Abstract
GABA-mediated synaptic inhibition is crucial in neural circuit operations. The development of GABAergic inhibitory synapses and innervation pattern in mammalian neocortex is a prolonged process, extending well into the postnatal period, and is regulated by neural activity and experience. Accumulating evidence supports the hypothesis that GABA signalling acts beyond synaptic transmission and regulates inhibitory synapse development; in other words, similar to glutamate signalling at developing excitatory synapses, GABA may coordinate pre- and post-synaptic maturation at inhibitory synapses. These findings raise numerous questions regarding the underlying mechanisms, including the role of GABA receptors and their link to synaptic adhesion molecules. Since synapse formation is a crucial component of axon growth, GABA signalling may also shape the axon arbor and innervation pattern of inhibitory neurons. A mechanism unique to GABAergic neurons is activity-dependent GABA synthesis, largely mediated through activity-regulated transcription of the rate-limiting enzyme GAD67. Such cell-wide as well as synaptic regulation of GABA signalling may constitute a mechanism by which input levels and patterns onto GABAergic neurons shape their innervation pattern during circuit development.
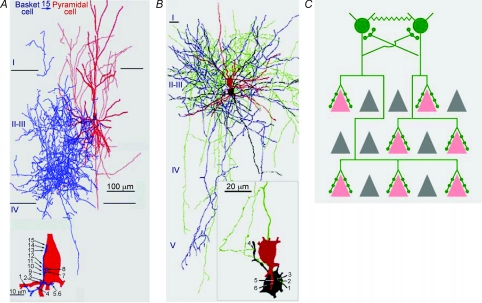
In many areas of the mammalian brain, such as the neocortex, neural circuits rely on inhibition mediated by γ-aminobutyric acid (GABA) from diverse cell types to control the spatiotemporal patterns of electrical signalling (Markram et al. 2004). The inhibitory output of GABAergic neurons is distributed in the network through their axons and synapses, which constitute elaborate and cell-type-specific innervation patterns (Huang et al. 2007). A prominent feature of GABAergic axon arbors in neocortex is their local exuberance: a single interneuron often produces extensive local arbors that innervate hundreds of neurons in its vicinity and form multiple clustered synapses onto each target neuron (Tamas et al. 1997; Wang et al. 2002). Such an innervation pattern probably contributes to their efficient control over the activity patterns in local cell populations. For example, a single parvalbumin-containing (PV) basket interneuron innervates hundreds of pyramidal neurons at the soma and proximal dendrites, and controls the output and synchrony of pyramidal neurons (Fig. 1; Cobb et al. 1995; Miles et al. 1996; Tamas et al. 1997). Furthermore, PV basket cells form extensive mutual innervation (Tamas et al. 2000) and, together with their unique physiological properties, contribute to the generation of coherent network oscillations that might organize functional neural ensembles (Bartos et al. 2007).
Figure 1. Perisomatic innervation pattern of the neocortical basket interneurons.
A, highly exuberant axonal arborization of a neocortical basket interneuron (blue) and one of its many postsynaptic pyramidal cells (red). Although the basket axon overlaps with a large part of the pyramidal basal dendritic tree, all 15 electron microscopically verified synaptic junctions (bottom panel, right) are clustered around the soma or the most proximal dendrites (bottom panel, left) (adapted from Tamas et al. 1997). B, reconstructions of the two PV basket cells connected by both chemical and electrical synapses (presynaptic cell: soma and dendrites, red; axon, green; postsynaptic cell: soma, dendrites, black; axon blue). Cortical layers (I−V) are indicated on the left. The electron microscopically identified synaptic junctions (1 − 4) and gap junctions (5, 6) mediating the interaction between the coupled cells were found nearby on the soma and a proximal dendrite (inset) (adapted from Tamas et al. 2000). C, a schematic showing prominent features of the innervation pattern of cortical basket interneurons. A single basket cell axon (green) innervating the many pyramidal neurons (pink) in its vicinity with clusters of perisomatic synapses (green dots). Basket cells also innervate other basket cells via chemical and electrical (zigzaged lines) synapses. Grey triangles represent pyramidal neurons that are not innervated by these basket cells.
The development of a mature GABAergic innervation pattern is often a prolonged process, extending well into the postnatal period. In the dentate gyrus of hippocampus, basket cell axon arbors undergo marked maturation between the first and fourth week, and increased connectivity among basket cells contributes to the enhanced coherence of gamma oscillation in local networks (Doischer et al. 2008). In primary visual cortex, the maturation of perisomatic inhibition by basket interneurons proceeds into the fifth postnatal week and may contribute to the regulation of the critical period of plasticity (Huang et al. 1999; Morales et al. 2002). Importantly, the maturation of inhibitory innervation in visual and somatosensory cortex is regulated by sensory experience (Morales et al. 2002; Chattopadhyaya et al. 2004; Jiao et al. 2006). Such activity-dependent development of inhibitory synapses and innervation pattern is a major component of neural circuit assembly, yet the underlying cellular and molecular mechanisms are poorly understood.
GABA signalling regulates inhibitory synapse development
As key mediators of neural activity, neurotransmitters are particularly well suited to couple synaptic signalling with synaptic wiring (Zhang & Poo, 2001; Hua & Smith, 2004). Glutamate, the major excitatory transmitter in vertebrate brain, has been implicated in regulating many aspects of synapse formation, maturation and plasticity (Zheng et al. 1994; Shi et al. 1999; Carroll et al. 1999; Wong & Wong, 2001; Bonhoeffer & Yuste, 2002; Malinow & Malenka, 2002; Tashiro et al. 2003). In addition, through regulating synaptogenesis, glutamate receptor signalling contributes to activity-dependent development of axonal and dendritic arbors (Ruthazer et al. 2003; Hua & Smith, 2004; Hua et al. 2005; Cline & Haas, 2008).
Initially discovered as an inhibitory transmitter, GABA has since been implicated in multiple processes of neural development, from cell proliferation to circuit formation (Owens & Kriegstein, 2002). The trophic effects of GABA on neuronal migration and neurite growth during the embryonic and perinatal period are largely explained by its depolarizing action in immature neurons, resulting from chloride ion efflux through the GABAA receptor, which triggers calcium influx and signalling (Ben-Ari et al. 1989; Leinekugel et al. 1995). During the postnatal period, the up-regulation of the chloride transporter KCC2 in neurons results in increased extrusion of intracellular chloride (Rivera et al. 1999), and GABA assumes its classic role as an inhibitory transmitter (Ben-Ari et al. 2007).
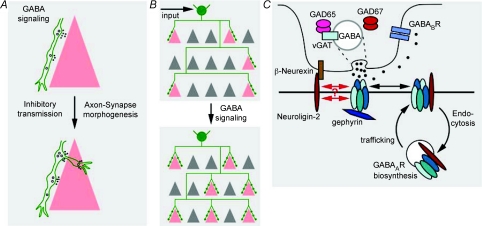
Recently, several studies converge and suggest that, in addition to mediating synaptic inhibition in more mature circuits, GABA signalling promotes and coordinates pre- and post- synaptic maturation during activity-dependent development of inhibitory synapses and innervation (Fig. 2). A main line of evidence came from studying the effects of altering GABA synthesis on the development of perisomatic synapses from PV basket interneurons in the visual cortex. The maturation of many features of basket cell axon arbors and perisomatic synapses can be recapitulated in cortical organotypic cultures (Di Cristo et al. 2004) and is strongly regulated by neuronal activity (Klostermann & Wahle, 1999; Chattopadhyaya et al. 2004). Genetic knockdown of GABA synthesis implicates GABA signalling itself in the development of perisomatic synapses (Chattopadhyaya et al. 2007). GABA is synthesized by two glutamate decarboxylases, GAD67 and GAD65 (Soghomonian & Martin, 1998). Of these two enzymes, GAD67 is the rate-limiting enzyme and influences cellular GABA contents in a dosage-dependent manner (Asada et al. 1997; Ji et al. 1999). Knockdown of GAD67 in single GABAergic interneurons, which should have minimum impact on circuit activity levels, results in profound cell autonomous deficits in synapse formation, axon branching and innervation field in cortical organotypic cultures; such deficits were partially rescued by blocking GABA re-uptake or enhancing GABAA or GABAB receptor function (Chattopadhyaya et al. 2007). Similar deficits were found in visual cortex of Gad67 germline heterozygotes, which show ∼40% reduction of GABA levels (Chattopadhyaya et al. 2007). Conversely, overexpression of Gad67 in single basket interneurons promotes the maturation of perisomatic synapses (Chattopadhyaya et al. 2007). These results demonstrate that GABA acts beyond inhibitory transmission in juvenile and adolescent brain, and regulates the maturation of inhibitory synapses and innervation patterns (Fig. 2), thus revealing a new facet of GABA function distinct from its early tropic action in neonatal brain.
Figure 2. GAD67 and GABA act beyond inhibitory transmission and regulate inhibitory synapse development and innervation patterns.
A, GABA signalling may regulate the morphogenesis of inhibitory synapses. B, since synapse formation is an integral part of axon growth and branching, activity-dependent GABA signalling may further influence the development of GABAergic axon arbor and innervation pattern. C, a hypothetical model depicting how GABA–GABA receptor signalling and neuroligin–neurexin adhesion may interact and co-operate to regulate the development of inhibitory synapses. Pentameric GABAARs are assembled in the endoplasmic reticulum. Most GABAARs are first delivered to extrasynaptic locations, they then either diffuse to and become trapped at postsynaptic sites or undergo endocytosis. NL2 and synaptic GABAARs stabilize each other, either through intracellular reciprocal interactions aided by scaffolding proteins such as gephyrin or through extracellular cis interaction. In addition, GABA activation of GABAARs might further stabilize synaptic GABAARs through structural changes or signalling mechanisms. Such activity- and GABA-mediated stabilization of GABAAR might further increase the levels of NL2 at cell–cell contacts and, in turn, stabilize presynaptic terminals through trans-synaptic interactions with neurexins.
Structural role of GABAA receptors: coupling transmission to synapse maturation and stability
Another line of evidence supporting a role of GABA on the development of inhibitory synapses came from studying the effects of manipulating GABAA receptor (GABAAR) subunits. GABAARs are heteropentameric chloride channels composed of several classes of subunits (Michels & Moss, 2007). Although over 19 subunits have been identified, giving rise to a large number of possible subunit combinations, the vast majority of GABAARs consist of α, β and γ2 subunits in a 2 : 2 : 1 stoichiometry. In the mature brain, GABAARs are primarily localized at postsynaptic and extrasynaptic membranes where they mediate phasic and tonic inhibition, respectively.
The γ2 subunit is essential for accumulation of cell surface GABAARs at postsynaptic sites (Essrich et al. 1998; Schweizer et al. 2003). Acute suppression of γ2 expression in cultured hippocampal neurons not only disrupts GABAAR clustering but also results in a profound reduction of GABAergic innervation of γ2-deficient neurons (Li et al. 2005; Fang et al. 2006). Moreover, when palmitoylation of the γ2 subunit was suppressed by knockdown of the Asp-His-His-Cys (DHHC) family palmitoyltransferase GODZ, trafficking of GABARs to postsynaptic sites was perturbed and GABAergic innervation was reduced (Fang et al. 2006). As both presynaptic GABA and postsynaptic GABAA receptors influence GABAergic synapse development, a simple hypothesis is that activity-dependent GABA signalling promotes the differentiation of pre- and post-synaptic sites, and coordinates the maturation and stabilization of inhibitory synapses.
Further evidence regarding the role of GABAARs in synapse formation came from studies of Purkinje neurons in the cerebellum. Purkinje cells are themselves GABAergic neurons but also receive two types of GABAergic inputs: the axo-somatic synapses from basket interneurons and the axo-dendritic synapses from stellate interneurons, both with GABAARs containing the α1 subunit. Deletion of the α1 subunit gene results in a complete loss of functional GABAARs in Purkinje cells by postnatal day 18 (Fritschy et al. 2006). In these α1−/− mice, GABAergic terminals from stellate axons are initially formed normally onto the Purkinje dendritic shaft. However, starting from postnatal day 11, synaptogenesis is significantly reduced and perturbed (Fritschy et al. 2006; Patrizi et al. 2008). Instead, stellate cell terminals form aberrant and mismatched contacts with postsynaptic specialization on the spines of Purkinje dendrites. These results suggest that initial steps of GABAergic synapse formation can proceed in the absence of α1, but GABAA receptors appear crucial for activity-dependent regulation of synapse density, possibly through promoting the stabilization of transient axodendritic contact into mature synapses. The mechanism linking GABA signalling to synapse maturation are still unclear. Activation of GABAARs may result in the local release of trophic factors which promote inhibitory synapse maturation, and/or act as protective signals that prevent synapses elimination. The failure to stabilize presynaptic terminals after postsynaptic loss of GABAARs suggests the presence of a retrograde signal that is regulated by synaptic activity or by association with postsynaptic GABAARs. Amongst the molecular mechanisms that may contribute to such an activity-regulated trans-synaptic signal, the neuroligin and neurexin complex represents one of the plausible candidates.
From GABAA receptors to synaptic adhesion and activity-dependent retrograde signalling
Neuroligins and neurexins are heterophilic synaptic adhesion molecules broadly expressed in the central nervous system (Brose, 1999; Sudhof, 2008). Cell biological studies have revealed potent ‘synaptogenic’ or synapse-organizing activities for these proteins (recently reviewed in (Levinson & El-Husseini, 2005; Craig & Kang, 2007). Postsynaptic neuroligins promote assembly of functional presynaptic specializations in axons, while presynaptic neurexins – through interaction with neuroligins – recruit postsynaptic scaffolding proteins and transmitter receptors in dendrites.
While neuroligin–neurexin complexes are common building blocks of glutamatergic and GABAergic synapses, analysis of mutant mice so far support their particularly critical roles in the organization of GABAergic synapses. Triple knockout mice lacking the three alpha-neurexin transcripts, although they die at birth, show a 50% reduction in the density of GABAergic synapses in the brainstem (Missler et al. 2003). In double knockout mice, some of which reach adulthood, GABAergic synapse density is reduced by 30% whereas glutamatergic synapse density is apparently unchanged (Dudanova et al. 2007). As for neuroligins, mice lacking the three major isoforms (NL1, 2 and 3), also perinatal lethal, show only a relatively small (15–20%) reduction in the number of synapses in the brainstem, but a severe loss of GABAARs and the scaffolding protein gephyrin from postsynaptic sites (Varoqueaux et al. 2006). Among the different isoforms, NL2 is exclusively localized to GABAergic synapses. NL2−/− mice display a selective decrease in the number of inhibitory synapses in the postnatal neocortex (Chubykin et al. 2007). In addition, layer 2/3 neurons in acute cortical slices from NL2−/− mice show a selective impairment of GABAergic transmission whereas glutamatergic transmission is normal. Overexpression of NL2 in cultured neurons increases the density GABAergic terminals (Chih et al. 2005) and the amplitude of inhibitory postsynaptic currents (Chubykin et al. 2007). Notably, this overexpression-induced increase in GABAergic transmission is blocked by pharmacologically reducing network activity in the culture. Therefore, neuronal and synaptic activity might either regulate the presynaptic response to NL2 or postsynaptic stabilization induced by NL2.
As both neuroligins and GABAA receptors play important roles in the maturation of postsynaptic specializations and the differentiation and stabilization of presynaptic terminals at inhibitory synapses, an obvious question is: how do GABA/GABAAR-mediated synaptic signalling and neuroligin/neurexin-mediated synaptic adhesion interact and cooperate to regulate activity-dependent development of inhibitory synapse? It is currently unknown at what stage of their biosynthetic pathway GABAARs first interact with NLs, and how such interactions might be regulated. One possibility is that NL2 and synaptic GABAARs would stabilize each other, either through intracellular reciprocal interactions aided by scaffolding proteins such as gephyrin or through extracellular cis interactions (Fig. 2). In addition, GABA activation of GABAARs might further stabilize GABAARs at synapses through as yet unknown structural or signalling mechanisms. Such activity- and GABA-mediated stabilization of GABAARs might further increase the levels of NL2 at postsynaptic sites; this, in turn, would stabilize the presynaptic terminals through trans-synaptic interactions with neurexins. Evidence consistent with this model include: (1) in vitro studies demonstrated a co-aggregation of NL2 and the GABAARα2 subunit in heterologous cells (Dong et al. 2007); (2) the residence time of GABAARs on the plasma membrane and their targeting to synapses is regulated by synaptic activity (Saliba et al. 2007); (3) pharmacological blockade of neuronal activity in cultured neurons diminishes the synaptogenic activity of NL2 (Chubykin et al. 2007); (4) reduced GABA synthesis and release result in a reduction of inhibitory synapses (Chattopadhyaya et al. 2007). Moreover, there is precedent for such mechanisms in activity-dependent recruitment of glutamate receptor and trans-synaptic signalling at glutamatergic synapses. Local spontaneous activity and glutamate release reduce diffusion exchange of GluR1 between synaptic and extrasynaptic domains, resulting in postsynaptic accumulation of GluR1 (Ehlers et al. 2007). In addition, PSD-95 and NL1 retrogradely modulate presynaptic release probability and may coordinate post- and pre-synaptic morphological changes (Ehrlich et al. 2007; Futai et al. 2007). It remains to be seen whether analogous mechanisms for GABA and NL2 signalling exist at inhibitory synapses.
In an alternative model, the expression and/or localization of NL2 might be regulated by GABA signalling, either through regulating NL2 protein levels or NL2-interacting proteins involved in its synaptic localization. It is also possible that GABA binding to GABAARs might modulate their coupling to NL2, thereby increasing the potency and affinity of NL2 towards neurexin in the presynaptic terminals.
Activity-regulated Gad67 transcription as a cell-wide mechanism for modulating GABA signalling and innervation pattern
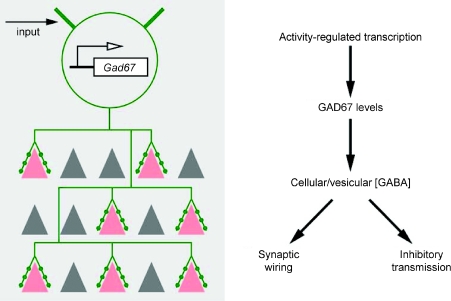
A mechanism unique to GABAergic neurons is activity-dependent GABA synthesis. Unlike glutamate, which is both the precursor and product of many essential metabolic and signalling processes in the cell, GABA can only be synthesized by two glutamate decarboxylases, and the main function of GABA is intercellular signalling (Soghomonian & Martin, 1998). In most brain regions, GAD67 activity is rate-limiting for GABA synthesis (Asada et al. 1996; Kash et al. 1997). Since GAD67 is produced at a limiting level in the brain (Asada et al. 1997), alterations in GAD67 levels influence cellular and vesicular GABA content (Murphy et al. 1998; Engel et al. 2001). Unlike GAD65, which is relatively stable, GAD67 protein has a rather quick turn-over rate, with a half-life of several hours (Christgau et al. 1991; Pinal & Tobin, 1998). The major step in the physiological regulation of GAD67 activity is Gad1 transcription, which is dynamically regulated during development (Kiser et al. 1998), by neural activity (Patz et al. 2003; Kinney et al. 2006) and experience (Benson et al. 1989; Benevento et al. 1995; Liang et al. 1996; Gierdalski et al. 2001; Kobori & Dash, 2006). Therefore, activity-dependent transcription may result in adjustment of GAD67 levels and the intracellular GABA pool for release. As alterations in GAD67 and GABA levels profoundly influence interneuron axon growth and synapse formation during the development of inhibitory circuits, neuronal activity might shape the pattern of inhibitory synaptic innervation through GAD67-mediated GABA synthesis (Fig. 3). Such activity-dependent and cell-wide regulation of a ‘transmitter resource’ implies a novel logic for the maturation of inhibitory synapses and innervation pattern. This hypothesis needs to be tested by disrupting the activity regulation of GAD67 transcription in GABAergic neurons and examining the impact on inhibitory synapse development.
Figure 3.
A scheme showing that the level and pattern of neuronal activity may regulate inhibitory synaptic morphogenesis and innervation patterns through GAD67-mediated GABA synthesis and signalling.
More questions than answers
The converging findings that GABA and GABA receptor signalling regulate inhibitory synapse development raise numerous questions regarding the underlying mechanisms and their functional implications. The many steps from GABA signalling to receptor trafficking/stability and neuroligin–neurexin function remain to be defined. In addition, it is unknown whether and how postsynaptic activity in pyramidal neuron might influence the action of GABA signalling on inhibitory synapse development. Furthermore, because cortical GABAergic neurons not only innervate pyramidal neurons but also other GABAergic neurons, an obvious question is whether and how GABA signalling might regulate the development of inhibitory synapses onto inhibitory neurons. Addressing such questions will require methods to visualize inhibitory synapses onto inhibitory neurons. Finally, although activity regulation of GAD67 transcription has been well demonstrated in numerous developmental and plasticity paradigms, its impact on GABA signalling and inhibitory synapse development and plasticity remains to be established in vivo. Compared with our understanding of the role of glutamate in excitatory synapse development, we are only beginning to scratch the surface of the role of GABA in the development of inhibitory synapses. Progress in this area will not only enhance our understanding of activity-dependent development of inhibitory synapses, axon arbors and innervation patterns, but also might have implications in the construction of cortical subnetworks, such as reciprocally connected groups of excitatory and inhibitory neurons (Yoshimura & Callaway, 2005).
References
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS, Port JD. γ-Aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res. 1995;689:172–182. doi: 10.1016/0006-8993(95)00553-3. [DOI] [PubMed] [Google Scholar]
- Benson DL, Isackson PJ, Hendry SH, Jones EG. Expression of glutamic acid decarboxylase mRNA in normal and monocularly deprived cat visual cortex. Brain Res Mol Brain Res. 1989;5:279–287. doi: 10.1016/0169-328x(89)90062-4. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T, Yuste R. Spine motility. Phenomenology, mechanisms, and function. Neuron. 2002;35:1019–1027. doi: 10.1016/s0896-6273(02)00906-6. [DOI] [PubMed] [Google Scholar]
- Brose N. Synaptic cell adhesion proteins and synaptogenesis in the mammalian central nervous system. Naturwissenschaften. 1999;86:516–524. doi: 10.1007/s001140050666. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Christgau S, Schierbeck H, Aanstoot HJ, Aagaard L, Begley K, Kofod H, Hejnaes K, Baekkeskov S. Pancreatic beta cells express two autoantigenic forms of glutamic acid decarboxylase, a 65-kDa hydrophilic form and a 64-kDa amphiphilic form which can be both membrane-bound and soluble. J Biol Chem. 1991;266:21257–21264. [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, A, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Qi J, Chen G. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABAA receptors. Mol Cell Neurosci. 2007;35:14–23. doi: 10.1016/j.mcn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Dudanova I, Tabuchi K, Rohlmann A, Sudhof TC, Missler M. Deletion of alpha-neurexins does not cause a major impairment of axonal pathfinding or synapse formation. J Comp Neurol. 2007;502:261–274. doi: 10.1002/cne.21305. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Pahner I, Schulze K, Frahm C, Jarry H, Ahnert-Hilger G, Draguhn A. Plasticity of rat central inhibitory synapses through GABA metabolism. J Physiol. 2001;535:473–482. doi: 10.1111/j.1469-7793.2001.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABAA receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoe-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the α1 subunit in Purkinje cells. J Neurosci. 2006;26:3245–3255. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierdalski M, Jablonska B, Siucinska E, Lech M, Skibinska A, Kossut M. Rapid regulation of GAD67 mRNA and protein level in cortical neurons after sensory learning. Cereb Cortex. 2001;11:806–815. doi: 10.1093/cercor/11.9.806. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Ji F, Kanbara N, Obata K. GABA and histogenesis in fetal and neonatal mouse brain lacking both the isoforms of glutamic acid decarboxylase. Neurosci Res. 1999;33:187–194. doi: 10.1016/s0168-0102(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PJ, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of rat somatosensory barrel cortex. J Comp Neurol. 1998;402:62–74. [PubMed] [Google Scholar]
- Klostermann O, Wahle P. Patterns of spontaneous activity and morphology of interneuron types in organotypic cortex and thalamus-cortex cultures. Neuroscience. 1999;92:1243–1259. doi: 10.1016/s0306-4522(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Kobori N, Dash PK. Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. J Neurosci. 2006;26:4236–4246. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Tseeb V, Ben-Ari Y, Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol. 1995;487:319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson JN, El-Husseini A. Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron. 2005;48:171–174. doi: 10.1016/j.neuron.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie S, Miralles CP, Bai J, Loturco JJ, De Blas AL. Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J Neurochem. 2005;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Liang F, Isackson PJ, Jones EG. Stimulus-dependent, reciprocal up- and downregulation of glutamic acid decarboxylase and Ca2+/calmodulin-dependent protein kinase II gene expression in rat cerebral cortex. Exp Brain Res. 1996;110:163–174. doi: 10.1007/BF00228548. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit Rev Biochem Mol Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Patrizi A, Scelfo B, Viltono L, Briatore F, Fukaya M, Watanabe M, Strata P, Varoqueaux F, Brose N, Fritschy JM, Sassoe-Pognetto M. Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors. Proc Natl Acad Sci U S A. 2008;105:13151–13156. doi: 10.1073/pnas.0802390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz S, Wirth MJ, Gorba T, Klostermann O, Wahle P. Neuronal activity and neurotrophic factors regulate GAD-65/67 mRNA and protein expression in organotypic cultures of rat visual cortex. Eur J Neurosci. 2003;18:1–12. doi: 10.1046/j.1460-9568.2003.02702.x. [DOI] [PubMed] [Google Scholar]
- Pinal CS, Tobin AJ. Uniqueness and redundancy in GABA production. Perspect Dev Neurobiol. 1998;5:109–118. [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl– co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301:66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. Activity-dependent ubiquitination of GABAA receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27:13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. The γ2 subunit of GABAA receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol. 1997;500:715–738. doi: 10.1113/jphysiol.1997.sp022054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Dunaevsky A, Blazeski R, Mason CA, Yuste R. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors: a two-step model of synaptogenesis. Neuron. 2003;38:773–784. doi: 10.1016/s0896-6273(03)00299-x. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 2002;12:395–410. doi: 10.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- Wong WT, Wong RO. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nat Neurosci. 2001;4:351–352. doi: 10.1038/85987. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4:1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]