific manner during cortical development (Laurie et al. 1992). Because these diverse receptor subunits have different sensitivities to GABA, their interactions with extrinsic environmental cues may differentially regulate the migration of newborn neurons. Recently, a study questioned the necessity of GABA-mediated depolarization in cortical neuron migration. The authors used in utero electroporation to overexpress KCC2 in proliferating neuronal progenitors to render immature neurons devoid of depolarizing GABAergic responses (Cancedda et al. 2007). While morphological maturation was markedly impaired in KCC2-expressing immature neurons, neuronal migration was not affected. It is unknown whether GABA-mediated depolarization regulates the migration of deeper-layer, earlier-born neurons, as the study only manipulated neurons born at E18–19. Future studies targeting the earlier-born neurons would help address the current discrepancy.
A GABAergic signalling system influencing neuronal migration is recapitulated in the adult neurogenic regions as well. In the adult SVZ, several studies have identified the contribution of GABA in regulating the migration of immature neurons from the SVZ to the olfactory bulb (Nguyen et al. 2003; Bolteus & Bordey, 2004; Liu et al. 2005; Ge et al. 2006). Bolteus and Bordey studied young neuron migration in acute sagittal brain slices to determine if GABA signalling between stem cells and immature neurons regulates the speed of neuronal migration in the rostral migratory stream (RMS). It was found that ambient GABA slowed down the migration of young neurons by causing an increase in intracellular Ca2+, independent of membrane depolarization (Bolteus & Bordey, 2004). Interestingly, stem cells ensheathing migrating neurons express the high-affinity GABA transporter GAT4. This suggests a model wherein neural precursors can fine-tune the speed of neuronal migration within the chains by regulating ambient GABA levels (Bolteus & Bordey, 2004).
GABA and synaptogenesis
Neural circuit development is a complicated process whereby a neuron extends its axon and receives inputs from targets. For a young neuron to become a functional unit of the cortical circuit, it must establish a mature morphology, make synaptic contacts with other cells, and refine those connections. Increasing evidence suggests that neuronal activity regulates circuit formation. In many brain regions examined in rodents and primates, newborn neurons express GABAA receptors before glutamate receptors. Moreover, young neurons first receive GABAergic inputs before forming glutamatergic synapses in the neocortex (Owens et al. 1999; Tyzio et al. 1999; Hennou et al. 2002; Ben-Ari, 2006). These spontaneous GABA depolarizations may provide the first excitatory drive necessary for activity-dependent synapse formation (Ben-Ari, 2006). Why is a GABA signalling system established before a glutamatergic one? GABA can at least partially substitute for glutamate as an excitatory neurotransmitter by providing enough membrane depolarization to activate voltage-gated calcium channels (VGCC) (Owens & Kriegstein, 2002; Ben-Ari, 2006). However, unlike glutamate, GABA exerts a shunting effect by clamping the membrane potential near the Cl− reversal potential (approximately −40 mV in immature neurons), thereby preventing long-lasting activation of voltage-gated channels that might engender toxic amounts of Ca2+ influx. This makes GABA an ideal regulator for synaptogenesis because this neurotransmitter can excite neurons without directly producing excitotoxicity.
Several recent studies have manipulated the Cl− gradient to study the effect of GABA-depolarization on synaptogenesis. By prematurely shifting the GABA reversal potential to a more hyperpolarizing potential (by knocking down NKCC1 or overexpressing KCC2), it has recently been shown that reversing the excitatory effect of GABA in cortical neurons results in fewer and shorter dendrites (Cancedda et al. 2007) and less mature dendritic spines (decreased density and increased lengths) (Wang & Kriegstein, 2008). Likewise, in the adult hippocampus, disturbing GABA-mediated depolarization altered the morphology of newly generated granule neurons (Ge et al. 2006). Evidence suggests that morphological changes could be a result of disrupted glutamatergic signalling in these neurons. Glutamatergic activity, via AMPA receptor-mediated transmission, has been known to promote dendritic arbor growth by mobilizing intracellular signalling cascades that stabilize and maintain branch formation (Wu et al. 1996; Rajan & Cline, 1998; Shi et al. 1999; Haas et al. 2006).
Moreover, increasing evidence shows that GABA-mediated depolarization regulates synaptic development of young neurons. Recently, by altering the Cl− gradient of newborn cortical neurons in vivo, we showed that GABA-induced excitation via GABAA receptor activation plays a permissive role in the formation of synaptic inputs on newborn cortical neurons (Wang & Kriegstein, 2008). Our results complement the findings of a study in newly generated granule neurons in the adult dentate gyrus (Ge et al. 2006), providing evidence that GABA-mediated excitation drives synaptic integration of newborn neurons in both embryos and adults. Intriguingly, GABA not only regulates development of synapses in the nervous system, but it also regulates the balance between excitation and inhibition in a developing circuit. A premature hyperpolarizing shift in the Cl− reversal potential has been shown to increase the ratio of inhibitory to excitatory inputs in Xenopus tectal neurons and rat cortical neurons in culture (Chudotvorova et al. 2005; Akerman & Cline, 2006). This occurs in the mammalian cortex as well, as GABA regulates the formation of excitatory synapses in the neocortical circuit (Wang & Kriegstein, 2008). In addition, the mechanism for GABA's regulation of excitatory synapse formation entails the cooperation between GABAA and NMDA receptor activation. Cortical neurons begin to express functional NMDA receptors when they migrate to the cortical plate, but the initial glutamatergic synapses are ‘silent’ due to the Mg2+ block of NMDA receptors at the resting membrane potential (LoTurco et al. 1991; Akerman & Cline, 2006). GABAergic depolarization can facilitate relief of this voltage-dependent Mg2+ block and allow Ca2+ entry to initiate intracellular signalling cascades (Leinekugel et al. 1997; Owens & Kriegstein, 2002; Ben-Ari, 2006). Cooperation with NMDA receptor activation represents an early form of coincidence detection between GABAergic and glutamatergic inputs. The degree of GABA activation reflects the level of GABAergic inputs onto a newborn neuron, and GABA depolarizations permit the development of glutamatergic synapses, thus ensuring a balance between excitation and inhibition from the beginning of synaptogenesis.
Unanswered questions
Despite these findings, in vivo evidence for a role of GABA in development is somewhat controversial. In GAD 65−/− : 67−/− mice (double knockout for glutamic acid decarboxylase 65 and 67), GABA levels in the brain were reduced to less than 5%, but the size and shape of the brains at E14 and P0 were indistinguishable from heterozygous littermates (Ji et al. 1999). While these GAD 65/67−/− mice die at birth due to cleft palate, no discernable neural developmental defects were detected in the neocortex, cerebellum, or hippocampus (Ji et al. 1999). This study seems to suggest that GAD activity and GABA are not crucial for trophic support during neocortical development. Possibly other substances such as glutamate, taurine, or glycine could compensate for the lack of GABA. Glutamate has been shown to produce a similar increase in intracellular Ca2+ concentration as GABA and to inhibit progenitor DNA synthesis in the VZ (LoTurco et al. 1995). Similar to GABA, glycine and taurine gate Cl− channels and can depolarize immature neurons (Flint et al. 1998). Furthermore, because the GAD 65/67 double knockout animals die young, it is unknown whether synaptogenesis and circuit development is normal in these mice.
Another major mystery is how GABA is released at early stages. In adult neurons, vesicles containing GABA fuse with the presynaptic membrane to release GABA into the cleft. However, in the absence of vesicular release, such as in the case of mice lacking Munc13, a protein necessary for synaptic vesicle maturation, neurons form normal synapses (Varoqueaux et al. 2002). Thus, synaptogenesis during CNS development is not dependent on synaptic secretory activity. In addition, tonic, spontaneous and evoked GABA currents can be detected in CA1 pyramidal neurons in Munc18-1-deficient mice in which vesicular release is abolished (Demarque et al. 2002). Blocking GABAA receptors in these mice also results in abnormal neuroblast migration, providing further evidence that GABA is released in a non-canonical, paracrine fashion (Manent et al. 2005). Furthermore, studies in the immature neurons of the adult SVZ suggest that GABA is not released through synapses, GABA transporter reversal, or hemichannels (Liu et al. 2005), leaving few alternative possibilities. One possible mechanism of GABA release is unconventional exocytosis triggered by intracellular Ca2+ release. Further studies are required to define the mechanism of non-synaptic GABA release.
Finally, the correlation between chloride transport by NKCC1 and KCC2 and the polarity of GABA-mediated responses might not pertain to all brain areas. For instance, in the lateral superior olive (LSO) of the auditory brainstem, neuronal responses to GABA and glycine shift from depolarizing to hyperpolarizing at P4–5 (Lohrke et al. 2005). However, the Cl− exporter KCC2 is already present at birth, at a time when glycine still exerts a depolarization effect (Lohrke et al. 2005). This seems to suggest that the mere emergence of KCC2 appears to be unrelated to the depolarization–hyperpolarization shift. This was further confirmed in KCC2 knockout mice, where the glycine reversal potential, which depends on the Cl− gradient, did not differ significantly from that of wild-type controls at P3, demonstrating that KCC2 is not active in neonates despite its early presence (Balakrishnan et al. 2003). Even more intriguing is the lack of NKCC1 mRNA during the depolarizing phase in the LSO, implying that this transporter does not contribute to the high intracellular Cl− (Balakrishnan et al. 2003). These data suggest that Cl− regulation in the brainstem differs significantly from that in the forebrain. Perhaps there are other active Cl− transporters that regulate Cl− gradients in these areas.
Conclusion
GABA has proven to be a versatile molecule with multiple functions during neocortical development. Most of GABA's trophic functions rely on its ability to depolarize progenitors, which depends upon the Cl− gradient. In fact, the high intracellular Cl− gradient in immature neurons has been observed in different species from Xenopus to primates and thus is highly evolutionarily conserved (Owens et al. 1996; Khazipov et al. 2001; Akerman & Cline, 2006). Activation of GABAA receptors in progenitor cells and immature neurons can depolarize the membrane sufficiently to activate VGCC or NMDA receptors. Activation of VGCCs in radial glia results in an influx of Ca2+ that can inhibit DNA synthesis and cell proliferation (Fig. 1) (LoTurco et al. 1995), whereas in immature neurons, GABA-mediated signalling can decrease the speed of neuronal migration (Fig. 2A) (Bolteus & Bordey, 2004). Finally, during synaptogenesis, GABA can cooperate with NMDA receptor activation to regulate the stabilization of AMPA receptors at the synapse (see Figs 1 and 2B). Depending on the phase of development, depolarizing GABA can have different effects mediated through calcium channel activation and second messengers interacting with a variety of signalling pathways.
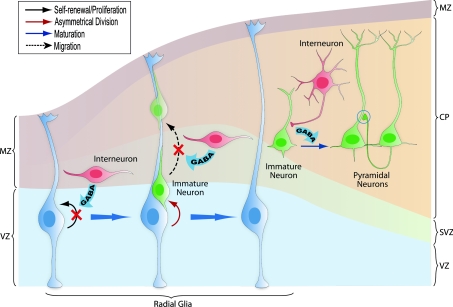
Figure 1. GABA's role in regulating embryonic cortical development.
During corticogenesis, interneurons migrating in the subventricular zone (SVZ) can release GABA and activate GABAA receptors on the radial glia, depolarizing these progenitors and decreasing their proliferation. Radial glia generates immature pyramidal neurons through asymmetrical division, and the migration of these immature neurons along the radial fibres is decreased by GABA signalling. As young neurons assume their position in the cortex and begin to mature, GABA-mediated depolarization by the interneurons is required for the development of dendritic arbors and excitatory synaptic inputs from other pyramidal neurons. MZ: marginal zone; VZ: ventricular zone; SVZ: subventricular zone; CP: cortical plate.
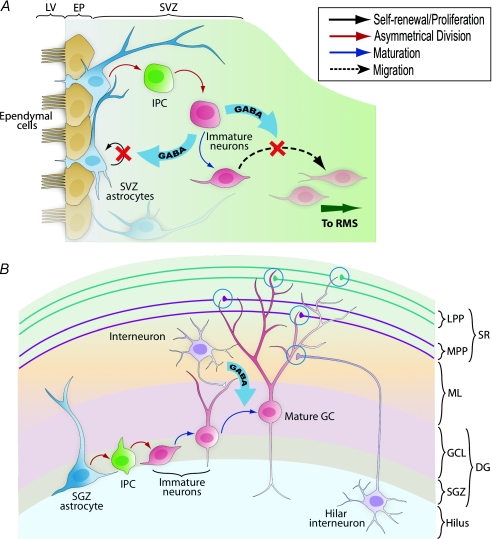
Figure 2. GABA's role in adult neurogenesis in the subventricular zone (A) and the dentate gyrus of the hippocampus (B).
A, in this adult neurogenic niche, astrocytes divide asymmetrically to give rise to intermediate progenitors (IPC), which divide again to generate immature neurons. These immature neurons produce GABA, which activates GABAA receptors in the subventricular zone astrocytes and depolarizes their membrane to negatively regulate their proliferation. GABA produced by the young neurons decreases their migration to the rostral migratory stream (RMS), where they will eventually become granule cells or periglomerular cells in the olfactory bulb. B, astrocytes of the subgranular zone are the stem cells that can produce IPCs through asymmetrical division. These IPCs generate immature neurons, which express GABAA receptors and respond tonically to GABA released from local interneurons. GABA-mediated depolarization regulates the synaptic maturation and integration of granule cells into the existing circuit, allowing them to receive inhibitory inputs from hilar interneurons and excitatory glutamatergic inputs from the lateral and medial perforant pathways. LV: lateral ventricle; EP: ependymal cell layer; SVZ: subventricular zone: SGZ: subgranular zone; GCL: granule cell layer; DG: dentate gyrus; ML: molecular layer; MPP: medial perforant pathway; LPP: lateral perforant pathway; SR: stratum radiatum.
These diverse roles of GABA, from proliferation and migration to synaptogenesis and circuit formation, seem to depend both on cell-intrinsic properties and on extrinsic factors. GABA's various functions also stem from its unique physiology and the changes that accompany its actions over time. With new tools and technological advances, we will undoubtedly see further studies that will deepen our understanding of this interesting and versatile molecule.
References
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, Wilbertz J, Arenas E, Koltzenburg M, Charnay P, El Manira A, Ibanez CF, Ernfors P. Histone H2AX-dependent GABAA receptor regulation of stem cell proliferation. Nature. 2008;451:460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- Andang M, Lendahl U. Ion fluxes and neurotransmitters signaling in neural development. Curr Opin Neurobiol. 2009 doi: 10.1016/j.conb.2008.06.001. in press. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Basic developmental rules and their implications for epilepsy in the immature brain. Epileptic Disord. 2006;8:91–102. [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudotvorova I, Ivanov A, Rama S, Hubner CA, Pellegrino C, Ben-Ari Y, Medina I. Early expression of KCC2 in rat hippocampal cultures augments expression of functional GABA synapses. J Physiol. 2005;566:671–679. doi: 10.1113/jphysiol.2005.089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR. Nonsynaptic glycine receptor activation during early neocortical development. Neuron. 1998;20:43–53. doi: 10.1016/s0896-6273(00)80433-x. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci U S A. 2006;103:12127–12131. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck N, Kilb W, Reiprich P, Kubota H, Furukawa T, Fukuda A, Luhmann HJ. GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb Cortex. 2007;17:138–148. doi: 10.1093/cercor/bhj135. [DOI] [PubMed] [Google Scholar]
- Hennou S, Khalilov I, Diabira D, Ben-Ari Y, Gozlan H. Early sequential formation of functional GABAA and glutamatergic synapses on CA1 interneurons of the rat foetal hippocampus. Eur J Neurosci. 2002;16:197–208. doi: 10.1046/j.1460-9568.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- Ji F, Kanbara N, Obata K. GABA and histogenesis in fetal and neonatal mouse brain lacking both the isoforms of glutamic acid decarboxylase. Neurosci Res. 1999;33:187–194. doi: 10.1016/s0168-0102(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Esclapez M, Caillard O, Bernard C, Khalilov I, Tyzio R, Hirsch J, Dzhala V, Berger B, Ben-Ari Y. Early development of neuronal activity in the primate hippocampus in utero. J Neurosci. 2001;21:9770–9781. doi: 10.1523/JNEUROSCI.21-24-09770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Li H, Tornberg J, Kaila K, Airaksinen MS, Rivera C. Patterns of cation-chloride cotransporter expression during embryonic rodent CNS development. Eur J Neurosci. 2002;16:2358–2370. doi: 10.1046/j.1460-9568.2002.02419.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrke S, Srinivasan G, Oberhofer M, Doncheva E, Friauf E. Shift from depolarizing to hyperpolarizing glycine action occurs at different perinatal ages in superior olivary complex nuclei. Eur J Neurosci. 2005;22:2708–2722. doi: 10.1111/j.1460-9568.2005.04465.x. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Blanton MG, Kriegstein AR. Initial expression and endogenous activation of NMDA channels in early neocortical development. J Neurosci. 1991;11:792–799. doi: 10.1523/JNEUROSCI.11-03-00792.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci. 2005;25:4755–4765. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, Rigo JM. Autocrine/paracrine activation of the GABAA receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Owens DF, Liu X, Kriegstein AR. Changing properties of GABAA receptor-mediated signaling during early neocortical development. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl– co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Hoge GJ, Zigova T, Luskin MB. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABAA receptors. J Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci. 2008;28:5547–5558. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]